Cost-Effectiveness Analysis of the Helicobacter Pylori Screening Programme in an Asymptomatic Population in China
Abstract
1. Introduction
2. Materials and Methods
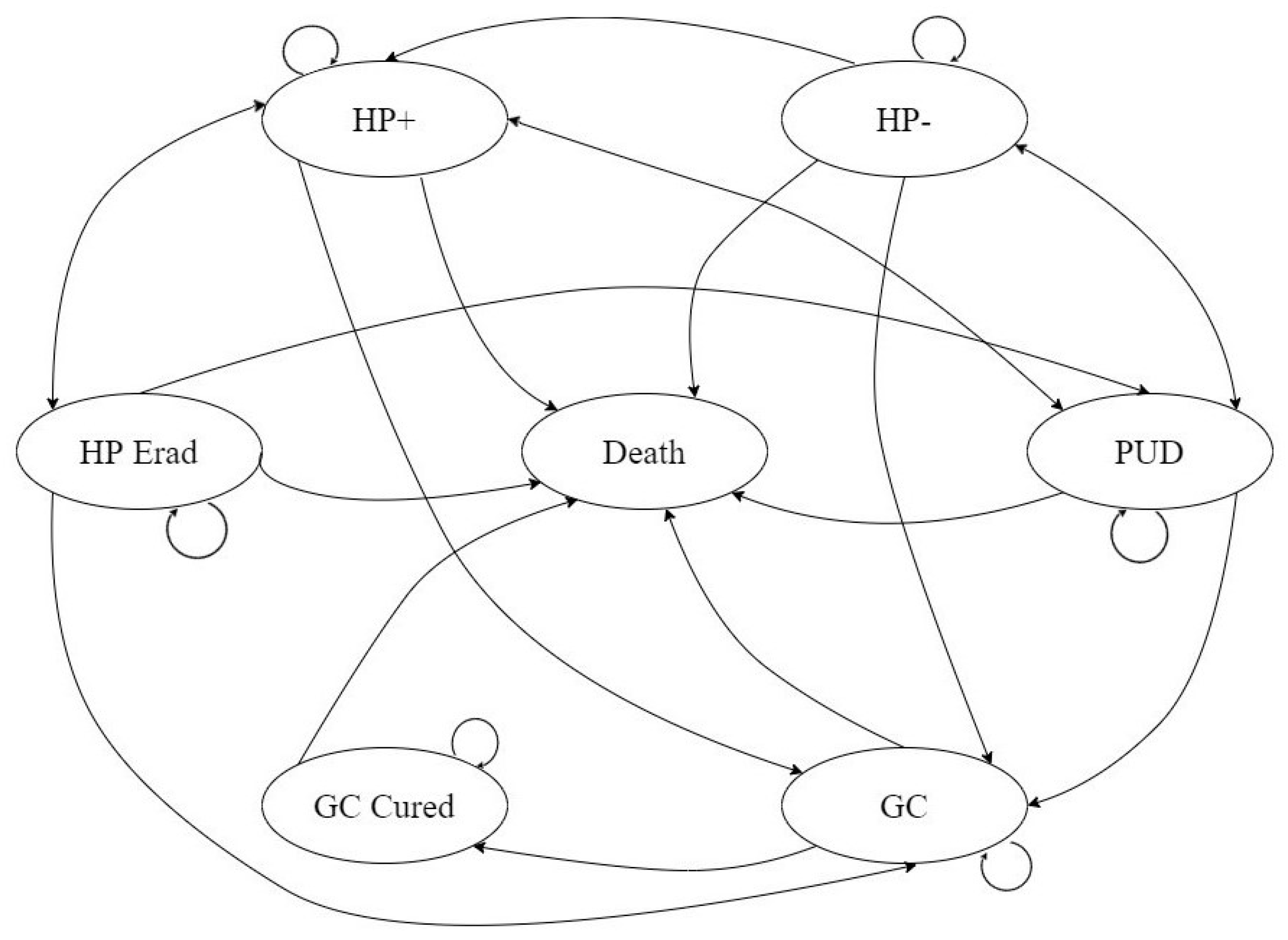
2.1. Model Design
2.2. Model Population
2.3. Transition Probabilities
2.4. Treatment Costs and Health Utility
2.5. Model Outcome
2.6. Uncertainty and Scenario Analysis
- It is assumed that screening for H. pylori is initiated at different ages in asymptomatic people. This hypothesis is used to explore the optimal screening regimen for different age groups.
- Compare the cost effectiveness of no repeat screening with that of no screening. This scenario examines the cost-effectiveness of increasing screening participation in asymptomatic people who are not screened.
3. Result
3.1. Cost-Effectiveness Analysis
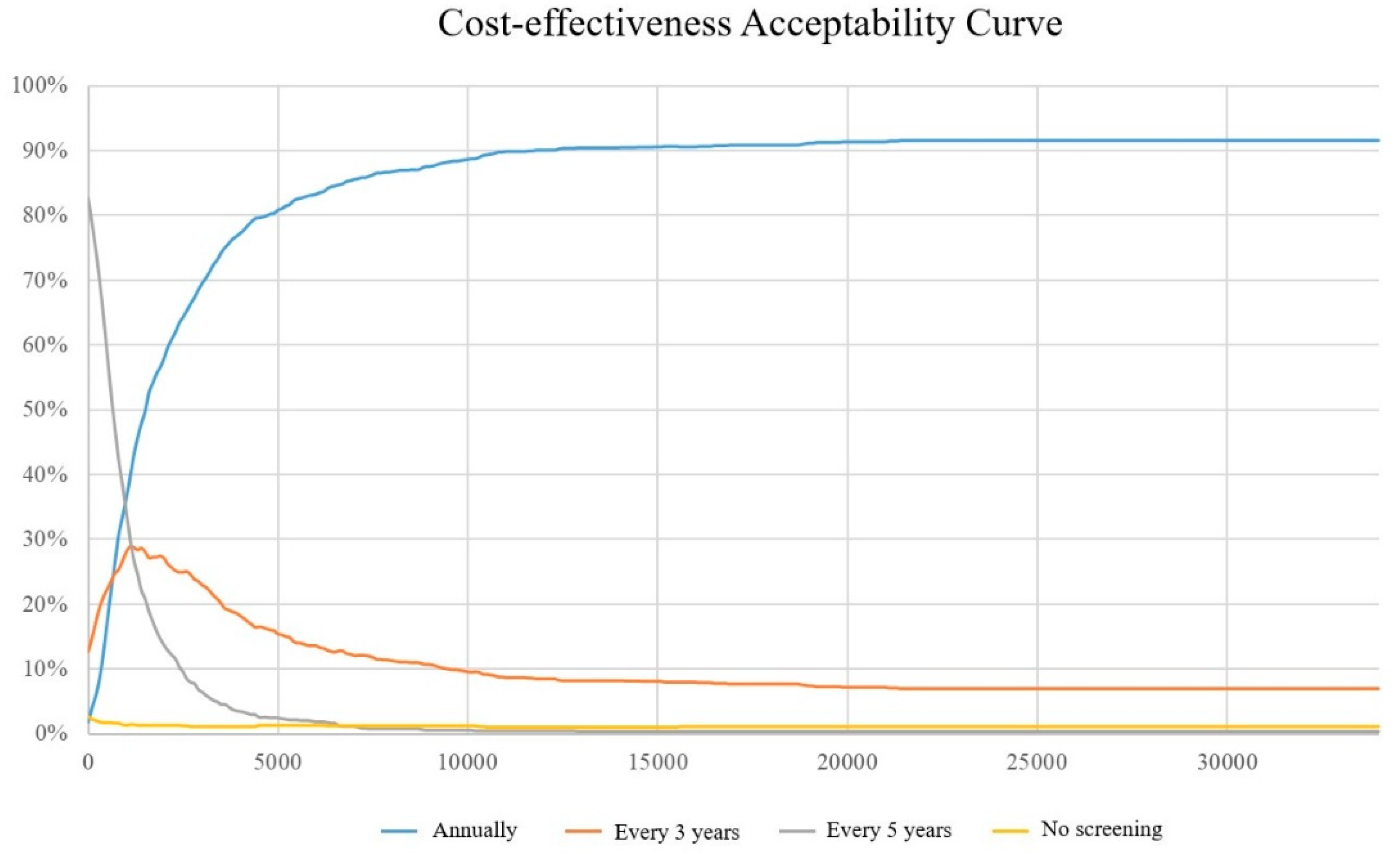
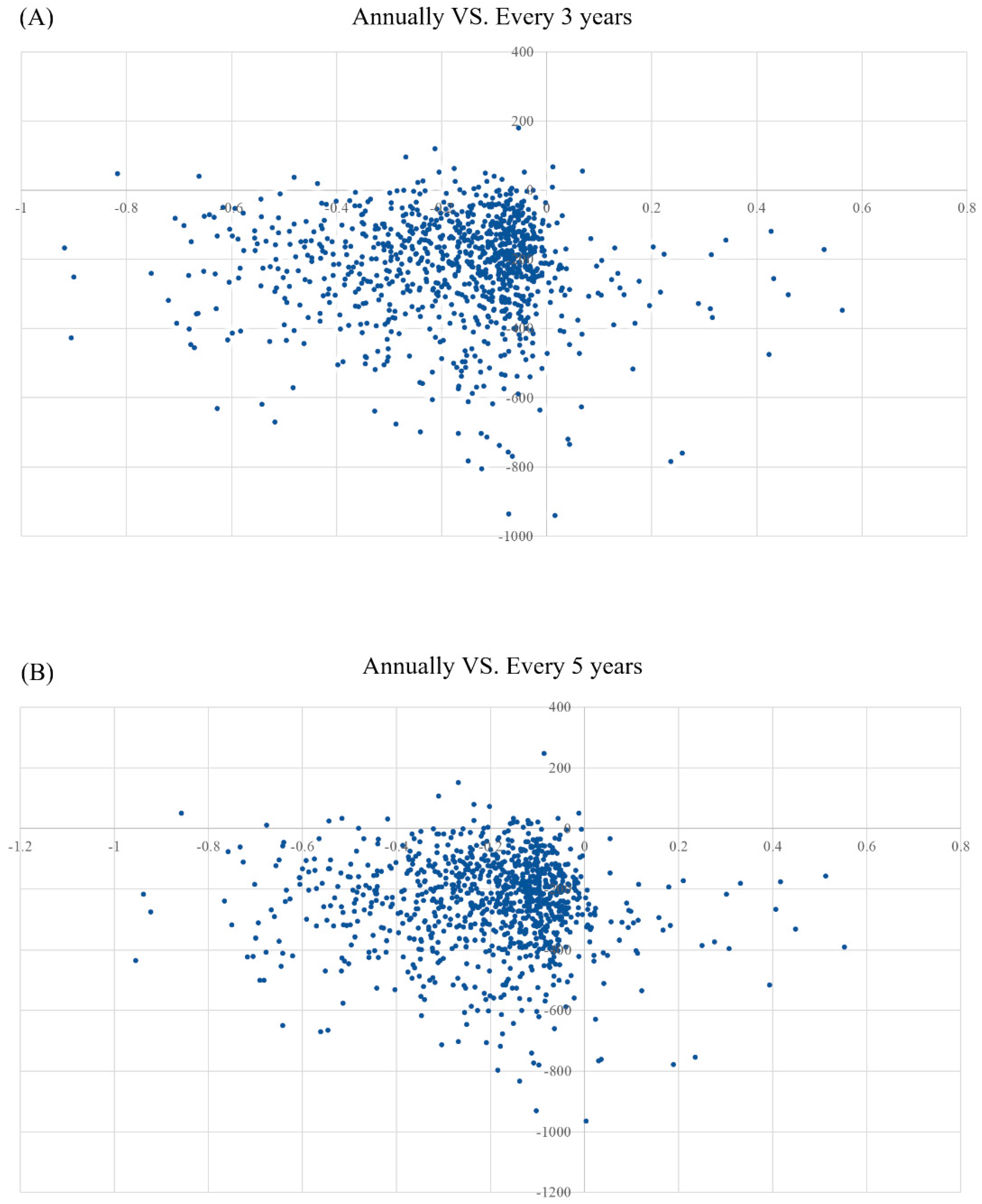
3.2. Sensitivity Analyses
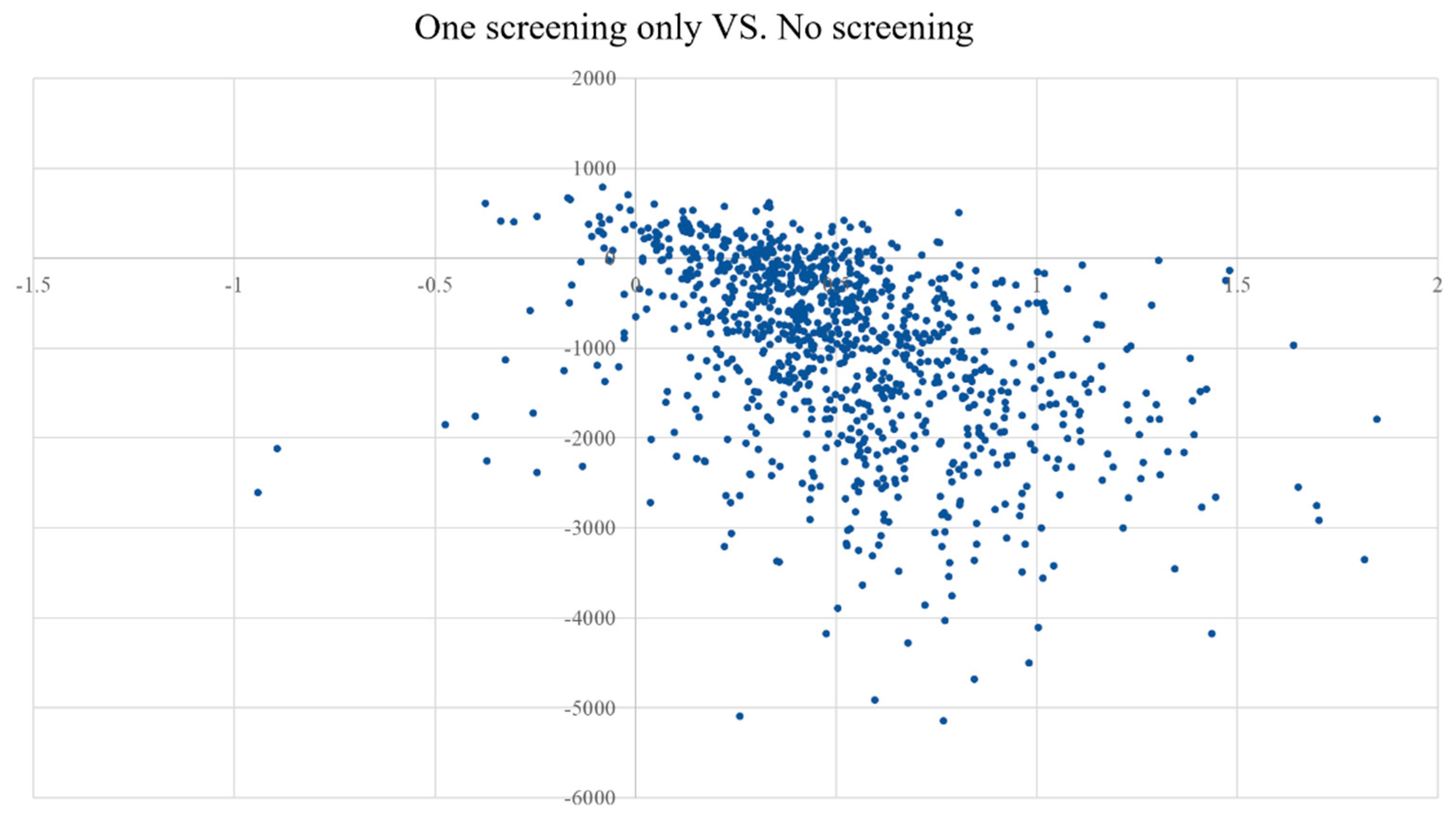
3.3. Scenario Analyses
4. Discussion
4.1. Control of Helicobacter Pylori Infection
4.2. Effectiveness in the Prevention of Peptic Ulcers
4.3. Preventive Effect on Gastric Cancer
4.4. Screening Strategy Recommendations
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Z.; Du, Y.-Q.; Lu, H.; Wang, W.-H.; Cheng, H.; Chen, S.Y.; Chen, M.-H.; Chen, W.-C.; Chen, Y.; Fang, J.-Y.; et al. Chinese Consensus Report on Family-Based Helicobacter pylori Infection Control and Management (2021 Edition). Gut 2022, 71, 238–253. [Google Scholar]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P.; et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lu, N.-H. Review: Clinical management of Helicobacter pylori infection in China. Helicobacter 2015, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Johansson, S.; Molloy-Bland, M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016, 8, 8. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, e12475. [Google Scholar] [CrossRef]
- Axon, A. Helicobacter pylori and public health. Helicobacter 2014, 19 (Suppl. 1), 68–73. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, Q.; Zhan, M.; Jin, C.; Li, Q. Cost-effectiveness Analysis of Helicobacter pylori Eradication Therapy in First-Degree Relatives of Patients with Gastric Cancer. Patient Prefer. Adherence 2021, 15, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yan, T.; Ma, H.; Yao, X.; Lu, C.; Li, Y.; Li, L. Cost-Effectiveness Analysis of Helicobacter pylori Eradication Therapy for Prevention of Gastric Cancer: A Markov Model. Dig. Dis. Sci. 2020, 65, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, X.; Long, X.; Yu, L.; Liu, W.; Lu, H. Cost-effectiveness analysis of screen-and-treat strategy in asymptomatic Chinese for preventing Helicobacter pylori-associated diseases. Helicobacter 2019, 24, e12563. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T. Analysis of ABC (D) stratification for screening patients with gastric cancer. World J. Gastroenterol. 2011, 17, 4793–4798. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Lee, Y.C.; Graham, D.Y. Reliability of pepsinogen combined with H. pylori antibody test in the screening of early gastric cancer. Pract. Integr. Chin. West. Med. Clin. 2019, 19, 3. [Google Scholar]
- Wang, X.; Shu, X.; Li, Q.; Li, Y.; Chen, Z.; Wang, Y.; Pu, K.; Zheng, Y.; Ye, Y.; Liu, M.; et al. Prevalence and risk factors of Helicobacter pylori infection in Wuwei, a high-risk area for gastric cancer in northwest China: An all-ages population-based cross-sectional study. Helicobacter 2021, 26, e12810. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, J.-H.; Li, X.-Y.; Zhu, Y.; Graham, D.Y.; Lu, N.-H. Systematic review with meta-analysis: The global recurrence rate of Helicobacter pylori. Aliment. Pharmacol. Ther. 2017, 46, 773–779. [Google Scholar] [CrossRef]
- Ferwana, M.; Abdulmajeed, I.; Alhajiahmed, A.; Madani, W.; Firwana, B.; Hasan, R.; Altayar, O.; Limburg, P.J.; Murad, M.H.; Knawy, B. Accuracy of urea breath test in Helicobacter pylori infection: Meta-analysis. World J. Gastroenterol. 2015, 21, 1305–1314. [Google Scholar] [CrossRef]
- Pan, K.F.; Zhang, L.; Gerhard, M.; Ma, J.L.; Liu, W.D.; Ulm, K.; Wang, J.X.; Zhang, L.; Zhang, Y.; Bajbouj, M.; et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: Baseline results and factors affecting the eradication. Gut 2016, 65, 9–18. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Yan, T.L.; Hu, Q.D.; Zhang, Q.; Li, Y.M.; Liang, T.B. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment. Pharmacol. Ther. 2013, 37, 963–968. [Google Scholar] [CrossRef]
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chen, T.H.; Chiu, H.M.; Shun, C.T.; Chiang, H.; Liu, T.Y.; Wu, M.S.; Lin, J.T. The benefit of mass eradication of Helicobacter pylori infection: A community-based study of gastric cancer prevention. Gut 2013, 62, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Sougioultzis, S.; Archimandritis, A.J. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: A systematic review. Clin. Gastroenterol. Hepatol. 2006, 4, 130–142. [Google Scholar] [CrossRef]
- Hansson, L.-E.; Nyrén, O.; Hsing, A.W.; Bergström, R.; Josefsson, S.; Chow, W.-H.; Fraumeni, J.F.; Adami, H.-O. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 1996, 335, 242–249. [Google Scholar] [CrossRef]
- Malmi, H.; Kautiainen, H.; Virta, L.J.; Färkkilä, M. Increased short- and long-term mortality in 8146 hospitalised peptic ulcer patients. Aliment. Pharmacol. Ther. 2016, 44, 234–245. [Google Scholar] [CrossRef]
- Ford, A.C.; Gurusamy, K.S.; Delaney, B.; Forman, D.; Moayyedi, P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst. Rev. 2016, 4, CD003840. [Google Scholar] [CrossRef]
- Lahat, A.; on behalf of the Israeli IBD research Network (IIRN); Kopylov, U.; Neuman, S.; Levhar, N.; Yablecovitch, D.; Avidan, B.; Weiss, B.; Ben-Horin, S.; Eliakim, R. Helicobacter pylori prevalence and clinical significance in patients with quiescent Crohn’s disease. BMC Gastroenterol. 2017, 17, 27. [Google Scholar] [CrossRef]
- Lopez, A.D.; Murray, C.C. The global burden of disease, 1990–2020. Nat. Med. 1998, 4, 1241–1243. [Google Scholar] [CrossRef]
- Schlesinger-Raab, A.; Mihaljevic, A.L.; Egert, S.; Emeny, R.; Jauch, K.-W.; Kleeff, J.; Novotny, A.; Nüssler, N.C.; Rottmann, M.; Schepp, W.; et al. Outcome of gastric cancer in the elderly: A population-based evaluation of the Munich Cancer Registry. Gastric Cancer 2016, 19, 713–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beck, J.R.; Pauker, S.G.; Gottlieb, J.E.; Klein, K.; Kassirer, J.P. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am. J. Med. 1982, 73, 889–897. [Google Scholar] [CrossRef]
- Ma, X.W. China Health Statistics Yearbook 2020; China Union Medical University Press: Beijing, China, 2020. [Google Scholar]
- Wang, Q.L.G.X. The measurement of health utility value in chronic gastritis, peptic ulcers and gastric cancer patient. Chin. J. Dig. 2000, 20, 273–274. [Google Scholar] [CrossRef]
- Eichler, H.G.; Kong, S.X.; Gerth, W.C.; Mavros, P.; Jönsson, B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: How are cost-effectiveness thresholds expected to emerge? Value Health 2004, 7, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Specialty Committee of Evaluation of Postmarketing Chinese Medicines of World Federation of Chinese Medicine Societies; Wang, Z.-F.; Xie, Y.-M.; Zhang, W.; Liao, X.; Chang, Y.-P. Guideline for postmarketing Chinese medicine pharmacoeconomic evaluation. Chin. J. Integr. Med. 2015, 21, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.-G.; Singer, A.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Shokri-Shirvani, J.; Zamani, V.; Zamani, M. Letter: Global emergence of Helicobacter pylori antibiotic resistance-unanswered questions. Aliment. Pharmacol. Ther. 2016, 43, 1249. [Google Scholar] [CrossRef]
- Ford, A.; Delaney, B.; Forman, D.; Moayyedi, P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: Systematic review and economic analysis. Am. J. Gastroenterol. 2004, 99, 1833–1855. [Google Scholar] [CrossRef]
- You, J.H.S.; Wong, P.-L.; Wu, J.C.Y. Cost-effectiveness of Helicobacter pylori “test and treat” for patients with typical reflux symptoms in a population with a high prevalence of H. pylori infection: A Markov model analysis. Scand. J. Gastroenterol. 2006, 41, 21–29. [Google Scholar] [CrossRef]
- Feng, R.M.; Zong, Y.N.; Cao, S.M.; Xu, R.H. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Sharp, L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 933–947. [Google Scholar] [CrossRef] [PubMed]

| Variables | Baseline | Range | Distribution | References |
|---|---|---|---|---|
| Epidemiological parameters | ||||
| Annual H. pylori infection rate (%) | 2.2 | 0.8–4 | β | [17] |
| UBT sensitivity (%) | 96 | 95–97 | β | [18] |
| UBT specificity (%) | 94 | 92–95 | β | [18] |
| Eradication rate of bismuth quadruple therapy(%) | 85.51 | 74.71–96.41 | β | [19] |
| Five-year survival rate for gastric cancer (%) | 47 | 45.0–49.5 | β | [20] |
| Annual H. pylori reinfection rate after eradicated (%) | 2.82 | 2.6–2.9 | β | [21] |
| Development of H. pylori infection into gastric cancer (%) | 28 | 18–56 | β | [22] |
| Risk of gastric cancer development (%) | 0.1186 | 0.099–0.14 | β | [23] |
| Development of gastric cancer after H. pylori cure (%) | 0.17 | 0.07–0.36 | β | [23] |
| Development of PUD without H pylori infection (%) | 9 | 7.2–10.8 | β | [24] |
| Development of PUD with H pylori infection (%) | 14 | 13.3–14.7 | β | [25] |
| PUD developing into gastric cancer (%) | 0.7 | 0.16–2 | β | [26] |
| PUD mortality rate (%) | 2.53 | 2.44–2.63 | β | [27] |
| Relapse after PUD cure with H. pylori(%) | 16.3 | 10.5–22.0 | β | [28] |
| Relapse after PUD cure without H. pylori (%) | 11.899 | 7.665–16.06 | β | [28] |
| H. pylori infection rate (%) | 50 | 15.5–83.4 | β | [29] |
| Costs (US dollars) | ||||
| H. pylori screening test (UBT) | 20.87 | 12.04–40.14 | Gamma | Local data |
| H. pylori eradication treatment | 28.27 | 9.78–59.23 | Gamma | Local data |
| Average annual cost of PUD | 1288.3 | 257.66–6441.50 | Gamma | Local data |
| Average annual cost of Gastric cancer | 3182.33 | 636.47–15911.65 | Gamma | Local data |
| Health-state utility | ||||
| Health | 1 | NA | NA | Assumption |
| PUD | 0.886 | 0.841–0.922 | β | [30] |
| Gastric cancer | 0.603 | 0.470–0.730 | β | [30] |
| Cured gastric cancer | 0.951 | 0.928–0.969 | β | [30] |
| Death | 0 | NA | NA | |
| Cycles (Years) | Gastric Cancer | Death | ||||
|---|---|---|---|---|---|---|
| Annually | Every 3 Years | Every 5 Years | Annually | Every 3 Years | Every 5 Years | |
| 10 | 18 | 19 | 19 | 83 | 83 | 84 |
| 20 | 20 | 22 | 24 | 93 | 94 | 94 |
| 30 | 12 | 13 | 16 | 85 | 85 | 86 |
| 40 | 10 | 10 | 12 | 76 | 77 | 77 |
| 50 | 10 | 11 | 10 | 69 | 69 | 68 |
| 60 | 10 | 10 | 10 | 62 | 61 | 62 |
| Screening Programme | Cost | INCR Cost | Life Year | ICER YoLS | EFF | ICER |
|---|---|---|---|---|---|---|
| Annually | 2487.08 | 45.43 | 22.78 | |||
| Every 3 years | 2266.06 | −221.02 | 45.01 | 516.41 | 22.61 | 1317.48 |
| Every 5 years | 2241.19 | −245.88 | 44.96 | 522.05 | 22.59 | 1277.92 |
| Screening Age | Screening Programme | Cost | INCR Cost | EFF | INCR EFF | ICER |
|---|---|---|---|---|---|---|
| 20 | Annually | 2441.80 | 22.78 | |||
| Every 3 years | 2223.98 | −217.82 | 22.61 | −0.18 | 1238.48 | |
| Every 5 years | 2204.59 | −237.21 | 22.58 | −0.20 | 1163.71 | |
| 30 | Annually | 2406.57 | 21.65 | |||
| Every 3 years | 2184.48 | −222.09 | 21.5 | −0.15 | 1480.6 | |
| Every 5 years | 2159.73 | −246.84 | 21.48 | −0.17 | 1452 | |
| 40 | Annually | 2333.99 | 19.97 | |||
| Every 3 years | 2131.73 | −202.26 | 19.85 | −0.12 | 1685.5 | |
| Every 5 years | 2106.61 | −227.38 | 19.83 | −0.14 | 1624.14 | |
| 50 | Annually | 2102.4 | 17.46 | |||
| Every 3 years | 1916.41 | −185.99 | 17.37 | −0.09 | 2066.56 | |
| Every 5 years | 1895.36 | −207.04 | 17.36 | −0.1 | 2070.4 | |
| 60 | Annually | 1692.51 | 13.74 | |||
| Every 3 years | 1538.08 | −154.43 | 13.69 | −0.05 | 3088.6 | |
| Every 5 years | 1518.19 | −174.32 | 13.68 | −0.06 | 2905.33 | |
| 70 | Annually | 1084.71 | 8.21 | |||
| Every 3 years | 992.76 | −91.95 | 8.19 | −0.02 | 4597.5 | |
| Every 5 years | 970.41 | −114.3 | 8.18 | −0.03 | 3810 | |
| No screening | 3617.40 | 13.67 | ||||
| One screening only | 1591.63 | −2025.77 | 22.22 | 8.54 | −237.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Zheng, Z.; Xu, J.; Cao, P.; Gao, S.; Yu, X. Cost-Effectiveness Analysis of the Helicobacter Pylori Screening Programme in an Asymptomatic Population in China. Int. J. Environ. Res. Public Health 2022, 19, 9986. https://doi.org/10.3390/ijerph19169986
Feng T, Zheng Z, Xu J, Cao P, Gao S, Yu X. Cost-Effectiveness Analysis of the Helicobacter Pylori Screening Programme in an Asymptomatic Population in China. International Journal of Environmental Research and Public Health. 2022; 19(16):9986. https://doi.org/10.3390/ijerph19169986
Chicago/Turabian StyleFeng, Tianyu, Zhou Zheng, Jiaying Xu, Peng Cao, Shang Gao, and Xihe Yu. 2022. "Cost-Effectiveness Analysis of the Helicobacter Pylori Screening Programme in an Asymptomatic Population in China" International Journal of Environmental Research and Public Health 19, no. 16: 9986. https://doi.org/10.3390/ijerph19169986
APA StyleFeng, T., Zheng, Z., Xu, J., Cao, P., Gao, S., & Yu, X. (2022). Cost-Effectiveness Analysis of the Helicobacter Pylori Screening Programme in an Asymptomatic Population in China. International Journal of Environmental Research and Public Health, 19(16), 9986. https://doi.org/10.3390/ijerph19169986





