Assessment of Mancozeb Exposure, Absorbed Dose, and Oxidative Damage in Greenhouse Farmers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects Enrolled and Timeline of the Study
2.3. Pads, Blood and Urine Sampling, Transport and Conservation Procedures
2.4. Chemicals
2.5. Pads and Urine Sample Processing and Calibration Analyses
2.6. Serum Biomarkers for the Measurement of Oxidative Stress
2.7. Data Processing and Exposure Assessments
2.8. Statistical Analyses
3. Results
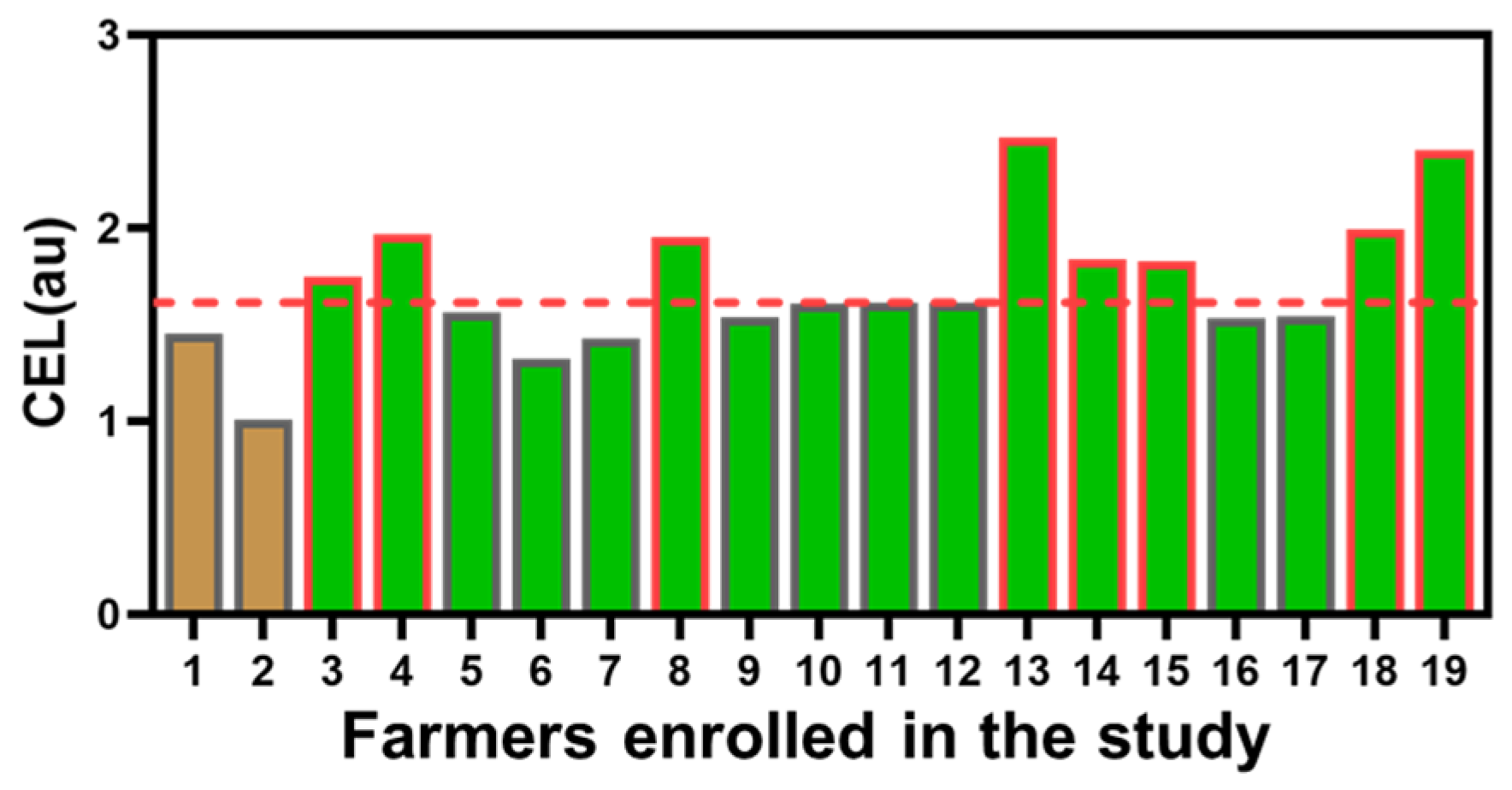
3.1. Chronic Exposure Levels Were Significantly Higher in the Seedling Group Compared with the Spreading Group
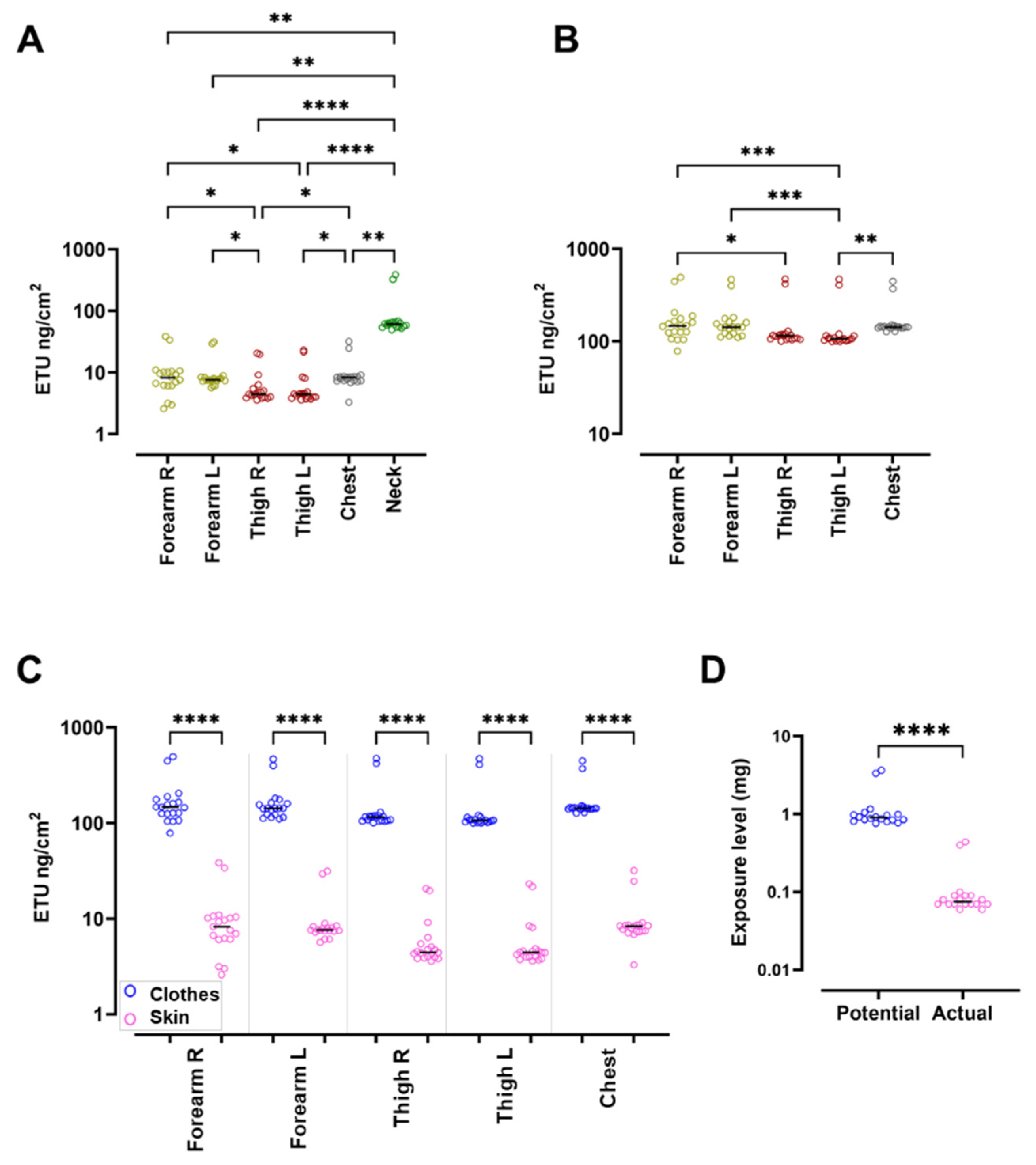
3.2. Exposure Assessment in Pads Shows Significantly Higher ETU Levels in the Spreading Group
3.3. ETU Excreted in Urine Was Significantly Higher in the Spreading Group
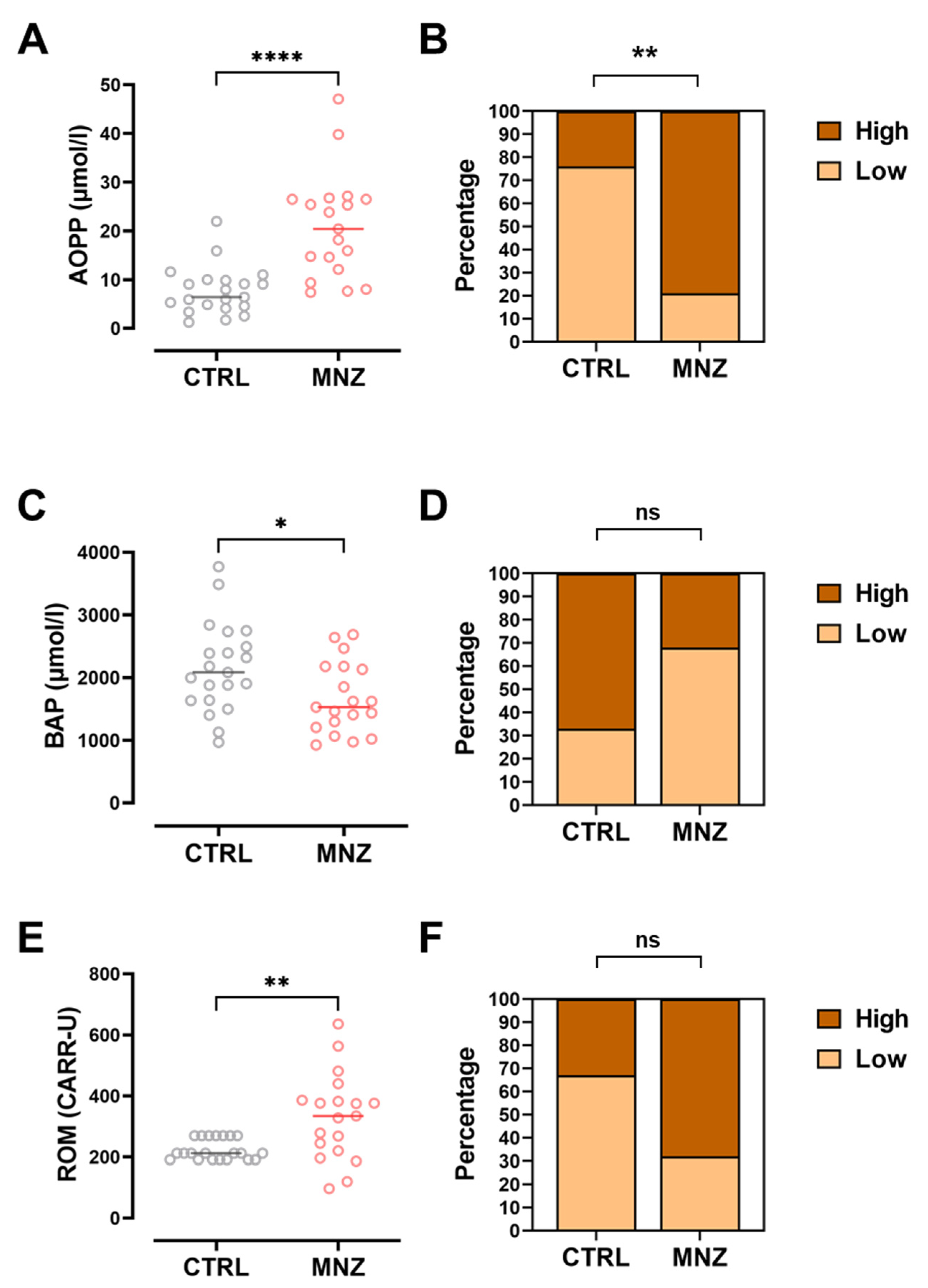
3.4. Oxidative Stress Marker Levels Are Associated with the Risk Evaluation from Pads and Urine Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NLM. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Mancozeb (accessed on 2 July 2022).
- Al-Alam, J.; Bom, L.; Chbani, A.; Fajloun, Z.; Millet, M. Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption Spectrometry. J. Chromatogr. Sci. 2016, 55, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/2087/oj (accessed on 2 July 2022).
- Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Mancozeb. EFSA J. 2020, 18, e05755. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, Y.; Wen, R.; Zhang, L.; Wang, X. Low Level of Mancozeb Exposure Affects Ovary in Mice. Ecotoxicol. Environ. Saf. 2022, 239, 113670. [Google Scholar] [CrossRef]
- Panis, C.; Candiotto, L.Z.P.; Gaboardi, S.C.; Gurzenda, S.; Cruz, J.; Castro, M.; Lemos, B. Widespread Pesticide Contamination of Drinking Water and Impact on Cancer Risk in Brazil. Environ. Int. 2022, 165, 107321. [Google Scholar] [CrossRef]
- Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union Report on Pesticide Residues in Food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef]
- Costa, C.; Briguglio, G.; Catanoso, R.; Giambò, F.; Polito, I.; Teodoro, M.; Fenga, C. New Perspectives on Cytokine Pathways Modulation by Pesticide Exposure. Curr. Opin. Toxicol. 2020, 19, 99–104. [Google Scholar] [CrossRef]
- Cocco, P. Time for Re-Evaluating the Human Carcinogenicity of Ethylenedithiocarbamate Fungicides? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2632. [Google Scholar] [CrossRef]
- Tait, S.; Lori, G.; Tassinari, R.; La Rocca, C.; Maranghi, F. In Vitro Assessment and Toxicological Prioritization of Pesticide Mixtures at Concentrations Derived from Real Exposure in Occupational Scenarios. Int. J. Environ. Res. Public Health 2022, 19, 5202. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global Trends in Pesticides: A Looming Threat and Viable Alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Thind, T.S.; Hollomon, D.W. Thiocarbamate Fungicides: Reliable Tools in Resistance Management and Future Outlook. Pest Manag. Sci. 2018, 74, 1547–1551. [Google Scholar] [CrossRef]
- Angela, M.; Wang, R. Biomonitoring of Contemporary Pesticides: Ethylenethiourea in Occupational Settings. In Pesticides in the Modern World-Effects of Pesticides Exposure; InTech: London, UK, 2011. [Google Scholar]
- Saraiva, M.A.; de Carvalho, N.R.; Martins, I.K.; Macedo, G.E.; Rodrigues, N.R.; de Brum Vieira, P.; Prigol, M.; Gomes, K.K.; Ziech, C.C.; Franco, J.L.; et al. Mancozeb Impairs Mitochondrial and Bioenergetic Activity in Drosophila Melanogaster. Heliyon 2021, 7, e06007. [Google Scholar] [CrossRef] [PubMed]
- Mandić-Rajčević, S.; Rubino, F.M.; Colosio, C. Establishing Health-Based Biological Exposure Limits for Pesticides: A Proof of Principle Study Using Mancozeb. Regul. Toxicol. Pharmacol. 2020, 115, 104689. [Google Scholar] [CrossRef] [PubMed]
- Colosio, C.; Fustinoni, S.; Birindelli, S.; Bonomi, I.; De Paschale, G.; Mammone, T.; Tiramani, M.; Vercelli, F.; Visentin, S.; Maroni, M. Ethylenethiourea in Urine as an Indicator of Exposure to Mancozeb in Vineyard Workers. Toxicol. Lett. 2002, 134, 133–140. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency EPA. Substance Registry Services (SRS). Available online: https://sor.epa.gov/sor_internet/registry/substreg/searchandretrieve/advancedsearch/externalSearch.do?p_type=CASNO&p_value=8018-01-7 (accessed on 2 July 2022).
- Dall’Agnol, J.; Ferri Pezzini, M.; Suarez Uribe, D.; Joveleviths, D. Systemic Effects of the Pesticide Mancozeb–A Literature Review. Eur. Rev. Med. Pharm. Sci. 2021, 25, 4113–4120. [Google Scholar] [CrossRef]
- Costa, C.; Teodoro, M.; Rugolo, C.A.; Alibrando, C.; Giambò, F.; Briguglio, G.; Fenga, C. MicroRNAs Alteration as Early Biomarkers for Cancer and Neurodegenerative Diseases: New Challenges in Pesticides Exposure. Toxicol. Rep. 2020, 7, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Axelstad, M.; Boberg, J.; Nellemann, C.; Kiersgaard, M.; Jacobsen, P.R.; Christiansen, S.; Hougaard, K.S.; Hass, U. Exposure to the Widely Used Fungicide Mancozeb Causes Thyroid Hormone Disruption in Rat Dams but No Behavioral Effects in the Offspring. Toxicol. Sci. 2011, 120, 439–446. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Advisory Group Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024. Available online: https://www.iarc.who.int/news-events/report-of-the-advisory-group-to-recommend-priorities-for-the-iarc-monographs-during-2020-2024/ (accessed on 2 July 2022).
- Ziech, C.C.; Rodrigues, N.R.; Macedo, G.E.; Gomes, K.K.; Martins, I.K.; Franco, J.L.; Posser, T. Pre-Imaginal Exposure to Mancozeb Induces Morphological and Behavioral Deficits and Oxidative Damage in Drosophila Melanogaster. Drug Chem. Toxicol. 2022, 1–13. [Google Scholar] [CrossRef]
- Fuhrimann, S.; Farnham, A.; Staudacher, P.; Atuhaire, A.; Manfioletti, T.; Niwagaba, C.B.; Namirembe, S.; Mugweri, J.; Winkler, M.S.; Portengen, L.; et al. Exposure to Multiple Pesticides and Neurobehavioral Outcomes among Smallholder Farmers in Uganda. Environ. Int. 2021, 152, 106477. [Google Scholar] [CrossRef]
- Bastías-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol. Neurobiol. 2019, 56, 1044–1055. [Google Scholar] [CrossRef]
- Cecconi, S.; Paro, R.; Rossi, G.; Macchiarelli, G. The Effects of the Endocrine Disruptors Dithiocarbamates on the Mammalian Ovary with Particular Regard to Mancozeb. Curr. Pharm. Des. 2007, 13, 2989–3004. [Google Scholar] [CrossRef]
- Saraiva, M.A.; da Rosa Ávila, E.; da Silva, G.F.; Macedo, G.E.; Rodrigues, N.R.; de Brum Vieira, P.; Nascimento, M.S.; Picoloto, R.S.; Martins, I.K.; de Carvalho, N.R.; et al. Exposure of Drosophila Melanogaster to Mancozeb Induces Oxidative Damage and Modulates Nrf2 and HSP70/83. Oxid. Med. Cell. Longev. 2018, 2018, 5456928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.-L.; He, S.; Chen, M.-H.; Zhang, Z.; Fu, X.-P.; Fu, B.-B.; Liao, B.-Q.; Lin, Y.-H.; Qi, Z.-Q.; et al. Protective Effects of Resveratrol against Mancozeb Induced Apoptosis Damage in Mouse Oocytes. Oncotarget 2017, 8, 6233–6245. [Google Scholar] [CrossRef] [PubMed]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A Systematic Review of Mancozeb as a Reproductive and Developmental Hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef]
- Chhabra, R. Comparative Carcinogenicity of Ethylene Thiourea with or without Perinatal Exposure in Rats and Mice. Fundam. Appl. Toxicol. 1992, 18, 405–417. [Google Scholar] [CrossRef]
- Calviello, G.; Piccioni, E.; Boninsegna, A.; Tedesco, B.; Maggiano, N.; Serini, S.; Wolf, F.; Palozza, P. DNA Damage and Apoptosis Induction by the Pesticide Mancozeb in Rat Cells: Involvement of the Oxidative Mechanism. Toxicol. Appl. Pharmacol. 2006, 211, 87–96. [Google Scholar] [CrossRef]
- Costa, C.; Miozzi, E.; Teodoro, M.; Fenga, C. Influence of Genetic Polymorphism on Pesticide-Induced Oxidative Stress. Curr. Opin. Toxicol. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Lijinsky, W. Induction of Tumors of the Nasal Cavity in Rats by Concurrent Feeding of Thiram and Sodium Nitrite. J. Toxicol. Environ. Health 1984, 13, 609–614. [Google Scholar] [CrossRef]
- Kumar, K.; Sabarwal, A.; Singh, R.P. Mancozeb Selectively Induces Mitochondrial-Mediated Apoptosis in Human Gastric Carcinoma Cells through ROS Generation. Mitochondrion 2019, 48, 1–10. [Google Scholar] [CrossRef]
- Santos, P.M.; Simões, T.; Sá-Correia, I. Insights into Yeast Adaptive Response to the Agricultural Fungicide Mancozeb: A Toxicoproteomics Approach. Proteomics 2009, 9, 657–670. [Google Scholar] [CrossRef]
- Corsini, E.; Viviani, B.; Birindelli, S.; Gilardi, F.; Torri, A.; Codecà, I.; Lucchi, L.; Bartesaghi, S.; Galli, C.L.; Marinovich, M.; et al. Molecular Mechanisms Underlying Mancozeb-Induced Inhibition of TNF-Alpha Production. Toxicol. Appl. Pharmacol. 2006, 212, 89–98. [Google Scholar] [CrossRef]
- Singh, S.K.; Bano, F.; Mohanty, B. Vitamin E Pretreatment Prevents the Immunotoxicity of Dithiocarbamate Pesticide Mancozeb in Vitro: A Comparative Age-Related Assessment in Mice and Chick. Pestic. Biochem. Physiol. 2016, 126, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Gök, E.; Deveci, E. Histopathological, Immunohistochemical and Biochemical Alterations in Liver Tissue after Fungicide-Mancozeb Exposures in Wistar Albino Rats. Acta Cir. Bras. 2022, 37, e370404. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Birindelli, S.; Fustinoni, S.; De Paschale, G.; Mammone, T.; Visentin, S.; Galli, C.L.; Marinovich, M.; Colosio, C. Immunomodulatory Effects of the Fungicide Mancozeb in Agricultural Workers. Toxicol. Appl. Pharmacol. 2005, 208, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Kim, C.S.; Kim, J.H. Human Exposure Assessment to Mancozeb During Treatment of Mandarin Fields. Bull. Environ. Contam. Toxicol. 2003, 70, 336–342. [Google Scholar] [CrossRef]
- Dereumeaux, C.; Mercier, F.; Soulard, P.; Hulin, M.; Oleko, A.; Pecheux, M.; Fillol, C.; Denys, S.; Quenel, P. Identification of Pesticides Exposure Biomarkers for Residents Living Close to Vineyards in France. Environ. Int. 2022, 159, 107013. [Google Scholar] [CrossRef]
- Hernández, A.F.; Gil, F.; Lacasaña, M.; Rodríguez-Barranco, M.; Tsatsakis, A.M.; Requena, M.; Parrón, T.; Alarcón, R. Pesticide Exposure and Genetic Variation in Xenobiotic-Metabolizing Enzymes Interact to Induce Biochemical Liver Damage. Food Chem. Toxicol. 2013, 61, 144–151. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Aprea, M.C. Environmental and Biological Monitoring in the Estimation of Absorbed Doses of Pesticides. Toxicol. Lett. 2012, 210, 110–118. [Google Scholar] [CrossRef]
- Rubino, F.M.; Mandic-Rajcevic, S.; Ariano, E.; Alegakis, A.; Bogni, M.; Brambilla, G.; De Paschale, G.; Firmi, A.; Minoia, C.; Micoli, G.; et al. Farmers’ Exposure to Herbicides in North Italy: Assessment under Real-Life Conditions in Small-Size Rice and Corn Farms. Toxicol. Lett. 2012, 210, 189–197. [Google Scholar] [CrossRef]
- Ross, R.D.; Crosby, D.G. Photolysis of Ethylenethiourea. J. Agric. Food Chem. 1973, 21, 335–337. [Google Scholar] [CrossRef]
- Kurttio, P.; Vartiainen, T.; Savolainen, K. Environmental and Biological Monitoring of Exposure to Ethylenebisdithiocarbamate Fungicides and Ethylenethiourea. Occup. Environ. Med. 1990, 47, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ekman, E.; Maxe, M.; Littorin, M.; Jönsson, B.A.G.; Lindh, C.H. High-Throughput Method for the Analysis of Ethylenethiourea with Direct Injection of Hydrolysed Urine Using Online on-Column Extraction Liquid Chromatography and Triple Quadrupole Mass Spectrometry. J. Chromatogr. B 2013, 934, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Kawabe, H.; Komiya, N.; Saito, I. Relations between Serum Reactive Oxygen Metabolites (ROMs) and Various Inflammatory and Metabolic Parameters in a Japanese Population. J. Atheroscler. Thromb. 2009, 16, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kakita, H.; Hussein, M.H.; Daoud, G.A.; Kato, T.; Murai, H.; Sugiura, T.; Mizuno, K.; Yamada, Y.; Ito, T.; Fukuda, S.; et al. Total Hydroperoxide and Biological Antioxidant Potentials in a Neonatal Sepsis Model. Pediatr. Res. 2006, 60, 675–679. [Google Scholar] [CrossRef]
- Costa, C.; Gangemi, S.; Giambò, F.; Rapisarda, V.; Caccamo, D.; Fenga, C. Oxidative Stress Biomarkers and Paraoxonase 1 Polymorphism Frequency in Farmers Occupationally Exposed to Pesticides. Mol. Med. Rep. 2015, 12, 6353–6357. [Google Scholar] [CrossRef]
- Fenske, R.A.; Lu, C.; Curl, C.L.; Shirai, J.H.; Kissel, J.C. Biologic Monitoring to Characterize Organophosphorus Pesticide Exposure among Children and Workers: An Analysis of Recent Studies in Washington State. Environ. Health Perspect. 2005, 113, 1651–1657. [Google Scholar] [CrossRef]
- Mandic-Rajcevic, S.; Rubino, F.M.; Ariano, E.; Cottica, D.; Neri, S.; Colosio, C. Environmental and Biological Monitoring for the Identification of Main Exposure Determinants in Vineyard Mancozeb Applicators. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 289–296. [Google Scholar] [CrossRef]
- Mandic-Rajcevic, S.; Rubino, F.M.; Ariano, E.; Cottica, D.; Negri, S.; Colosio, C. Exposure Duration and Absorbed Dose Assessment in Pesticide-Exposed Agricultural Workers: Implications for Risk Assessment and Modeling. Int. J. Hyg. Environ. Health 2019, 222, 494–502. [Google Scholar] [CrossRef]
- Colosio, C.; Visentin, S.; Birindelli, S.; Campo, L.; Fustinoni, S.; Mariani, F.; Tiramani, M.; Tommasini, M.; Brambilla, G.; Maroni, M. Reference Values for Ethylenethiourea in Urine in Northern Italy: Results of a Pilot Study. Toxicol. Lett. 2006, 162, 153–157. [Google Scholar] [CrossRef]
- Stadler, K.; Li, X.; Liu, B.; Bao, W.; Wang, K.; Lehmler, H.-J. Systematic Review of Human Biomonitoring Studies of Ethylenethiourea, a Urinary Biomarker for Exposure to Dithiocarbamate Fungicides. Environ. Pollut. 2022, 292, 118419. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Lacasaña, M.; Gil, F.; Rodríguez-Barranco, M.; Pla, A.; López-Guarnido, O. Evaluation of Pesticide-Induced Oxidative Stress from a Gene–Environment Interaction Perspective. Toxicology 2013, 307, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Kilk, K.; Meitern, R.; Härmson, O.; Soomets, U.; Hõrak, P. Assessment of Oxidative Stress in Serum by D-ROMs Test. Free Radic. Res. 2014, 48, 883–889. [Google Scholar] [CrossRef]
- Lubrano, V.; Vassalle, C.; L’Abbate, A.; Zucchelli, G. A New Method to Evaluate Oxidative Stress in Humans. Immuno-Anal. Biol. Spécial. 2002, 17, 172–175. [Google Scholar] [CrossRef]
- Paro, R.; Tiboni, G.M.; Buccione, R.; Rossi, G.; Cellini, V.; Canipari, R.; Cecconi, S. The Fungicide Mancozeb Induces Toxic Effects on Mammalian Granulosa Cells. Toxicol. Appl. Pharmacol. 2012, 260, 155–161. [Google Scholar] [CrossRef] [PubMed]


| All (N = 19) | Spreading (N = 2) | Seedling (N = 17) | p Value | |
|---|---|---|---|---|
| Males (N, %) | 2 (11%) | 2 (100%) | 0 | na |
| Females (N, %) | 17 (89%) | 0 | 2 (100%) | na |
| Age (years) | 44 (21–69) | 37 (31–42) | 45 (21–69) | 0.333 |
| Height (m) | 1.63 (1.50–1.77) | 1.72 (1.67–1.77) | 1.58 (1.50–1.75) | 0.078 |
| Weight (kg) | 65 (47–106) | 83 (80–85) | 65 (47–106) | 0.245 |
| BMI (kg/m2) | 24.7 (17.9–38.9) | 27.9 (27.1–28.7) | 24.1 (17.9–38.9) | 0.729 |
| BSA (m2) | 1.72 (1.40–2.20) | 1.99 (1.93–2.04) | 1.71 (1.40–2.20) | 0.125 |
| Working seniority (years) | 6 (3–19) | 8 (5–10) | 6 (3–19) | 0.994 |
| Exposure duration (days per year) | 220 (24–220) | 45 (24–66) | 220 | <0.0001 |
| Chronic exposure levels (au) | 1.61 (1.01–2.47) | 1.23 (1.01–1.45) | 1.61 (1.32–1.47) | 0.039 |
| Activity | No | Yes |
|---|---|---|
| Cigarette smoking | 11 (58%) | 8 (42%) |
| Alcohol consumption | 19 (100%) | 0 |
| Daily coffee/tea use | 4 (21%) | 15 (79%) |
| Daily vegetable consumption | 0 | 19 (100%) |
| Daily fruit consumption | 4 (21%) | 15 (79%) |
| Symptoms possibly related to exposure (last 12 months) | 14 (74%) | 5 (26%) * |
| Occurring pathologies | 13 (68%) | 6 (32%) ** |
| Medication usage in the last 30 days | 10 (53%) | 9 (47%) *** |
| All (N = 19) | Spreading (N = 2) | Seedling (N = 17) | p Value | |
|---|---|---|---|---|
| PSE (mg) | 0.90 (0.76–3.65) | 3.49 (3.33–3.65) | 0.87 (0.76–1.16) | <0.0001 |
| ASE (mg) | 0.07 (0.06–0.44) | 0.42 (0.40–0.44) | 0.07 (0.06–0.10) | <0.0001 |
| Protection percentage (%) | 92 (89–93) | 89 | 92 (92–93) | <0.0001 |
| ETU absorbed (µg/kg bw) | 0.31 (1.44–0.24) | 1.33 (1.23–1.44) | 0.30 (0.24–0.38) | <0.0001 |
| Risk ETUpads (%) | 15 (12–72) | 67 (62–72) | 15 (12–19) | <0.0001 |
| ETUurine (µg/g creatinine) | 8.0 (0.5–203.8) | 171.6 (139.5–203.8) | 6.3 (0.5–79.3) | <0.0001 |
| ETUurine above 2.5 µg/g creatinine (N, %) | 14 (74%) | 2 (100%) | 12 (71%) | na * |
| Risk ETUurine (%) | 2.8 (0.1–59.9) | 51.8 (43.6–59.9) | 2.5 (0.1–30.5) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.; Teodoro, M.; Giambò, F.; Catania, S.; Vivarelli, S.; Fenga, C. Assessment of Mancozeb Exposure, Absorbed Dose, and Oxidative Damage in Greenhouse Farmers. Int. J. Environ. Res. Public Health 2022, 19, 10486. https://doi.org/10.3390/ijerph191710486
Costa C, Teodoro M, Giambò F, Catania S, Vivarelli S, Fenga C. Assessment of Mancozeb Exposure, Absorbed Dose, and Oxidative Damage in Greenhouse Farmers. International Journal of Environmental Research and Public Health. 2022; 19(17):10486. https://doi.org/10.3390/ijerph191710486
Chicago/Turabian StyleCosta, Chiara, Michele Teodoro, Federica Giambò, Stefania Catania, Silvia Vivarelli, and Concettina Fenga. 2022. "Assessment of Mancozeb Exposure, Absorbed Dose, and Oxidative Damage in Greenhouse Farmers" International Journal of Environmental Research and Public Health 19, no. 17: 10486. https://doi.org/10.3390/ijerph191710486
APA StyleCosta, C., Teodoro, M., Giambò, F., Catania, S., Vivarelli, S., & Fenga, C. (2022). Assessment of Mancozeb Exposure, Absorbed Dose, and Oxidative Damage in Greenhouse Farmers. International Journal of Environmental Research and Public Health, 19(17), 10486. https://doi.org/10.3390/ijerph191710486









