Hybrid Exercise Program for Sarcopenia in Older Adults: The Effectiveness of Explainable Artificial Intelligence-Based Clinical Assistance in Assessing Skeletal Muscle Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
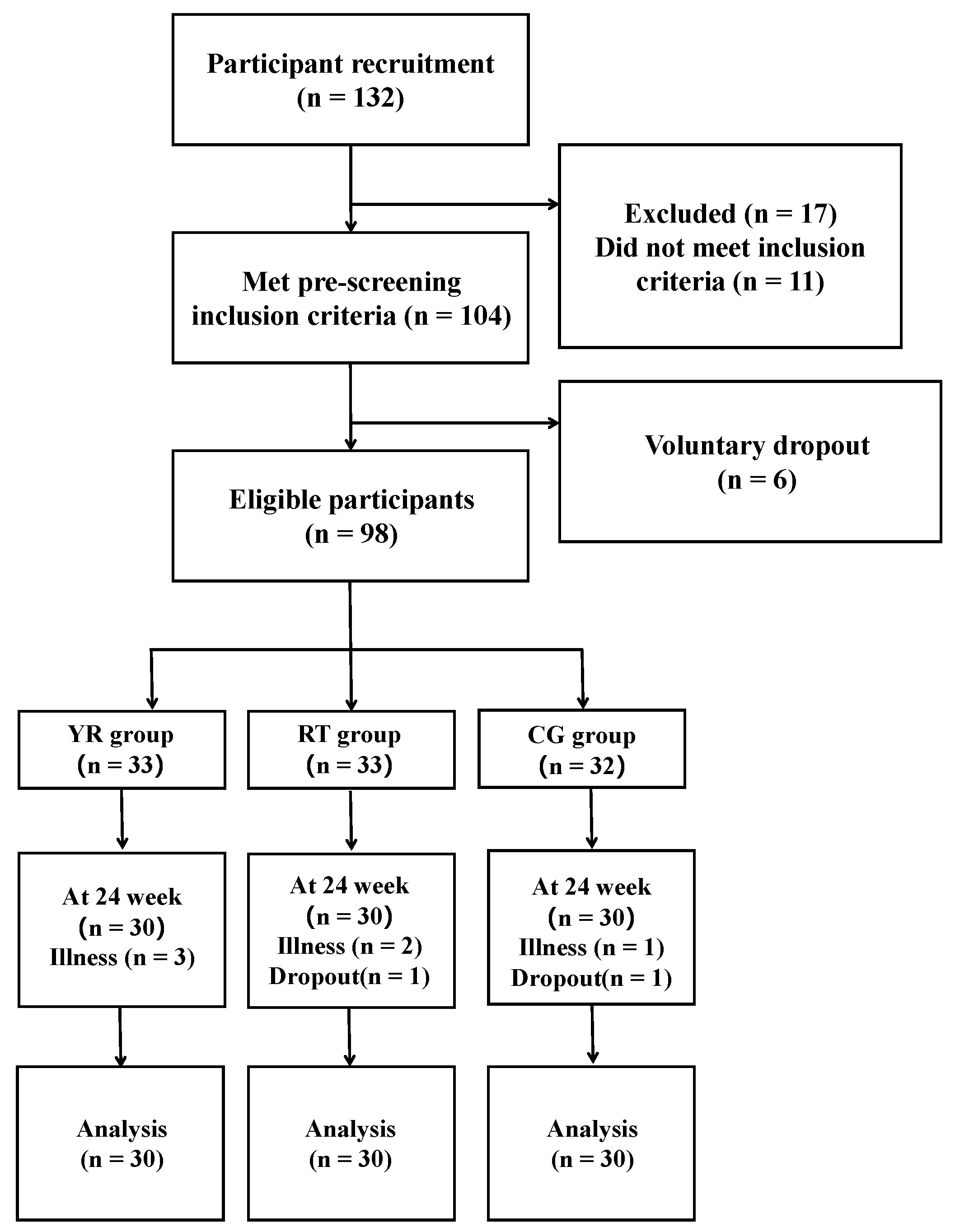
2.2. Study Design
2.2.1. Experimental Arrangement
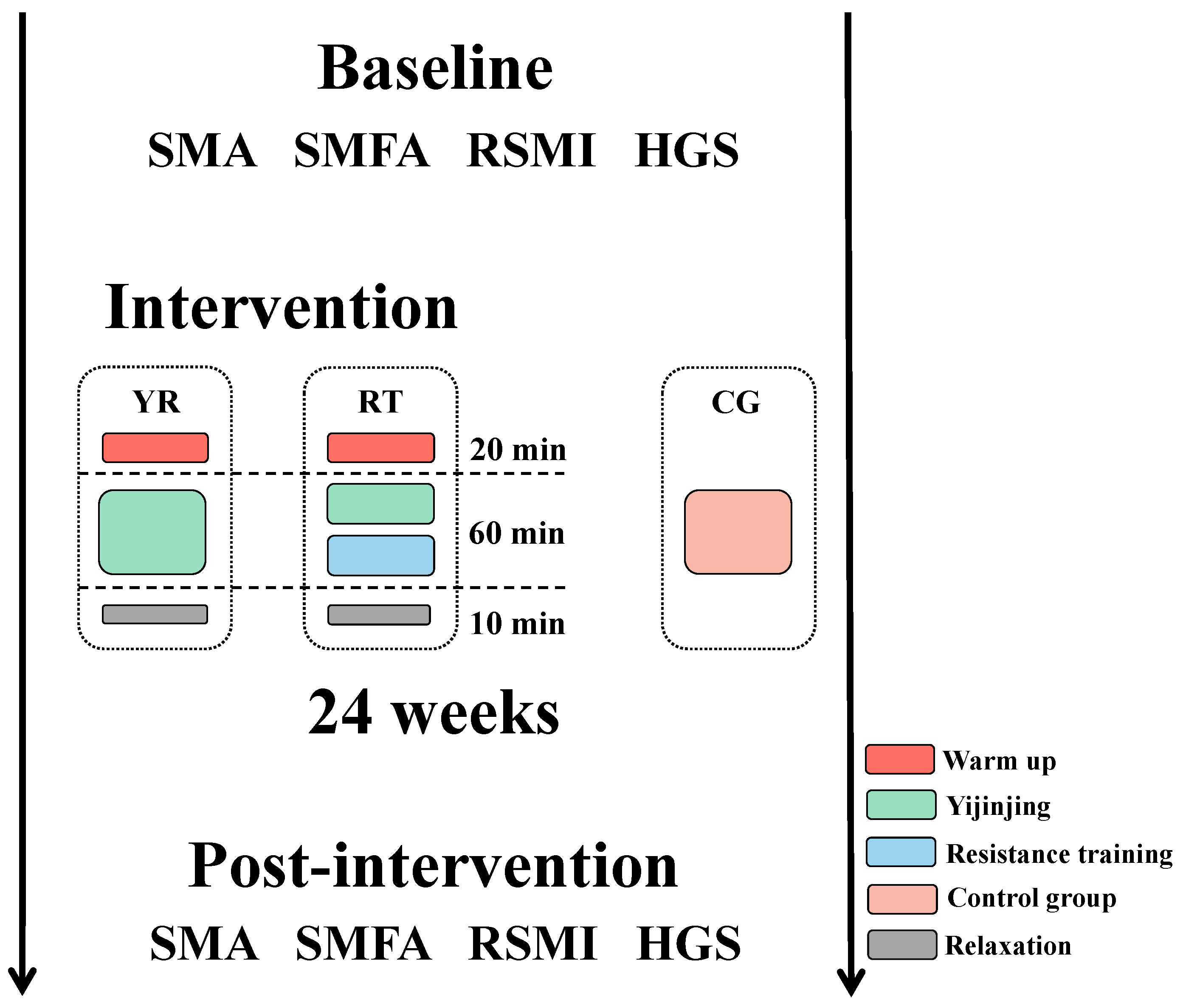
2.2.2. Intervention
- (1)
- Yi Jin Jing exercise: The training schedule of Yi Jin Jing consisted of two phases of 8 and 16 weeks, respectively. The first phase involved learning the movements: two Yi Jin Jing exercises were practiced in each session. During the second phase, the main goal was to consolidate learning of the exercise, and three Yi Jin Jing exercises were practiced in each session.
- (2)
- Resistance training: Resistance training consists of a total of five exercises which include two lower body exercises with supine elastic band resistance leg lifts and standing elastic band resistance leg raises, and three upper body exercises, including bicep curls, reverse grip curl, and seated pull down. To maximize strength gains the muscle hypertrophy, the training session was divided into three 8-week periods. The purpose of the first phase was to allow participants to learn and master the training movements using low loads (40.0–60.0% of 1 RM) and high repetitions (12–20) for 2–4 training sets. The second stage aimed to induce muscle hypertrophy and further improve muscle mass and reduce the fat content of the skeletal muscle interstitium by gradually increasing the load with a medium load of (60.0–80.0% of 1 RM), 5–12 repetitions, for 2–4 sets. In the third phase, we used a higher load (70.0–85.0% of 1 RM) and reduced the number of repetitions (5–8 repetitions) for 2–4 sets to further optimize muscle strength and increase maximal muscle power. The RT group underwent 4 sets for each movement, whereas the YR group completed 2 sets, with a 2 to 3 min interval between sets.
- (3)
- Control group: The CG group underwent conventional treatment, and the nurse educated the participants on sarcopenia and prevention methods, such as increasing protein intake in the diet and participating in more physical activities.
2.2.3. Assessment of Sarcopenia
- (1)
- Handgrip strength: Subjects’ handgrip strength was measured using a calibrated Jamar Hydraulic Hand Dynamometer (model SH5001, Saehan Corp, Masan, Korea, 2017). The handgrip strength test was in a standing position, and the shoulder was aligned with the torso, with the elbow fully extended, with the wrist maintaining a neutral position [44]. Each participant took two handgrip strength tests and the maximum value was used for this study. The handgrip strength of less than 28 kg for men and less than 18 kg for women met the screening criteria.
- (2)
- Physical performance: According to recommendations of AWGS 2016, we used a six-meter gait speed to assess physical performance. Participants stand with both feet on the starting line, and when hear the command “go”, walk along the 6-meter route at normal speed, and walk a few steps past the finish line [45]. The cut-off value of gait speed was 0.8 m/s.
- (3)
- Appendicular skeletal muscle mass (ASM): Appendicular skeletal muscle mass was performed on participants using a multifrequency with eight tactile electrodes (Inbody S10 Biospace, Biospace Co., Ltd., Seoul, Korea). When the ASM was less than 7.0 kg/m for men and less than 5.7 kg/m for women met the screening criteria.
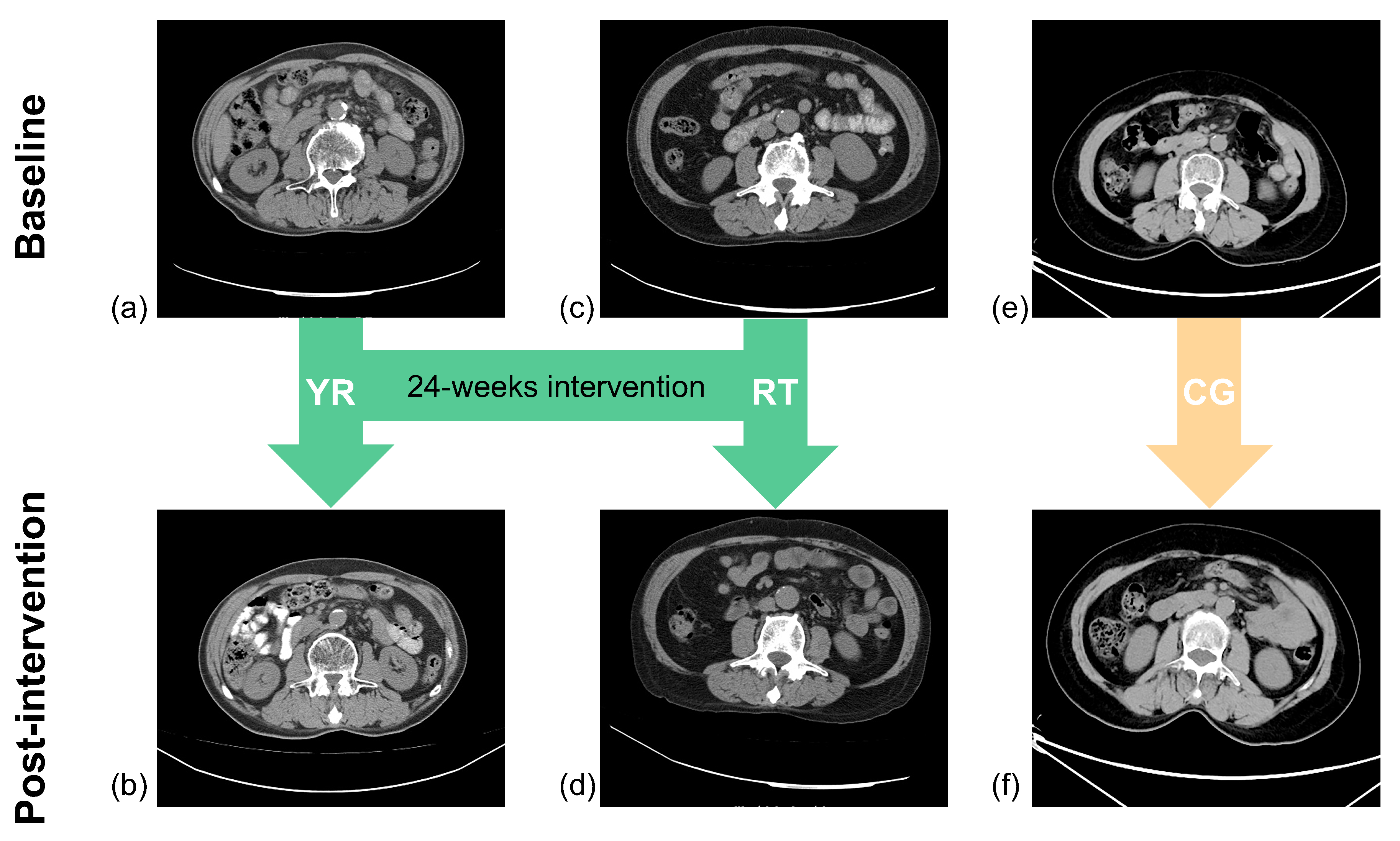
2.2.4. Assessment of Skeletal Muscle and Fat
Measurement of Skeletal Muscle
Measurement of Skeletal Muscle Interstitial Fat
Measurement of Relative Skeletal Muscle Mass Index
Measurement of Muscle Fat Infiltration
Measurement of Handgrip Strength
2.2.5. Data Analysis
2.2.6. Statistical Analysis
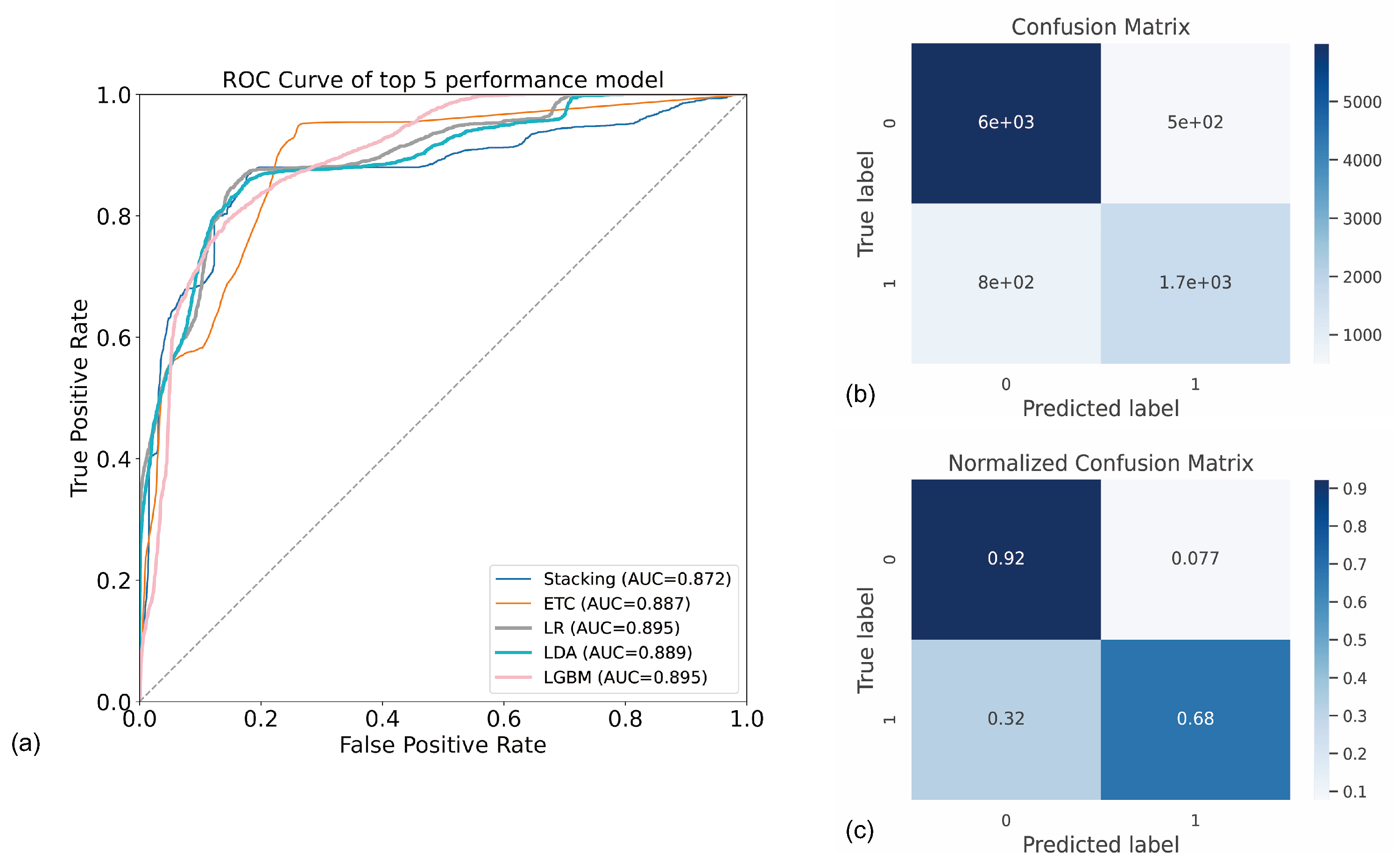
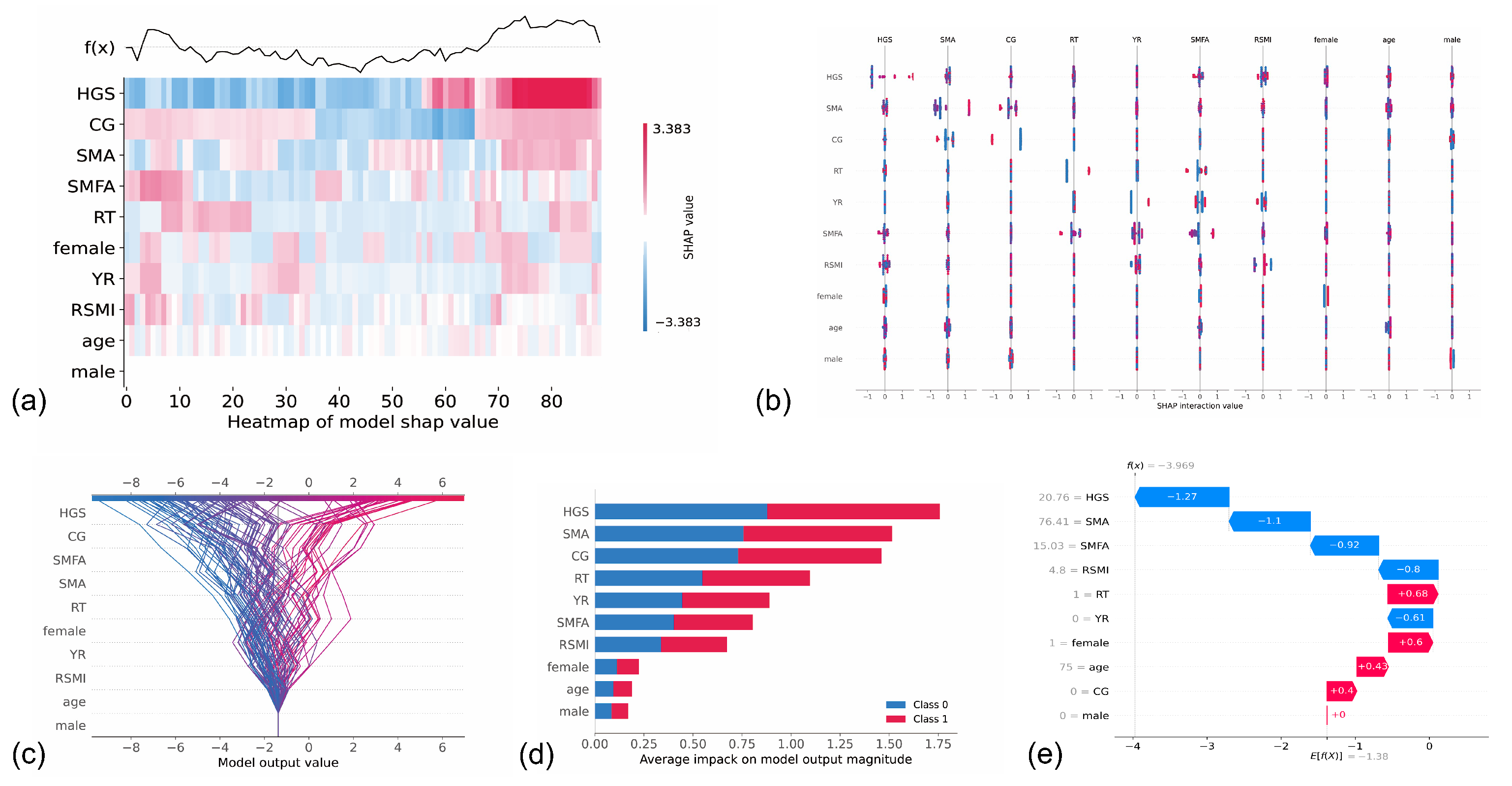
3. Results
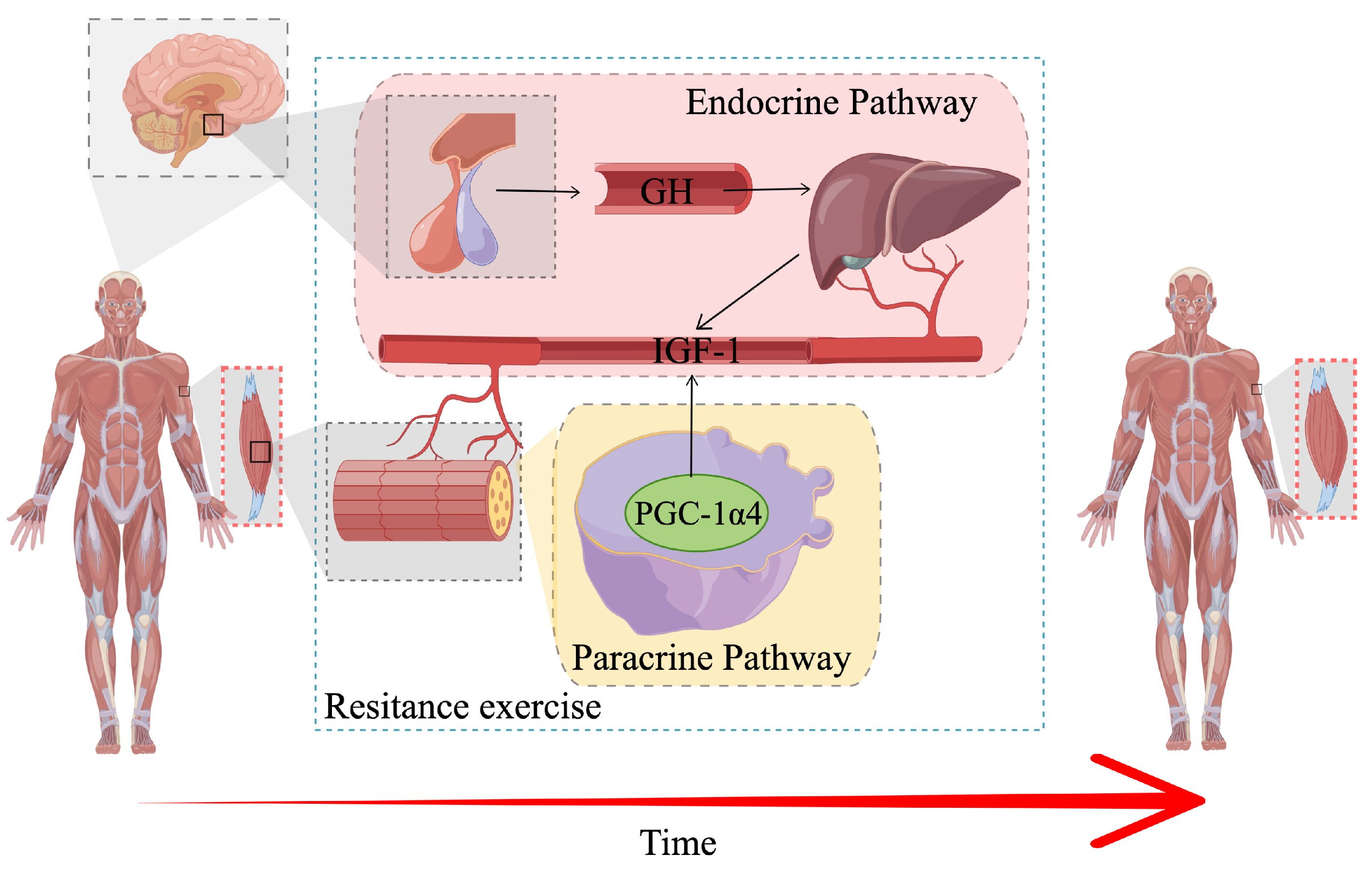
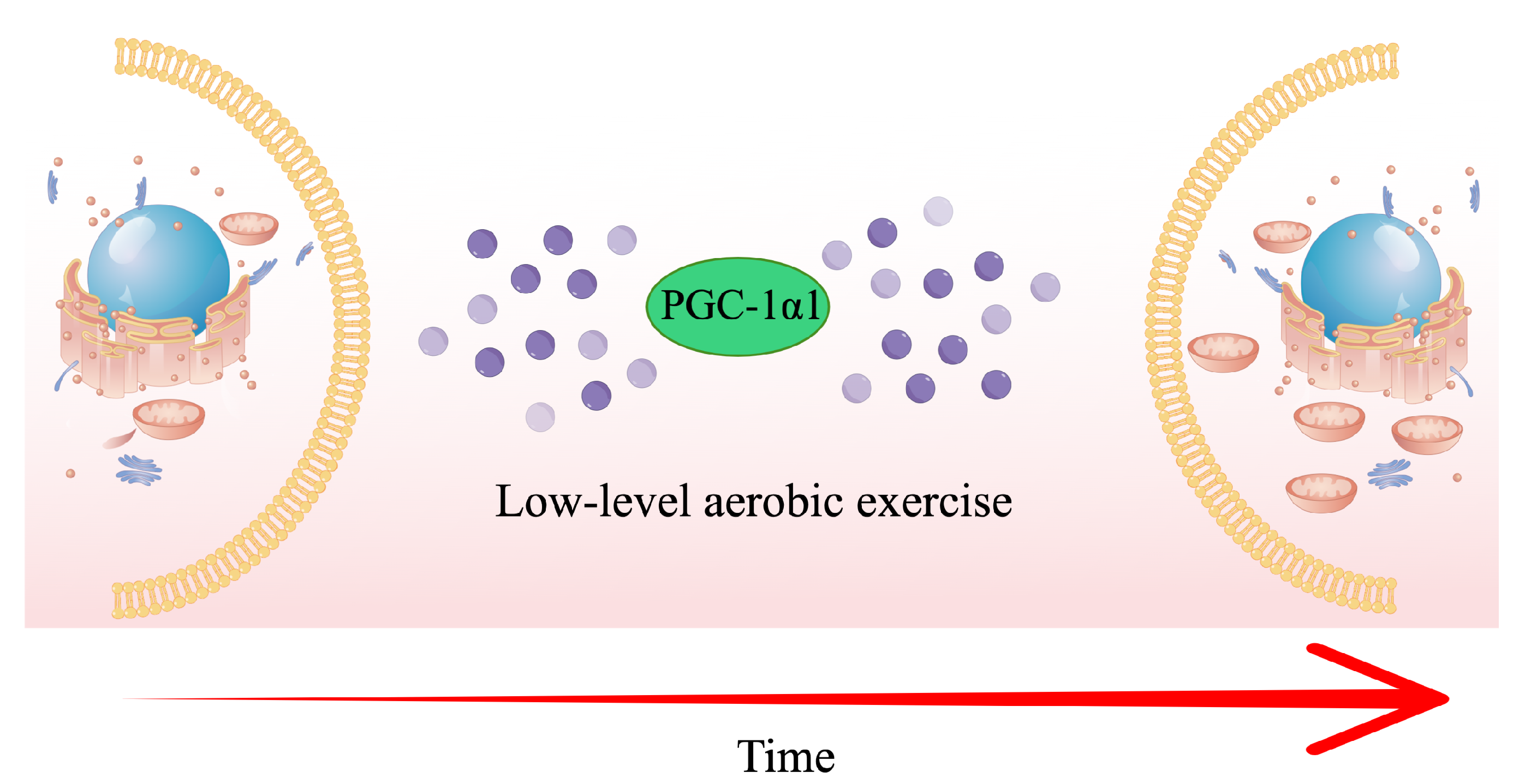
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faulkner, J.A.; Brooks, S.V.; Zerba, E. Skeletal Muscle Weakness and Fatigue in Old Age: Underlying Mechanisms. Annu. Rev. Gerontol. Geriatr. 1990, 10, 147–166. [Google Scholar]
- Metter, E.J.; Conwit, R.; Tobin, J.; Fozard, J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, B267–B276. [Google Scholar] [CrossRef]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015, 21, 854–862. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Hepple, R.T.; Rice, C.L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. 2016, 594, 1965–1978. [Google Scholar] [CrossRef]
- Dennison, E.M.; Sayer, A.A.; Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 340–347. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the obesity paradox: The association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Baracos, V.E.; Sawyer, M.B.; Bianchi, L.; Roberts, S.; Assenat, E.; Mollevi, C.; Senesse, P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016, 5, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Antoun, S.; Dalban, C.; Malka, D.; Mansourbakht, T.; Zaanan, A.; Latko, E.; Taieb, J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr. Cancer 2014, 66, 583–589. [Google Scholar] [CrossRef]
- Jung, H.W.; Kim, J.W.; Kim, J.Y.; Kim, S.W.; Yang, H.K.; Lee, J.W.; Lee, K.W.; Kim, D.W.; Kang, S.B.; Kim, K.i.; et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 2015, 23, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the C SCANS study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut–Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Geirsdottir, O.G.; Arnarson, A.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr. Res. 2013, 33, 608–612. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; Mclean, R.R.; Harris, T.B.; Luigi, F.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Apovian, C.M.; Travison, T.G.; Pencina, K.; Moore, L.L.; Huang, G.; Campbell, W.W.; Li, Z.; Howland, A.S.; Chen, R.; et al. Effect of Protein Intake on Lean Body Mass in Functionally Limited Older Men: A Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; Haehling, S.V. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef]
- Bunce, S.; Schroeder, K. Interventions for sarcopenia and muscle weakness in older people. Age Ageing 2005, 34, 414–415. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Lu, A.M.; Yan, W.; An, H.; Chen, Y.; Hu, J.; Li, X.N.; Qin, Z.H. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp. Gerontol. 2013, 48, 427–436. [Google Scholar]
- Liu, Y.C.; Wang, Z.Y.; Fang, L.; Yan, J.T.; Fang, M.; Zhu, Q.G.; Zhang, H.; Shang, Y.; Zhang, H.B. Effect of Yi Jin Jing on Dynamic balance of Patients with Senile Sarcopenia. J. Hebei Tradit. Chin. Med. Pharmacol. 2014, 29, 9–11. [Google Scholar]
- Zhu, G.F.; Shen, Z.F.; Shen, Q.H.; Jin, Y.Q.; Lou, Z.Y. Effect of Yi Jin Jing(Sinew-transforming Qigong Exercises) on skeletal muscle strength in the elderly. J. Acupunct. Tuina Sci. 2017, 15, 434–439. [Google Scholar] [CrossRef]
- Jin, D.P.; Jun, X.U.; Zhao, J.Z.; Ying, H.U.; Wang, D.Y. Effect of Yijinjing on Daily Living Function and Physical Function of Sarcopenia Patients. Chin. J. Inform. Tradit. Chin. Med. 2011, 18, 14–16. [Google Scholar]
- Liu, Y.C.; Yan, J.T.; Wang, Z.Y.; Zhu, Q.G.; Fang, M.; Zhang, H.; Fang, L.; Shang, Y.; Wang, Y.L.; Zhang, H.B.; et al. Influence of Yi Jin Jing on Skeletal Muscle Contractile Function in Gerontism with Sarcopenia. Acad. J. Shanghai Univ. Tradit. Chin. Med. 2016, 30, 42–45. [Google Scholar]
- Yang, J.; Chen, P. Clinical observation on the improvement of lower limb muscle strength and quality in elderly patients with sarcopenia by Tuina. Zhejiang J. Tradit. Chin. Med. 2016, 51, 753. [Google Scholar]
- Fang, L.; Li, Z.; Tao, X.; Luo, J. Clinical study on the effect of Yijinjing on the risk of fall in elderly patients with balance disorder. Chin. J. Rehabil. Med. 2020, 35, 319–323. [Google Scholar]
- Gong, L.; Yan, J.T.; Yc, L.; Fang, L.; Zhang, H.; Xu, J. Effect of the Tui Na gongfu method Yi Jin Jing on isometric muscle strength in elderly patients with sarcopenia. Acad. J. Shanghai Univ. Trad. Chin. Med. 2011, 25, 55–58. [Google Scholar]
- Qi, H.; Zhang, Z. Research on sarcopenia treatment with Chinese medicine for elderly patients. AIP Conf. Proc. 2020, 2252, 20020. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Guo, Y.; Dou, Y.; Zhao, J.; He, Y. Effect of tui na combined with resistance exercise on activities of daily living in patients with sarcopenia. Chin. J. Rehabil. Med. 2016, 31, 989–994. [Google Scholar]
- Shen, Z.F.; Zhu, G.F.; Qian, L.F.; Fu, Y.X. Yi Jin Jing (Sinew-transforming Qigong Exercises) for primary osteoporosis in the elderly: A clinical trial. J. Acupunct. Tuina Sci. 2018, 16, 104–108. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, G.; Li, X.; Yao, F.; Wu, Z.; Zhu, Q.; Min, F. The effectiveness of Traditional Chinese Yijinjing Qigong Exercise for the patients with Knee Osteoarthritis on the Pain, Dysfunction and Mood Disorder, a pilot randomized controlled trial. Front. Med. (Lausanne) 2021, 8, 2664. [Google Scholar] [CrossRef]
- Shirwaikar, R.D.; Acharya U, D.; Makkithaya, K.; M, S.; Srivastava, S.; Lewis U, L.E.S. Optimizing neural networks for medical data sets: A case study on neonatal apnea prediction. Artif. Intell. Med. 2019, 98, 59–76. [Google Scholar] [CrossRef]
- Vellido, A. The importance of interpretability and visualization in machine learning for applications in medicine and health care. Neural Comput. Appl. 2020, 32, 18069–18083. [Google Scholar] [CrossRef]
- Chen, L.K.; Lee, W.J.; Peng, L.N.; Liu, L.K.; Arai, H.; Akishita, M. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, 767.e1–767.e7. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Casas Herrero, A.; Zambom Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodríguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Guerri, S.; Mercatelli, D.; Aparisi Gomez, M.P.; Napoli, A.; Battista, G.; Guglielmi, G.; Bazzocchi, A. Quantitative imaging techniques for the assessment of osteoporosis and sarcopenia. Quant. Imaging Med. Surg. 2018, 8, 60–85. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.C.; Yoo, J.I.; Park, Y.J.; Lee, C.H.; Park, K.S. Measurement of uncertainty using standardized protocol of hand grip strength measurement in patients with sarcopenia. J. Bone Metab. 2018, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Penninx, B.W.H.J.; Nicklas, B.J.; Simonsick, E.M.; Newman, A.B.; Tylavsky, F.A.; Brach, J.S.; Satterfield, S.; Bauer, D.C.; et al. Prognostic Value of Usual Gait Speed in Well-Functioning Older People—Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005, 53, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Lieffers, J.; McCargar, L.; Reiman, T.; Sawyer, M.; Martin, L.; Baracos, V. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Baumgartner, R.; Koehler, K.; Gallagher, D.; Romero, L.; Heymsfield, S.; Ross, R.; Garry, P.; Lindeman, R. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Camastra, S.; Vitali, A.; Anselmino, M.; Gastaldelli, A.; Bellini, R.; Berta, R.; Severi, I.; Baldi, S.; Astiarraga, B.; Barbatelli, G.; et al. Muscle and adipose tissue morphology, insulin sensitivity and beta-cell function in diabetic and nondiabetic obese patients: Effects of bariatric surgery. Sci. Rep. 2017, 7, 9007. [Google Scholar] [CrossRef]
- Porto, J.M.; Nakaishi, A.P.M.; Cangussu-Oliveira, L.M.; Freire Júnior, R.C.; Spilla, S.B.; de Abreu, D.C.C. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch. Gerontol. Geriatr. 2019, 82, 273–278. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the NeurIPS Proceedings, Montreal, QC, Canada, 3–8 December 2018; pp. 3149–3157. [Google Scholar]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Chen, T.Q.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2012, 12, 2825–2830. [Google Scholar]
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X. Study of data mining algorithm based on decision tree. In Proceedings of the 2010 International Conference on Computer Design and Applications, Qinhuangdao, China, 25–27 June 2010; Volume 1, pp. V1-155–V1-158. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Szostak, D.; Walkowiak, K.; Włodarczyk, A. Short-Term Traffic Forecasting in Optical Network using Linear Discriminant Analysis Machine Learning Classifier. In Proceedings of the 2020 22nd International Conference on Transparent Optical Networks (ICTON), Bari, Italy, 19–23 July 2020; pp. 1–4. [Google Scholar]
- Corinna, C.; Vladimir, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar]
- Kumar, R.; Naik, S.M.; Naik, V.D.; Shiralli, S.; Sunil, V.G.; Husain, M. Predicting clicks: CTR estimation of advertisements using Logistic Regression classifier. In Proceedings of the 2015 IEEE International Advance Computing Conference (IACC), Bangalore, India, 12–13 June 2015; pp. 1134–1138. [Google Scholar]
- Lundberg, S.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Bamman, M.M.; Roberts, B.M.; Adams, G.R. Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb. Perspect. Med. 2018, 8, a029751. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α Isoform Induced by Resistance Training Regulates Skeletal Muscle Hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Meng, D.; Guo, H.; Liang, S.; Tian, Z.; Wang, R.; Yang, G.; Wang, Z. Effectiveness of a Hybrid Exercise Program on the Physical Abilities of Frail Elderly and Explainable Artificial-Intelligence-Based Clinical Assistance. Int. J. Environ. Res. Public Health 2022, 19, 6988. [Google Scholar] [CrossRef]
- Motl, R.W.; Smith, D.C.; Elliott, J.; Weikert, M.; Dlugonski, D.; Sosnoff, J.J. Combined training improves walking mobility in persons with significant disability from multiple sclerosis: A pilot study. J. Neurol. Phys. Ther. 2012, 36, 32–37. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, S.; Zhang, S.; Mao, Y.; Fang, L. Eight weeks of high-intensity interval static strength training improves skeletal muscle atrophy and motor function in aged rats via the PGC-1α/FNDC5/UCP1 pathway. Clin. Interv. Aging 2021, 16, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sport. Med. 2018, 48, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 2013, 12, 943–949. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance exercise as a countermeasure for aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wen, J.; Sun, W.; Li, T.; Yu, X.; Zhang, T.; Zhou, X.; Wu, W.; Li, R. Effects of traditional Chinese exercise on cardiac rehabilitation after percutaneous coronary intervention: Study protocol for network meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e023096. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Pi, Y.L.; Chen, P.J.; Liu, Y.; Wang, R.; Li, X.; Chen, B.L.; Zhu, Y.; Yang, Y.J.; Niu, Z.B. Traditional Chinese exercise for cardiovascular diseases: Systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016, 5, e002562. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, A.P.; Amati, F.; Smiley, M.A.; Goodpaster, B.; Wright, V. Chronic exercise preserves lean muscle mass in masters athletes. Phys. Sport. 2011, 39, 172–178. [Google Scholar] [CrossRef]
- Miljkovic, I.; Kuipers, A.L.; Cvejkus, R.; Bunker, C.H.; Patrick, A.L.; Gordon, C.L.; Zmuda, J.M. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity 2016, 24, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Tanabe, K.; Yokoyama, N.; Chijiki, S.; Tsuruzono, T.; Kuno, S. Effects of daily walking on intermuscular adipose tissue accumulation with age: A 5-year follow-up of participants in a lifestyle-based daily walking program. Eur. J. Appl. Physiol. 2018, 118, 785–793. [Google Scholar] [CrossRef]
| Items | YR 1 (n = 30) | RT 2 (n = 30) | CG 3 (n = 30) | p-Value |
|---|---|---|---|---|
| Sex (male/female) | 14/16 | 13/17 | 16/14 | 0.745 |
| Age (years) | 66.70 ± 4.10 | 66.87 ± 3.84 | 65.42 ± 3.97 | 0.301 |
| Body mass (cm) | 166.13 ± 7.67 | 168.11 ± 8.58 | 167.14 ± 8.35 | 0.217 |
| Stature (kg) | 63.13 ± 6.19 | 64.37 ± 7.16 | 61.05 ± 7.87 | 0.417 |
| BMI (kg/m) | 22.76 ± 2.19 | 22.80 ± 3.18 | 21.93 ± 2.86 | 0.870 |
| Parameters | YR 1 (n = 30) | RT 2 (n = 30) | CG 3 (n = 30) | Group × Time # | |||
|---|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | Baseline | 24 Weeks | Baseline | 24 Weeks | p-Value | |
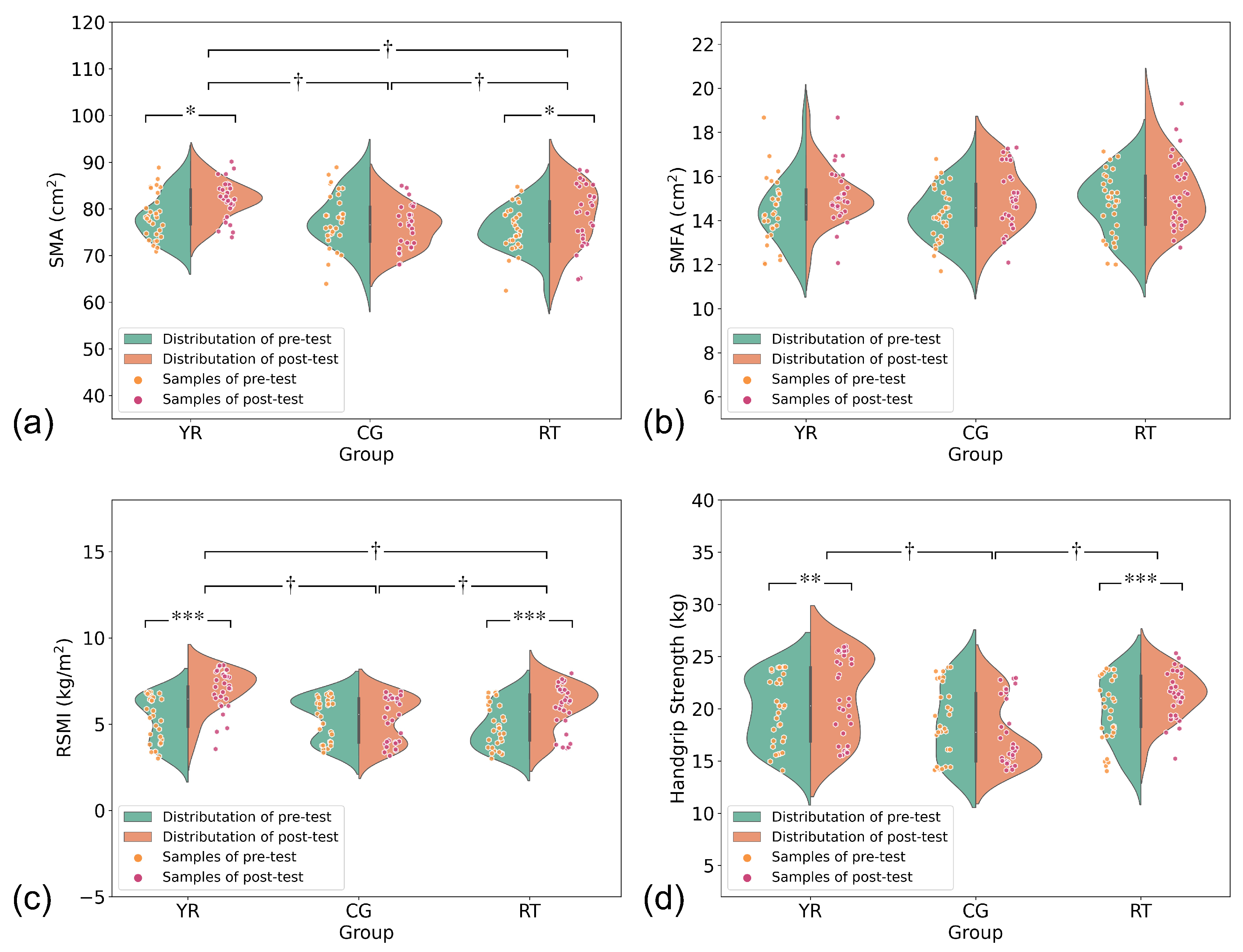
| SMA (cm) | 78.39 ± 4.71 | 81.92 ± 3.99 †,* | 75.79 ± 4.77 | 78.64 ± 6.36 †,* | 77.63 ± 5.81 | 77.06 ± 4.53 † | <0.05 |
| SMD (HU) | 32.53 ± 3.14 | 34.25 ± 3.15 †,** | 32.64 ± 3.03 | 34.72 ± 2.80 †,** | 32.69 ± 3.72 | 32.44 ± 3.31 † | >0.05 |
| SMFA (cm) | 14.43 ± 1.46 | 15.11 ± 1.22 | 14.73 ± 1.43 | 15.30 ± 1.58 | 14.28 ± 1.24 | 15.03 ± 1.39 | >0.05 |
| SMFD (HU) | −64.73 ± 5.90 | −65.05 ± 5.83 | −64.01 ± 5.43 | −64.48 ± 4.71 | −64.21 ± 5.69 | −64.68 ± 6.11 | >0.05 |
| RSMI (kg/m) | 5.25 ± 1.32 | 6.98 ± 1.18 †,*** | 4.84 ± 1.23 | 6.05 ± 1.30 †,*** | 5.32 ± 1.21 | 5.24 ± 1.28 † | <0.05 |
| MFI (%) | 15.57 ± 1.53 | 15.58 ± 1.24 | 16.30 ± 1.61 | 16.35 ± 1.93 | 15.60 ± 1.82 | 16.52 ± 1.52 ** | <0.05 |
| HGS (kg) | 19.70 ± 3.22 | 21.21 ± 3.80 †,** | 19.73 ± 3.13 | 21.35 ± 2.29 †,*** | 18.72 ± 3.48 | 17.67 ± 3.10 † | <0.05 |
| Models | Accuracy | Precision | Recall | F1 |
|---|---|---|---|---|
| SVC 1 (%) | 72.2 ± 11.6 | 60.2 ± 11.4 | 64.8 ± 12.3 | 65.2 ± 11.1 |
| KNN Classifier 2 (%) | 79.1 ± 11.4 | 61.3 ± 11.6 | 63.8 ± 12.1 | 68.6 ± 12.1 |
| XGB Classifier 3 (%) | 79.6 ± 11.9 | 64.0 ± 12.7 | 61.1 ± 11.3 | 60.1 ± 12.5 |
| Decision Tree (%) | 80.4 ± 12.5 | 66.1 ± 11.4 | 66.0 ± 11.3 | 63.4 ± 15.8 |
| Extra Tree Classifier (%) | 81.1 ± 11.2 | 67.6 ± 13.4 | 61.3 ± 10.5 | 60.2 ± 14.2 |
| GDB Classifier 4 (%) | 82.0 ± 11.7 | 70.5 ± 11.4 | 61.4 ± 11.2 | 63.0 ± 16.6 |
| RF Classifier 5 (%) | 82.9 ± 11.5 | 71.8 ± 11.9 | 61.9 ± 10.1 | 63.4 ± 13.1 |
| Logistic Regression (%) | 84.0 ± 10.9 | 74.6 ± 12.1 | 69.7 ± 11.7 | 68.5 ± 14.1 |
| LGBM Classifier 6 (%) | 85.0 ± 11.3 | 78.7 ± 10.8 | 64.8 ± 10.4 | 67.1 ± 10.8 |
| LDA 7 (%) | 85.4 ± 11.1 | 75.1 ± 11.6 | 77.3 ± 12.9 | 73.4 ± 12.8 |
| Stacking (%) | 85.7 ± 10.6 | 78.2 ± 13.2 | 74.5 ± 15.9 | 75.3 ± 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Meng, D.; Guo, H.; He, S.; Tian, Z.; Wang, Z.; Yang, G.; Wang, Z. Hybrid Exercise Program for Sarcopenia in Older Adults: The Effectiveness of Explainable Artificial Intelligence-Based Clinical Assistance in Assessing Skeletal Muscle Area. Int. J. Environ. Res. Public Health 2022, 19, 9952. https://doi.org/10.3390/ijerph19169952
Wei M, Meng D, Guo H, He S, Tian Z, Wang Z, Yang G, Wang Z. Hybrid Exercise Program for Sarcopenia in Older Adults: The Effectiveness of Explainable Artificial Intelligence-Based Clinical Assistance in Assessing Skeletal Muscle Area. International Journal of Environmental Research and Public Health. 2022; 19(16):9952. https://doi.org/10.3390/ijerph19169952
Chicago/Turabian StyleWei, Meiqi, Deyu Meng, Hongzhi Guo, Shichun He, Zhibo Tian, Ziyi Wang, Guang Yang, and Ziheng Wang. 2022. "Hybrid Exercise Program for Sarcopenia in Older Adults: The Effectiveness of Explainable Artificial Intelligence-Based Clinical Assistance in Assessing Skeletal Muscle Area" International Journal of Environmental Research and Public Health 19, no. 16: 9952. https://doi.org/10.3390/ijerph19169952
APA StyleWei, M., Meng, D., Guo, H., He, S., Tian, Z., Wang, Z., Yang, G., & Wang, Z. (2022). Hybrid Exercise Program for Sarcopenia in Older Adults: The Effectiveness of Explainable Artificial Intelligence-Based Clinical Assistance in Assessing Skeletal Muscle Area. International Journal of Environmental Research and Public Health, 19(16), 9952. https://doi.org/10.3390/ijerph19169952






