Lifestyle Factors Associated with Metabolic Syndrome in Urban Cambodia
Abstract
:1. Introduction
2. Materials and Methods
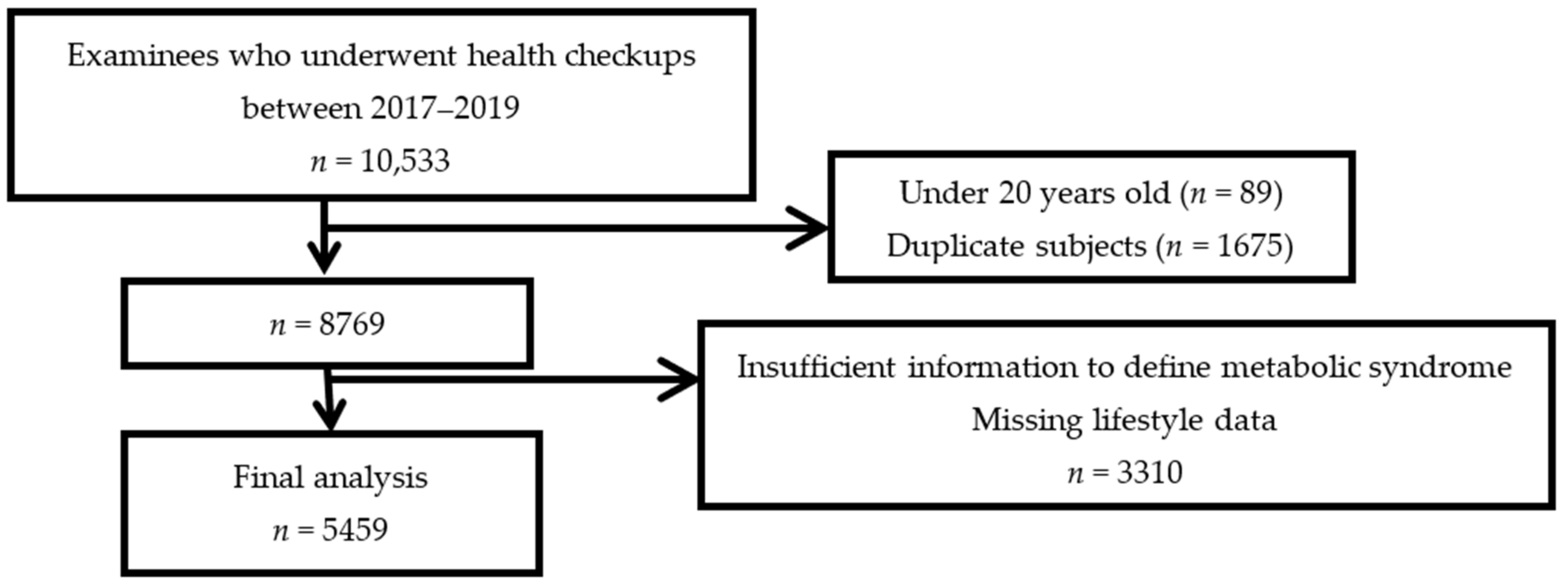
2.1. Data Source and Study Population
2.2. Variables
2.2.1. Definition of Metabolic Syndrome
2.2.2. Lifestyle Factors
2.2.3. Anthropometric and Biochemical Measurements
2.3. Statistical Analysis
2.4. Ethical Consideration
3. Results
3.1. Characteristics of Participants
3.2. Characteristics of Participants with and without MetS
3.3. Lifestyle Factors Associated with MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, C.H.; Kim, K.J.; Lee, Y.K.; Kwon, J.H.; Lee, B.W.; Kwon, H.S.; Park, J.Y.; Khun, T.; Cha, B.Y.; Cho, N.H.; et al. The Glycemic Status of Diabetes in an Urban Area of Cambodia. Diabetes Res. Clin. Pract. 2014, 104, e34–e37. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fact Sheets: Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 15 August 2022).
- World Health Organization. Top 10 Causes of Death in Cambodia for Both Sexes Aged All Ages. 2019. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 15 August 2022).
- Goryakin, Y.; Rocco, L.; Suhrcke, M. The Contribution of Urbanization to Non-Communicable Diseases: Evidence from 173 Countries from 1980 to 2008. Econ. Hum. Biol. 2017, 26, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A.; Dyerberg, J.; Selleck, M.; Stender, S. Nutrition Transition and Its Relationship to the Development of Obesity and Related Chronic Diseases. Obes. Rev. 2008, 9, 48–52. [Google Scholar] [CrossRef]
- Popkin, B.M. The Nutrition Transition and Obesity in the Developing World. J. Nutr. 2001, 131, 871S–873S. [Google Scholar] [CrossRef]
- Popkin, B.M. Urbanization, Lifestyle Changes and the Nutrition Transition. World Dev. 1999, 27, 1905–1916. [Google Scholar] [CrossRef]
- Monda, K.L.; Gordon-Larsen, P.; Stevens, J.; Popkin, B.M. China’s Transition: The Effect of Rapid Urbanization on Adult Occupational Physical Activity. Soc. Sci. Med. 2007, 64, 858–870. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome, a Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atheroscleroses Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Chu, A.H.Y.; Moy, F.M. Association between Physical Activity and Metabolic Syndrome among Malay Adults in a Developing Country, Malaysia. J. Sci. Med. Sport 2014, 17, 195–200. [Google Scholar] [CrossRef]
- Katano, S.; Nakamura, Y.; Nakamura, A.; Murakami, Y.; Tanaka, T.; Nakagawa, H.; Takebayashi, T.; Yamato, H.; Okayama, A.; Miura, K.; et al. Relationship among Physical Activity, Smoking, Drinking and Clustering of the Metabolic Syndrome Diagnostic Components. J. Atheroscler. Thromb. 2010, 17, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S.; Kohl, H.W.; Mokdad, A.H.; Ajani, U.A. Sedentary Behavior, Physical Activity, and the Metabolic Syndrome among U.S. Adults. Obes. Res. 2005, 13, 608–614. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Ning, G. Active Smoking and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef] [Green Version]
- Tajima, M.; Lee, J.S.; Watanabe, E.; Park, J.S.; Tsuchiya, R.; Fukahori, A.; Mori, K.; Kawakubo, K. Association Between Changes in 12 Lifestyle Behaviors and the Development of Metabolic Syndrome During 1 Year Among Workers in the Tokyo Metropolitan Area. Circ. J. 2014, 78, 1152–1159. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kim, H.J.; Min, K.; Lee, H.; Lee, S.-H.; Kim, S.; Kim, J.S.; Oh, B. The Relationship between Smoking Cigarettes and Metabolic Syndrome: A Cross-Sectional Study with Non-Single Residents of Seoul under 40 Years Old. PLoS ONE 2021, 16, e0256257. [Google Scholar] [CrossRef]
- Lin, C.H.; Chiang, S.L.; Heitkemper, M.M.L.; Hung, Y.J.; Lee, M.S.; Tzeng, W.C.; Chiang, L.C. Effects of Telephone-Based Motivational Interviewing in Lifestyle Modification Program on Reducing Metabolic Risks in Middle-Aged and Older Women with Metabolic Syndrome: A Randomized Controlled Trial. Int. J. Nurs. Stud. 2016, 60, 12–23. [Google Scholar] [CrossRef]
- Den Boer, A.T.; Herraets, I.J.T.; Stegen, J.; Roumen, C.; Corpeleijn, E.; Schaper, N.C.; Feskens, E.; Blaak, E.E. Prevention of the Metabolic Syndrome in IGT Subjects in a Lifestyle Intervention: Results from the SLIM Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1147–1153. [Google Scholar] [CrossRef] [Green Version]
- Saboya, P.P.; Bodanese, L.C.; Zimmermann, P.R.; Gustavo, A.D.S.; Macagnan, F.E.; Feoli, A.P.; Oliveira, M.D.S. Lifestyle Intervention on Metabolic Syndrome and Its Impact on Quality of Life: A Randomized Controlled Trial. Arq. Bras. Cardiol. 2017, 108, 60–69. [Google Scholar] [CrossRef]
- Tsushita, K.; Hosler, A.S.; Miura, K.; Ito, Y.; Fukuda, T.; Kitamura, A.; Tatara, K. Rationale and Descriptive Analysis of Specific Health Guidance: The Nationwide Lifestyle Intervention Program Targeting Metabolic Syndrome in Japan. J. Atheroscler. Thromb. 2018, 25, 308–322. [Google Scholar] [CrossRef] [Green Version]
- Nanri, A.; Miyaji, N.; Kochi, T.; Eguchi, M.; Kabe, I.; Mizoue, T. Eating Speed and Risk of Metabolic Syndrome among Japanese Workers: The Furukawa Nutrition and Health Study. Nutrition 2020, 78, 110962. [Google Scholar] [CrossRef]
- Lorzadeh, E.; Sangsefidi, Z.S.; Mirzaei, M.; Hosseinzadeh, M. Dietary Habits and Their Association with Metabolic Syndrome in a Sample of Iranian Adults: A Population-based Study. Food Sci. Nutr. 2020, 8, 6217–6225. [Google Scholar] [CrossRef]
- Okoro, C.A.; Zhong, Y.; Ford, E.S.; Balluz, L.S.; Strine, T.W.; Mokdad, A.H. Association between the metabolic syndrome and its components and gait speed among U.S. adults aged 50 years and older: A cross-sectional analysis. BMC Public Health 2006, 6, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozal, D.; Dumin, M.; Koren, D. Role of sleep quality in the metabolic syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 281–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preverence of Non-Communicable Disease Risk Factors in Cambodia: STEPS Survey Country Report; University of Health Sciences, Preventive Medicine Department of the Ministry of Health: Phnom Penh, Cambodia, 2010.
- Sim, S.; Laohasiriwong, W. Fast Food Consumption, Overweight and Obesity among Working Age Persons in Cambodia. J. Clin. Diagn. Res. 2019, 13, LC01–LC06. [Google Scholar] [CrossRef]
- General Population Census of the Kingdom of Cambodia 2019. 2020. Available online: http://nis.gov.kh/nis/Census2019/Final%20General%20Population%20Census%202019-English.pdf (accessed on 15 August 2022).
- Zhu, B.; Haruyama, Y.; Muto, T.; Yamazaki, T. Association Between Eating Speed and Metabolic Syndrome in a Three-Year Population-Based Cohort Study. J. Epidemiol. 2015, 25, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Yang, K.; Huang, F.; Liu, X.; Li, X.; Luo, Y.; Wu, L.; Guo, X. Association between Self-Reported Eating Speed and Metabolic Syndrome in a Beijing Adult Population: A Cross-Sectional Study. BMC Public Health 2018, 18, 855. [Google Scholar] [CrossRef] [Green Version]
- Shin, A.; Lim, S.Y.; Sung, J.; Shin, H.R.; Kim, J. Dietary Intake, Eating Habits, and Metabolic Syndrome in Korean Men. J. Am. Diet. Assoc. 2009, 109, 633–640. [Google Scholar] [CrossRef]
- Maruyama, K.; Sato, S.; Ohira, T.; Maeda, K.; Noda, H.; Kubota, Y.; Nishimura, S.; Kitamura, A.; Kiyama, M.; Okada, T.; et al. The Joint Impact on Being Overweight of Self Reported Behaviours of Eating Quickly and Eating until Full: Cross Sectional Survey. BMJ 2008, 337, a2002. [Google Scholar] [CrossRef] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Totsuka, K.; Maeno, T.; Saito, K.; Kodama, S.; Asumi, M.; Yachi, Y.; Hiranuma, Y.; Shimano, H.; Yamada, N.; Ono, Y.; et al. Self-Reported Fast Eating is a Potent Predictor of Development of Impaired Glucose Tolerance in Japanese Men and Women: Tsukuba Medical Center Study. Diabetes Res. Clin. Pract. 2011, 94, e72–e74. [Google Scholar] [CrossRef]
- Lee, S.W.; Jang, S.-I. Association of Alcohol Drinking Patterns with Metabolic Syndrome and Its Components in Korean Adults: The Korea National Health and Nutrition Examination Survey 2016–2018. Int. J. Environ. Res. Public Health 2021, 18, 6433. [Google Scholar] [CrossRef]
- Kikuchi, A.; Monma, T.; Ozawa, S.; Tsuchida, M.; Tsuda, M.; Takeda, F. Risk Factors for Multiple Metabolic Syndrome Components in Obese and Non-Obese Japanese Individuals. Prev. Med. 2021, 153, 106855. [Google Scholar] [CrossRef]
- Lin, Y.; Ying, Y.-Y.; Li, S.-X.; Wang, S.-J.; Gong, Q.-H.; Li, H. Association between Alcohol Consumption and Metabolic Syndrome among Chinese Adults. Public Health Nutr. 2020, 24, 4582–4590. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.; Lee, J.-K.; Choi, J.-Y.; Shin, A.; Park, S.K.; Kang, D.; Park, S.M. Association between Change in Alcohol Consumption and Metabolic Syndrome: Analysis from the Health Examinees Study. Diabetes Metab. J. 2019, 43, 615. [Google Scholar] [CrossRef]
- Fan, A.Z.; Russell, M.; Naimi, T.; Li, Y.; Liao, Y.; Jiles, R.; Mokdad, A.H. Patterns of Alcohol Consumption and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 3833–3838. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Ren, M.; Liu, D.; Wang, C.; Yang, C.; Yan, L. Alcohol Consumption and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. Clin. Nutr. 2014, 33, 596–602. [Google Scholar] [CrossRef]
- Yeomans, M.R. Alcohol, Appetite and Energy Balance: Is Alcohol Intake a Risk Factor for Obesity? Physiol. Behav. 2010, 100, 82–89. [Google Scholar] [CrossRef]
- Navia, B.; López-Sobaler, A.M.; Villalobos, T.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Serra-Majem, L.; Varela-Moreiras, G.; Ortega, R.M. Breakfast Habits and Differences Regarding Abdominal Obesity in a Cross-Sectional Study in Spanish Adults: The ANIBES Study. PLoS ONE 2017, 12, e0188828. [Google Scholar] [CrossRef]
- Monzani, A.; Ricotti, R.; Caputo, M.; Solito, A.; Archero, F.; Bellone, S.; Prodam, F. A Systematic Review of the Association of Skipping Breakfast with Weight and Cardiometabolic Risk Factors in Children and Adolescents. What Should We Better Investigate in the Future? Nutrients 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, A.O.; Jacobs, D.R.; Steffen, L.M.; Van Horn, L.; Ludwig, D.S.; Pereira, M.A. Breakfast Frequency and Development of Metabolic Risk. Diabetes Care 2013, 36, 3100–3106. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping Breakfast: Longitudinal Associations with Cardiometabolic Risk Factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef]
- Jung, J.; Kim, A.-S.; Ko, H.-J.; Choi, H.-I.; Hong, H.-E. Association between Breakfast Skipping and the Metabolic Syndrome: The Korea National Health and Nutrition Examination Survey, 2017. Medicina 2020, 56, 396. [Google Scholar] [CrossRef]
- Yoshimura, E.; Hatamoto, Y.; Yonekura, S.; Tanaka, H. Skipping Breakfast Reduces Energy Intake and Physical Activity in Healthy Women Who Are Habitual Breakfast Eaters: A Randomized Crossover Trial. Physiol. Behav. 2017, 174, 89–94. [Google Scholar] [CrossRef]
- Min, C.; Noh, H.; Kang, Y.S.; Sim, H.J.; Baik, H.W.; Song, W.O.; Yoon, J.; Park, Y.H.; Joung, H. Skipping Breakfast Is Associated with Diet Quality and Metabolic Syndrome Risk Factors of Adults. Nutr. Res. Pract. 2011, 5, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, A.M.; Bes-Rastrollo, M.; Gea, A.; Sayón-Orea, C.; Zazpe, I.; Lopez-Iracheta, R.; Martinez-Gonzalez, M.A. Snacking between Main Meals Is Associated with a Higher Risk of Metabolic Syndrome in a Mediterranean Cohort: The SUN Project (Seguimiento Universidad de Navarra). Public Health Nutr. 2016, 19, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Azizi, N.; Shab-Bidar, S.; Bazshahi, E.; Lesani, A.; Javanbakht, M.H.; Djafarian, K. Joint Association of Meal Frequency and Diet Quality with Metabolic Syndrome in Iranian Adults. BMC Nutr. 2022, 8, 12. [Google Scholar] [CrossRef]
- Fan, L.; Hao, Z.; Gao, L.; Qi, M.; Feng, S.; Zhou, G. Non-Linear Relationship between Sleep Duration and Metabolic Syndrome: A Population-Based Study. Medicine 2020, 99, e18753. [Google Scholar] [CrossRef]
- Kobayashi, D.; Takahashi, O.; Deshpande, G.A.; Shimbo, T.; Fukui, T. Relation between Metabolic Syndrome and Sleep Duration in Japan: A Large Scale Cross-Sectional Study. Intern. Med. 2011, 50, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Okubo, N.; Matsuzaka, M.; Takahashi, I.; Sawada, K.; Sato, S.; Akimoto, N.; Umeda, T.; Nakaji, S. Relationship between Self-Reported Sleep Quality and Metabolic Syndrome in General Population. BMC Public Health 2014, 14, 562. [Google Scholar] [CrossRef] [Green Version]
- Hung, H.-C.; Yang, Y.-C.; Ou, H.-Y.; Wu, J.-S.; Lu, F.-H.; Chang, C.-J. The Association between Self-Reported Sleep Quality and Metabolic Syndrome. PLoS ONE 2013, 8, e54304. [Google Scholar] [CrossRef]
- Keevil, V.L.; Wijndaele, K.; Luben, R.; Sayer, A.A.; Wareham, N.J.; Khaw, K.T. Television Viewing, Walking Speed, and Grip Strength in a Prospective Cohort Study. Med. Sci. Sports Exerc. 2015, 47, 735–742. [Google Scholar] [CrossRef] [Green Version]
- At, J.; Bryce, R.; Prina, M.; Acosta, D.; Ferri, C.P.; Guerra, M.; Huang, Y.; Rodriguez, J.J.L.; Salas, A.; Sosa, A.L.; et al. Frailty and the Prediction of Dependence and Mortality in Low- and Middle-Income Countries: A 10/66 Population-Based Cohort Study. BMC Med. 2015, 13, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, R.; Kuh, D.; Cooper, C.; Gale, C.R.; Lawlor, D.A.; Matthews, F.; Hardy, R. Objective Measures of Physical Capability and Subsequent Health: A Systematic Review. Age Ageing 2011, 40, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Men | Women | |
|---|---|---|
| Number (%) | 2845 (52.1) | 2614 (47.9) |
| Age (years) | 47.0 ± 14.4 | 49.4 ± 15.6 |
| MetS n (%) | 1717 (60.4) | 1375 (52.6) |
| BMI | 25.5 ± 3.6 | 23.9 ± 4.0 |
| WC (cm) | 88.7 ± 10.0 | 80.5 ± 10.7 |
| Systolic BP (mmHg) | 128.6 ± 16.4 | 122.2 ± 19.0 |
| Diastolic BP (mmHg) | 84.5 ± 11.1 | 77.8 ± 11.3 |
| Triglycerides (mg/dL) | 186.45 ± 150.23 | 135.13 ± 101.1 |
| FBG (mg/dL) | 110.0 ± 31.7 | 103.6 ± 27.0 |
| HbA1C (%) | 5.8 ± 0.98 | 5.8 ± 0.95 |
| HDL-C (mg/dL) | 41.9 ± 10.8 | 49.2 ± 13.0 |
| LDL-C (mg/dL) | 122.8 ± 34.4 | 125.3 ± 36.0 |
| Men (n = 2845) | Women (n = 2614) | |||||
|---|---|---|---|---|---|---|
| MetS | Non-MetS | p-Value | MetS | Non-MetS | p-Value | |
| Number (%) | 1717 (60.4) | 1128 (39.6) | 1375 (52.6) | 1239 (47.4) | ||
| Age (years) | 49.4 ±13.7 | 43.3 ± 14.5 | <0.001 a | 55.6 ± 14.0 | 42.5 ± 14.4 | <0.001 a |
| BMI | 26.7 ± 3.4 | 23.6 ± 3.0 | <0.001 b | 25.6 ± 3.7 | 22.0 ± 3.3 | <0.001 b |
| WC (cm) | 92.7 ± 9.0 | 82.7 ± 8.4 | <0.001 b | 85.8 ± 8.9 | 74.5 ± 9.2 | <0.001 b |
| Systolic BP (mmHg) | 133 ± 16.4 | 122.3 ± 14.3 | <0.001 b | 129.8 ± 18.6 | 113.7 ± 15.7 | <0.001 b |
| Diastolic BP (mmHg) | 87.3 ± 11.1 | 80.4 ± 14.3 | <0.001 b | 81.5 ± 11.2 | 73.8 ± 12.3 | <0.001 b |
| Triglycerides (mg/dL) | 232.2 ± 172.3 | 116.9 ± 61.0 | <0.001 b | 175.7 ± 115.9 | 90.1 ± 52.9 | <0.001 b |
| FBG (mg/dL) | 116.7 ± 36.8 | 99.9 ± 16.7 | <0.001 b | 112.0 ± 33.4 | 94.3 ± 11.7 | <0.001 b |
| HbA1c (%) | 6.0 ± 1.1 | 5.5 ± 0.7 | <0.001 b | 6.1 ± 1.1 | 5.4 ± 0.5 | <0.001 b |
| HDL-C (mg/dL) | 38.4 ± 9.2 | 47.2 ± 10.8 | <0.001 b | 43.6 ± 10.9 | 55.5 ± 12.3 | <0.001 b |
| LDL-C (mg/dL) | 129.4 ± 37.1 | 127.8 ± 32.7 | 0.061 b | 122.9 ± 37.3 | 120.1 ± 33.1 | <0.001 b |
| Lifestyle Items (10 Items) Men | Number (%) | Age-Adjusted Logistic Regression Model 1 OR (95% CI) | p-Value | Multivariable Logistic Regression Model 2 OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| MetS (n = 1717) | Non-MetS (n = 1128) | |||||
| Exercising with light sweat for at least 30 min per session more than twice a week for over a year | ||||||
| Yes | 1023 (59.6) | 664 (58.9) | 0.92 (0.79–1.08) | 0.324 | - | |
| No | 694 (40.4) | 464 (41.1) | 1 | |||
| Walking or equivalent physical activity in daily life for more than 1 h per day | ||||||
| Yes | 945 (55.0) | 619 (54.9) | 0.95 (0.81–1.11) | 0.528 | - | |
| No | 772 (45.0) | 509 (45.1) | 1 | |||
| Walking faster than the same sex of similar age | ||||||
| Yes | 718 (41.8) | 526 (46.6) | 0.86 (0.74–1.00) | 0.053 | 0.78 (0.67–0.92) | 0.003 |
| No | 999 (58.2) | 602 (53.4) | 1 | 1 | ||
| Eating quicker than others | ||||||
| Quickly | 646 (37.6) | 351 (31.1) | 2.23 (1.66–2.99) | <0.001 | 2.25 (1.68–3.03) | <0.001 |
| Normal | 953 (55.5) | 655 (58.1) | 1.74 (1.32–2.31) | <0.001 | 1.73 (1.30–2.31) | <0.001 |
| Slowly | 118 (6.9) | 122 (10.8) | 1 | 1 | ||
| Having dinner within 2 h before bedtime at least 3 days a week | ||||||
| Yes | 909 (52.9) | 562 (49.8) | 1.13 (0.97–1.32) | 0.105 | 1.12 (0.95–1.32) | 0.175 |
| No | 808 (47.1) | 566 (50.2) | 1 | 1 | ||
| Eating food after dinner ≥3 days a week | ||||||
| Yes | 382 (22.2) | 219 (19.4) | 1.23 (1.01–1.48) | 0.036 | 1.19 (0.98–1.46) | 0.086 |
| No | 1335 (77.8) | 909 (80.6) | 1 | 1 | ||
| Skipping breakfast ≥3 days a week | ||||||
| Yes | 576 (33.5) | 385 (34.1) | 1 (0.85–1.18) | 0.974 | ||
| No | 1141 (66.5) | 743 (65.9) | 1 | |||
| Sufficient rest with sleep | ||||||
| Yes | 1069 (62.3) | 686 (60.8) | 1.05 (0.90–1.23) | 0.53 | ||
| No | 648 (37.7) | 442 (39.2) | 1 | |||
| Alcohol drinking habit | ||||||
| Current drinker | 1241 (72.3) | 811 (71.9) | 1.34 (1.11–1.62) | 0.002 | 1.33 (1.10–1.61) | 0.004 |
| Former drinker | 90 (5.2) | 49 (4.3) | 1.04 (0.70–1.54) | 0.854 | 1.03 (0.69–1.53) | 0.895 |
| Never | 386 (22.5) | 268 (23.8) | 1 | 1 | ||
| Smoking status | ||||||
| Current smoker | 169 (9.8) | 94 (8.3) | 1.13 (0.86–1.48) | 0.388 | ||
| Former smoker | 236 (13.8) | 102 (9.1) | 1.02 (0.78–1.34) | 0.86 | ||
| Never | 1312 (76.4) | 932 (82.6) | 1 | |||
| Lifestyle Items (10 Items) Women | Number (%) | Age-Adjusted Logistic Regression Model 1 OR (95% CI) | p-Value | Multivariable Logistic Regression Model 2 OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| MetS (n = 1375) | Non-MetS (n = 1239) | |||||
| Exercising with light sweat for at least 30 min per session more than twice a week for over a year | ||||||
| Yes | 683 (49.7) | 543 (43.8) | 0.99 (0.81–1.15) | 0.694 | - | |
| No | 692 (50.3) | 696 (56.2) | 1 | |||
| Walking or equivalent physical activity in daily life for more than 1 h per day | ||||||
| Yes | 755 (54.9) | 650 (52.5) | 0.92 (0.77–1.09) | 0.312 | - | |
| No | 620 (45.1) | 589 (47.5) | 1 | |||
| Walking faster than the same sex of similar age | ||||||
| Yes | 448 (32.6) | 504 (40.7) | 0.81 (0.68–0.97) | 0.022 | 0.75 (0.62–0.89) | 0.002 |
| No | 927 (67.4) | 735 (59.3) | 1 | 1 | ||
| Eating quicker than others | ||||||
| Quickly | 401 (29.2) | 292 (23.6) | 1.82 (1.34–2.46) | <0.001 | 1.92 (1.41–2.60) | <0.001 |
| Normal | 820 (59.6) | 795 (64.2) | 1.31 (0.99–1.73) | 0.056 | 1.28 (0.97–1.28) | 0.082 |
| Slowly | 154 (11.2) | 152 (12.2) | 1 | 1 | ||
| Having dinner within 2 h before bedtime at least 3 days a week | ||||||
| Yes | 706 (51.3) | 645 (52.1) | 0.96 (0.81–1.13) | 0.591 | ||
| No | 669 (48.7) | 594 (47.9) | 1 | |||
| Eating food after dinner ≥3 days a week | ||||||
| Yes | 302 (22.0) | 219 (19.2) | 1.24 (1.01–1.53) | 0.043 | 1.25 (1.01–1.55) | 0.041 |
| No | 1073 (78.0) | 909 (80.8) | 1 | 1 | ||
| Skipping breakfast ≥3 days a week | ||||||
| Yes | 424 (30.8) | 436 (35.2) | 0.84 (0.70–1.00) | 0.053 | 0.83 (0.69–0.99) | 0.045 |
| No | 951 (69.2) | 803 (64.8) | 1 | 1 | ||
| Sufficient rest with sleep | ||||||
| Yes | 811 (59.0) | 691 (55.8) | 1.19 (1.01–1.42) | 0.043 | 1.19 (1.01–1.42) | 0.043 |
| No | 564 (41.0) | 548 (44.2) | 1 | 1 | ||
| Alcohol drinking habit | ||||||
| Current drinker | 355 (25.8) | 414 (33.4) | 1.33 (1.09–1.61) | 0.004 | 1.33 (1.09–1.62) | 0.004 |
| Former drinker | 19 (1.4) | 7 (0.6) | 1.96 (0.78–4.97) | 0.155 | 1.95 (0.77–4.94) | 0.159 |
| Never | 1001 (72.8) | 818 (66.0) | 1 | 1 | ||
| Smoking status | ||||||
| Current smoker | 7 (0.5) | 2 (0.2) | 2.97 (0.56–15.91) | 0.203 | - | |
| Former smoker | 5 (0.4) | 2 (0.2) | 1.17 (0.18–7.54) | 0.87 | ||
| Never | 1363 (99.1) | 1235 (99.6) | 1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaoki, M.; Honda, I.; Nakanishi, K.; Nakajima, M.; Cheam, S.; Okawada, M.; Sakakibara, H. Lifestyle Factors Associated with Metabolic Syndrome in Urban Cambodia. Int. J. Environ. Res. Public Health 2022, 19, 10481. https://doi.org/10.3390/ijerph191710481
Tamaoki M, Honda I, Nakanishi K, Nakajima M, Cheam S, Okawada M, Sakakibara H. Lifestyle Factors Associated with Metabolic Syndrome in Urban Cambodia. International Journal of Environmental Research and Public Health. 2022; 19(17):10481. https://doi.org/10.3390/ijerph191710481
Chicago/Turabian StyleTamaoki, Miharu, Ikumi Honda, Keisuke Nakanishi, Maki Nakajima, Sophathya Cheam, Manabu Okawada, and Hisataka Sakakibara. 2022. "Lifestyle Factors Associated with Metabolic Syndrome in Urban Cambodia" International Journal of Environmental Research and Public Health 19, no. 17: 10481. https://doi.org/10.3390/ijerph191710481
APA StyleTamaoki, M., Honda, I., Nakanishi, K., Nakajima, M., Cheam, S., Okawada, M., & Sakakibara, H. (2022). Lifestyle Factors Associated with Metabolic Syndrome in Urban Cambodia. International Journal of Environmental Research and Public Health, 19(17), 10481. https://doi.org/10.3390/ijerph191710481






