Association of a Healthy Lifestyle with All-Cause, Cause-Specific Mortality and Incident Cancer among Individuals with Metabolic Syndrome: A Prospective Cohort Study in UK Biobank
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Participants
2.2. Ascertaining MetS
2.3. Assessment of Healthy Lifestyles
2.4. Ascertaining the Outcomes
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
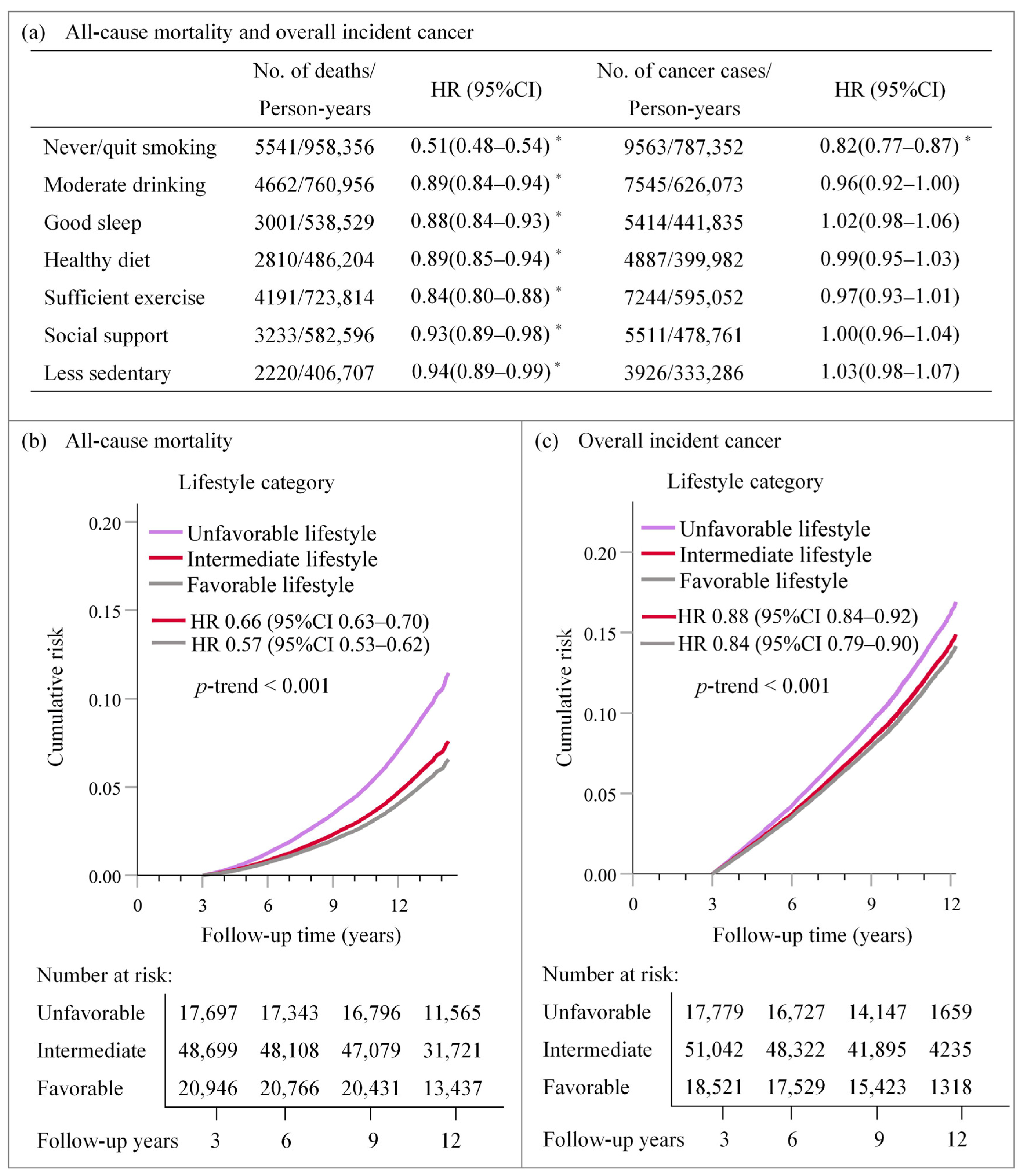
3.2. Associations of Single and Integrated Lifestyle Factors with All-Cause Mortality and Overall Cancer Incidence
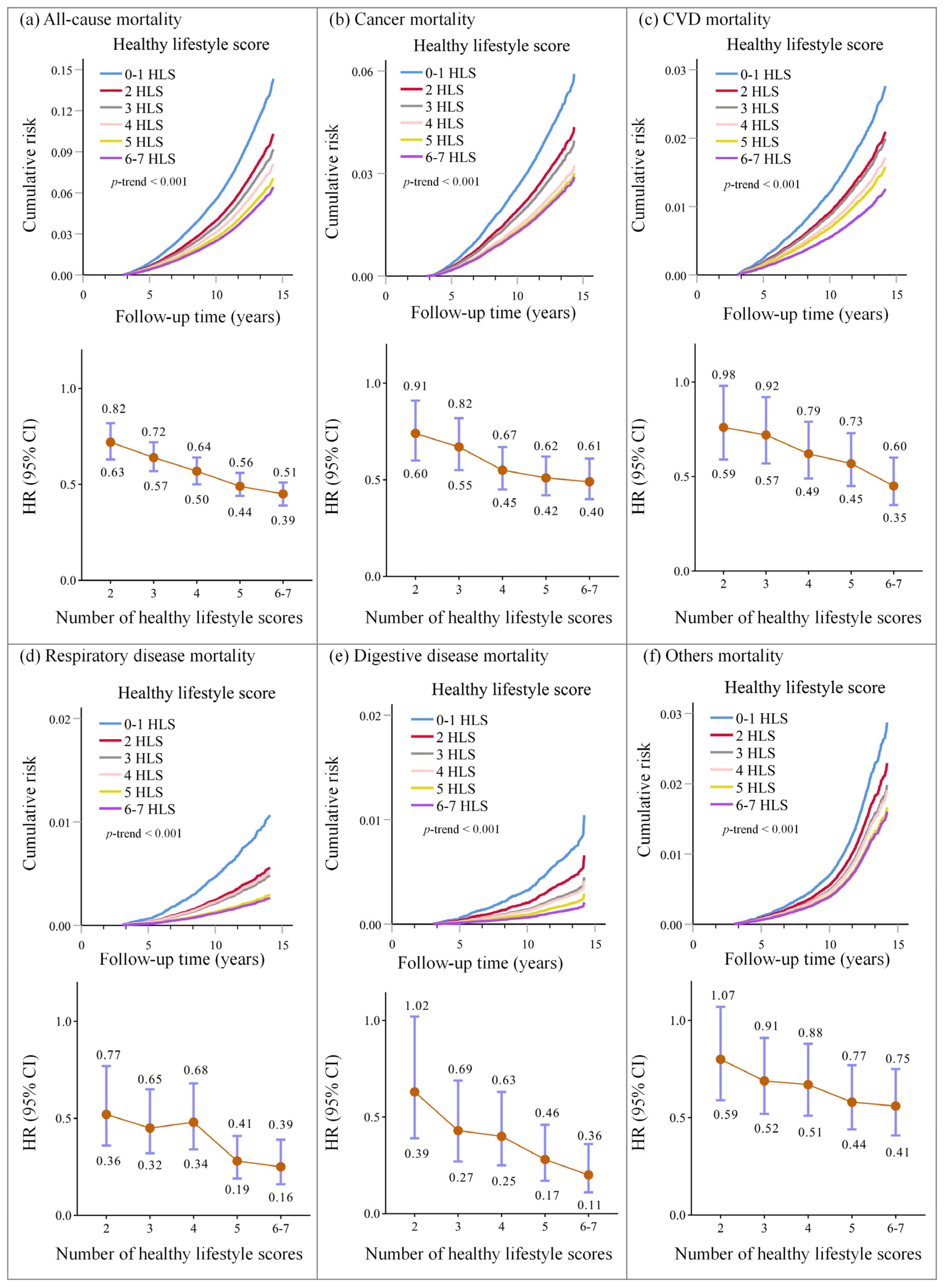
3.3. Associations of Healthy Lifestyle Scores with Cause-Specific Mortality
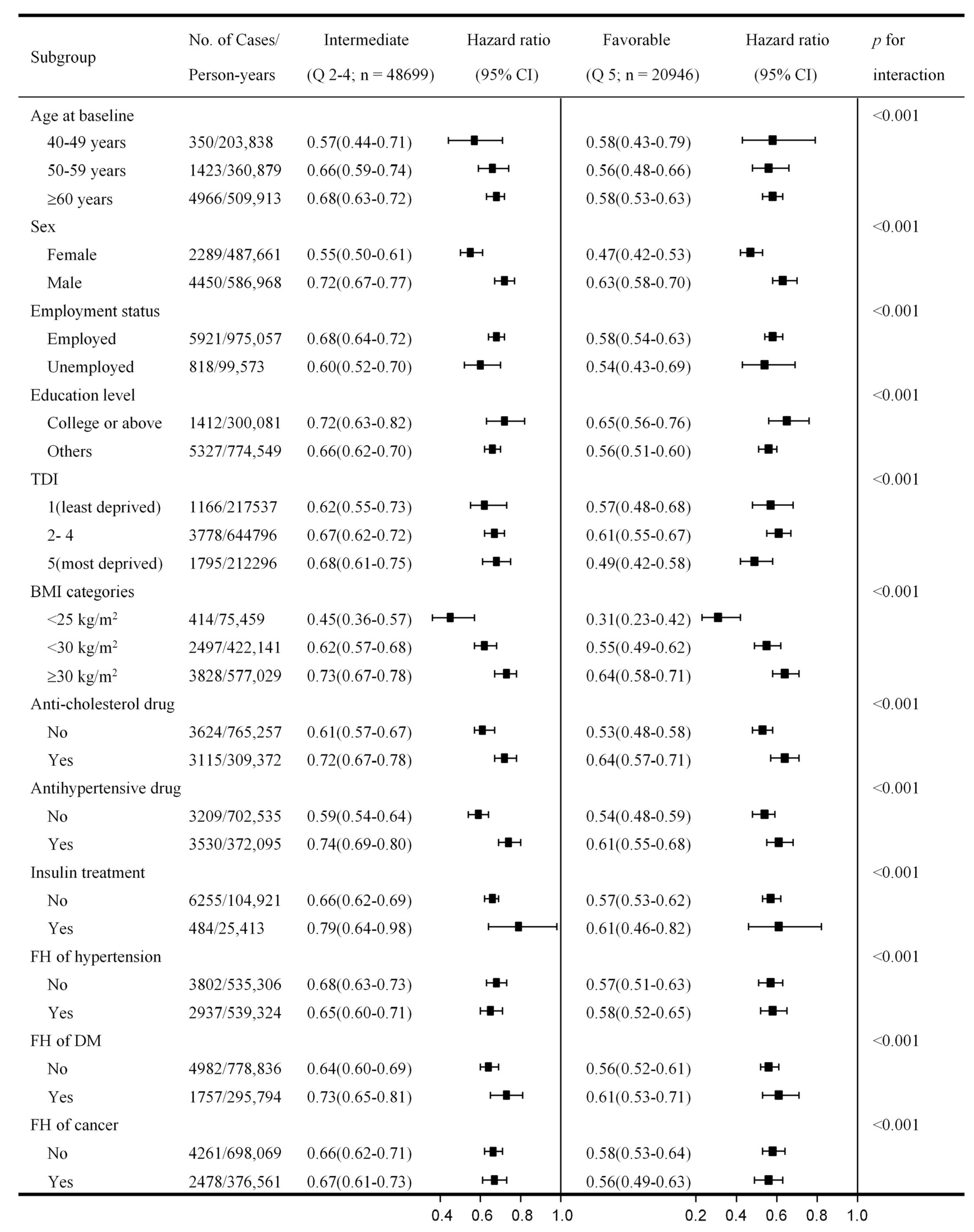
3.4. Subgroup Analysis by Baseline Characteristics
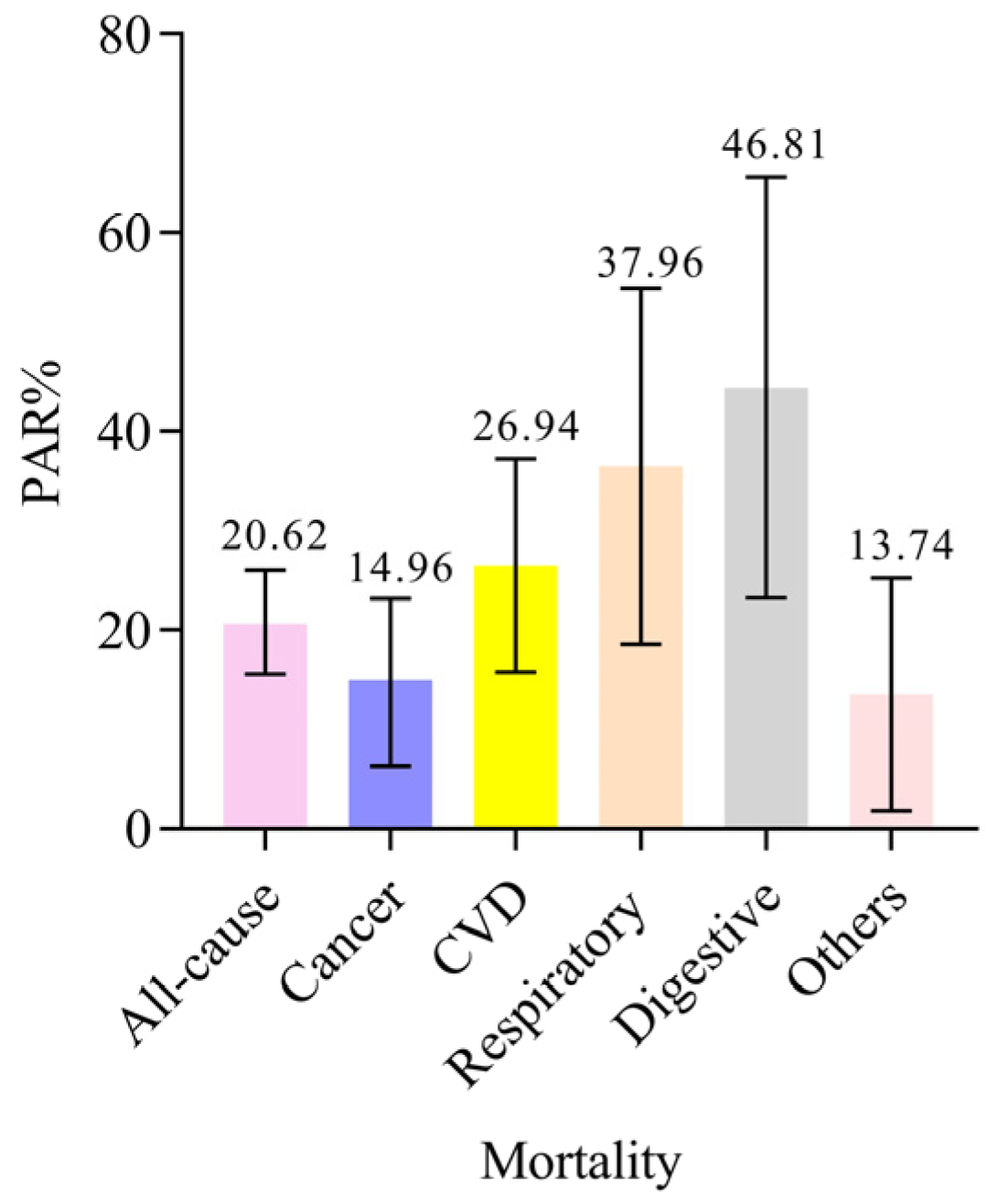
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Saklayen; Mohammad, G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef]
- Barati, E.; Ghazizadeh, H.; Sadabadi, F.; Kazemi, E.; Ferns, G.A.; Avan, A.; Ghayour-Mobarhan, M. Association of the IL6 Gene Polymorphism with Component Features of Metabolic Syndrome in Obese Subjects. Biochem. Genet. 2019, 57, 695–708. [Google Scholar] [CrossRef]
- Boudreau, D.M.; Malone, D.C.; Raebel, M.A.; Fishman, P.A.; Nichols, G.A.; Feldstein, A.C.; Boscoe, A.N.; Ben-Joseph, R.H.; Magid, D.J.; Okamoto, L.J. Health care utilization and costs by metabolic syndrome risk factors. Metab. Syndr. Relat. Disord. 2009, 7, 305–314. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Obes. Rev. 2005, 24, 283–296. [Google Scholar]
- Xu, Z.J.; Shi, J.P.; Yu, D.R.; Zhu, L.J.; Jia, J.D.; Fan, J.G. Evaluating the Relationship Between Metabolic Syndrome and Liver Biopsy-Proven Non-Alcoholic Steatohepatitis in China: A Multicenter Cross-Sectional Study Design. Adv. Ther. 2016, 33, 2069–2081. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Diaz-Lopez, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Lambert, C.T.; Gordon, N.F.; Fletcher, G.F.; Franklin, B.A.; Wenger, N.K.; Sperling, L. Cardiac Rehabilitation and Risk Reduction: Time to “Rebrand and Reinvigorate”. J. Am. Coll. Cardiol. 2015, 65, 389–395. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Peiris, C.L.; van Namen, M.; O’Donoghue, G. Education-based, lifestyle intervention programs with unsupervised exercise improve outcomes in adults with metabolic syndrome. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 877–890. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Ortolá, R.; Ruiz-Canela, M.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F. Association between the Mediterranean lifestyle, metabolic syndrome and mortality: A whole-country cohort in Spain. Cardiovasc. Diabetol. 2021, 20, 5. [Google Scholar] [CrossRef]
- Heath, L.; Jebb, S.A.; Aveyard, P.; Piernas, C. Obesity, metabolic risk and adherence to healthy lifestyle behaviours: Prospective cohort study in the UK Biobank. BMC Med. 2022, 20, 65. [Google Scholar] [CrossRef]
- Yadav, R.; Yadav, R.K.; Khadgawat, R.; Pandey, R.M.; Upadhyay, A.D.; Mehta, N. Randomized Controlled Trial of A 12-Week Yoga-Based (Including Diet) Lifestyle vs. Dietary Intervention on Cardio-Metabolic Risk Factors and Continuous Risk Score in Indian Adults with Metabolic Syndrome. Behav. Med. 2020, 46, 9–20. [Google Scholar] [CrossRef]
- Perez-Martin, A.R.; Castro-Eguiluz, D.; Cetina-Perez, L.; Velasco-Torres, Y.; Bahena-Gonzalez, A.; Montes-Servin, E.; Gonzalez-Ibarra, E.; Espinoza-Romero, R.; Gallardo-Rincon, D. Impact of metabolic syndrome on the risk of endometrial cancer and the role of lifestyle in prevention. Bosn. J. Basic. Med. Sci. 2022, 22, 499–510. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Yin, S.; Wang, T.; Zhu, M.; Liu, L.; Jin, G. Genetic risk, metabolic syndrome, and gastrointestinal cancer risk: A prospective cohort study. Cancer Med. 2022, 1–9. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Marchini, J. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Magkos, F.; Yannakoulia, M.; Chan, J.L.; Mantzoros, C.S. Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Annu. Rev. Nutr. 2009, 29, 223–256. [Google Scholar] [CrossRef]
- Klarich, D.; Brasser, S.M.; Hong, M.Y. Moderate Alcohol Consumption and Colorectal Cancer Risk. Alcohol. Clin. Exp. Res. 2015, 39, 1280–1291. [Google Scholar] [CrossRef]
- Peppone, L.J.; Mahoney, M.C.; Cummings, K.M.; Michalek, A.M.; Reid, M.E.; Moysich, K.B.; Hyland, A. Colorectal cancer occurs earlier in those exposed to tobacco smoke: Implications for screening. J. Cancer Res. Clin. Oncol. 2008, 134, 743–751. [Google Scholar] [CrossRef]
- Li, X.; Xue, Q.; Wang, M.; Zhou, T.; Qi, L. Adherence to a Healthy Sleep Pattern and Incident Heart Failure: A Prospective Study of 408802 UK Biobank Participants. Circulation 2020, 143, 97–99. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–255. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA J. Am. Med. Assoc. 2019, 322, 430–437. [Google Scholar] [CrossRef]
- Oru, Z.; Kaplan, M.A. Effect of exercise on colorectal cancer prevention and treatment. World J. Gastrointest. Oncol. 2019, 11, 348–366. [Google Scholar]
- Gronewold, J.; Hermann, D.M. Social isolation and risk of fatal cardiovascular events. Lancet Public Health 2021, 6, e197–e198. [Google Scholar] [CrossRef]
- Smith, R.W.; Barnes, I.; Green, J.; Reeves, G.K.; Floud, S. Social isolation and risk of heart disease and stroke: Analysis of two large UK prospective studies. Lancet Public Health 2021, 6, e232–e239. [Google Scholar] [CrossRef]
- Weber, A.; Leitzmann, M.F.; Sedlmeier, A.M.; Baurecht, H.; Jochem, C.; Haferkamp, S.; Baumeister, S.E. Association between physical activity, grip strength and sedentary behaviour with incidence of malignant melanoma: Results from the UK Biobank. Br. J. Cancer 2021, 125, 593–600. [Google Scholar] [CrossRef]
- Kunzmann, A.; Mallon, K.; Hunter, R.; Cardwell, C.; Mcmenamin, U.; Spence, A.; Coleman, H. Physical activity, sedentary behaviour and risk of oesophago-gastric cancer: A prospective cohort study within UK Biobank. UEG J. 2018, 6, 1144–1154. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Chen, C.; Pan, X.F.; Guo, J.; Li, Y.; Franco, O.H.; Liu, G.; Pan, A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: Two prospective cohort studies. BMJ 2021, 373, n604. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.H.; Shen, D.; Zhang, P.D.; Song, W.Q.; Zhang, W.T.; Huang, Q.M.; Chen, P.L.; Zhang, X.R.; Mao, C. Association of Sugar-Sweetened, Artificially Sweetened, and Unsweetened Coffee Consumption With All-Cause and Cause-Specific Mortality: A Large Prospective Cohort Study. Ann. Intern. Med. 2022, 175, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.L.; Perez-Cornago, A.; Fensom, G.K.; Smith-Byrne, K.; Noor, U.; Andrews, C.D.; Gunter, M.J.; Holmes, M.V.; Martin, R.M.; Tsilidis, K.K.; et al. Circulating insulin-like growth factors and risks of overall, aggressive and early-onset prostate cancer: A collaborative analysis of 20 prospective studies and Mendelian randomization analysis. Int. J. Epidemiol. 2022, 1–16. [Google Scholar] [CrossRef]
- Pei, Y.F.; Zhang, L. Is the Townsend Deprivation Index a Reliable Predictor of Psychiatric Disorders? Biol. Psychiatry. 2021, 89, 839–841. [Google Scholar] [CrossRef]
- Lin, X.J.; Wang, C.P.; Liu, X.D.; Yan, K.K.; Li, S.; Bao, H.H.; Zhao, L.Y.; Liu, X. Body mass index and risk of gastric cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2014, 44, 783–791. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Fang, Z.; Hang, D.; Wang, F.; Polychronidis, G.; Wang, L.; Lo, C.H.; Wang, K.; Zhong, R.; Knudsen, M.D.; et al. Circulating liver function markers and colorectal cancer risk: A prospective cohort study in the UK Biobank. Int. J. Cancer 2021, 148, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yang, R.; Wang, W.; Huang, N.; Zhuang, Z.; Han, Y.; Qi, L.; Xu, M.; Tang, Y.D.; Huang, T. Association of healthy lifestyle including a healthy sleep pattern with incident type 2 diabetes mellitus among individuals with hypertension. Cardiovasc. Diabetol. 2021, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cao, Y.; Feng, C.; Zheng, Y.; Dhana, K.; Zhu, S.; Shang, C.; Yuan, C.; Zong, G. Association of a Healthy Lifestyle With All-Cause and Cause-Specific Mortality Among Individuals With Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care 2022, 45, 319–329. [Google Scholar] [CrossRef]
- Botteri, E.; Berstad, P.; Sandin, S.; Weiderpass, E. Lifestyle changes and risk of cancer: Experience from the Swedish women’s lifestyle and health cohort study. Acta Oncol. 2021, 60, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Rezende, L.; Murata, E.; Giannichi, B.; Tomita, L.Y.; Ferrari, G. Cancer cases and deaths attributable to lifestyle risk factors in Chile. BMC Cancer 2020, 20, 693. [Google Scholar] [CrossRef]
- Van Namen, M.; Prendergast, L.; Peiris, C. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: A systematic review and meta-analysis. Metabolism 2019, 101, 153988. [Google Scholar] [CrossRef]
- Hastert, T.A.; Ruterbusch, J.J.; Beresford, S.A.A.; Sheppard, L.; White, E. Contribution of health behaviors to the association between area-level socioeconomic status and cancer mortality. Soc. Sci. Med. 2016, 148, 52–58. [Google Scholar] [CrossRef]
- Soghra, J.; Yan, Y.; Mario, S.; William, C.D. Income Disparity and Risk of Death: The Importance of Health Behaviors and Other Mediating Factors. PLoS ONE 2012, 7, e49929. [Google Scholar]
- Laaksonen, M.; Talala, K.; Martelin, T.; Rahkonen, O.; Roos, E.; Helakorpi, S.; Laatikainen, T.; Prattala, R. Health behaviours as explanations for educational level differences in cardiovascular and all-cause mortality: A follow-up of 60,000 men and women over 23 years. Eur. J. Public Health 2008, 18, 38–43. [Google Scholar] [CrossRef]
- Schrijvers, C.T.; Stronks, K.; Van, D.; Mackenbach, J.P. Explaining educational differences in mortality: The role of behavioral and material factors. Am. J. Public Health 1999, 89, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Stringhini, S.; Dugravot, A.; Shipley, M.; Goldberg, M.; Zins, M.; Kivim?Ki, M.; Marmot, M.; Sabia, S.; Singh-Manoux, A.; Lopez, A.D. Health Behaviours, Socioeconomic Status, and Mortality: Further Analyses of the British Whitehall II and the French GAZEL Prospective Cohorts. PLoS Med. 2011, 8, e1000419. [Google Scholar] [CrossRef]
- Hecht, S.S.; Hatsukami, D.K. Smokeless tobacco and cigarette smoking: Chemical mechanisms and cancer prevention. Nat. Rev. Cancer 2022, 22, 143–155. [Google Scholar] [CrossRef]
- Schneider, S.; Merfort, I.; Idzko, M.; Zech, A. Blocking P2X purinoceptor 4 signalling alleviates cigarette smoke induced pulmonary inflammation. Respir. Res. 2022, 23, 148. [Google Scholar] [CrossRef]
- Battey, J.N.D.; Szostak, J.; Phillips, B.; Teng, C.; Tung, C.K.; Lim, W.T.; Yeo, Y.S.; Ouadi, S.; Baumer, K.; Thomas, J.; et al. Impact of 6-Month Exposure to Aerosols From Potential Modified Risk Tobacco Products Relative to Cigarette Smoke on the Rodent Gastrointestinal Tract. Front. Microbiol. 2021, 12, 587745. [Google Scholar] [CrossRef]
- Ude, A.N.; Edem, V.F.; Onifade, A.A.; Arinola, O.G. Serum Levels of Selected Vitamins and Trace Elements in Nigerian Consumers of Alcoholic Beverage: A Suggestion for DNA Hypomethylation. Niger. J. Physiol. Sci. Off. Publ. Physiol. Soc. Niger. 2016, 31, 101–104. [Google Scholar]
- Rossi, M.; Anwar, M.J.; Usman, A.; Keshavarzian, A.; Bishehsari, F. Colorectal Cancer and Alcohol Consumption—Populations to Molecules. Cancers 2018, 10, 38. [Google Scholar] [CrossRef]
- Barve, S.; Chen, S.Y.; Kirpich, I.; Watson, W.H.; Mcclain, C. Development, Prevention, and Treatment of Alcohol-Induced Organ Injury: The Role of Nutrition. Alcohol Res. Curr. Rev. 2017, 38, 289–302. [Google Scholar]
- Anuradha, R.; Pranoti, M. Alcohol and Cancer: Mechanisms and Therapies. Biomolecules 2017, 7, 61. [Google Scholar]
- Happel, M.; Zhang, P.; Kolls, J.K.; Nelson, S.; Molinaespeja, P. Focus On: Alcohol and the Immune System. Alcohol Res. Health 2010, 33, 97–108. [Google Scholar]
- Bujanda, L. The effects of alcohol consumption upon the gastrointestinal tract. Am. J. Gastroenterol. 2000, 95, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Trevejo-Nunez, G.; Kolls, J.K.; de Wit, M. Alcohol Use As a Risk Factor in Infections and Healing: A Clinician’s Perspective. Alcohol Res. Curr. Rev. 2015, 37, 177–184. [Google Scholar]
- Brzecka, A.; Sarul, K.; Dya, T.; Avila-Rodriguez, M.; Aliev, G. The Association of Sleep Disorders, Obesity, and Sleep-Related Hypoxia with Cancer. Curr. Top. Med. Chem. 2020, 21, 444–453. [Google Scholar] [CrossRef]
- Kurin, M.; Shibli, F.; Kitayama, Y.; Kim, Y.; Fass, R. Sorting out the Relationship between Gastroesophageal Reflux Disease and Sleep. Curr. Gastroenterol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Turati, F.; Bravi, F.; Polesel, J.; Bosetti, C.; Negri, E.; Garavello, W.; Taborelli, M.; Serraino, D.; Libra, M.; Montella, M. Adherence to the Mediterranean diet and nasopharyngeal cancer risk in Italy. Cancer Causes Control. 2017, 28, 89–95. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Sorli-Aguilar, M.; Martin-Lujan, F.; Flores-Mateo, G.; Arija-Val, V.; Basora-Gallisa, J.; Sola-Alberich, R. Dietary patterns are associated with lung function among Spanish smokers without respiratory disease. BMC Pulm. Med. 2016, 16, 162. [Google Scholar] [CrossRef]
- Turner, J.E.; Brum, P.C. Does Regular Exercise Counter T Cell Immunosenescence Reducing the Risk of Developing Cancer and Promoting Successful Treatment of Malignancies? Oxidative Med. Cell. Longev. 2017, 2017, 4234765. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Zhao, W.; Qian, H.; Yan, X.; Zong, R.; Zhang, Y.; Lao, K. Aerobic exercise promotes the expression of ERCC1 to prolong lifespan: A new possible mechanism. Med. Hypotheses 2019, 122, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Raynor, H.A.; Ciccolo, J.T. A Review of TV Viewing and Its Association With Health Outcomes in Adults. Am. J. Lifestyle Med. 2008, 2, 250–259. [Google Scholar] [CrossRef]
- Vila, J. Social Support and Longevity: Meta-Analysis-Based Evidence and Psychobiological Mechanisms. Front. Psychol. 2021, 12, 717164. [Google Scholar] [CrossRef]
| Characteristic | All-Cause Mortality | Overall Cancer Incidence | ||
|---|---|---|---|---|
| No (n = 80,603) | Yes (n = 6739) | No (n = 76,540) | Yes (n = 10,802) | |
| Age, mean (SD), years t | 57.2 (7.7) | 62.0 (6.1) | 57.1 (7.8) | 60.8 (6.3) |
| Male, n (%) * | 43,384 (53.8) | 4450 (66.4) | 41,261 (53.9) | 6573 (60.8) |
| BMI, mean (SD) t | 30.9 (4.7) | 31.5 (5.1) | 31.0 (4.8) | 30.8 (4.5) |
| White Ethnicity, n (%) * | 74,642 (92.6) | 6397 (94.9) | 70,615 (92.3) | 10,424 (96.5) |
| Employed, n (%) * | 73,219 (90.8) | 5921 (87.9) | 69,163 (90.4) | 9977 (92.4) |
| College/university, n (%) * | 22,862 (28.4) | 1412 (21.0) | 21,540 (28.1) | 2734 (25.3) |
| TDI, n (%) w | ||||
| 1 (least deprived) | 16,314 (20.2) | 1166 (17.3) | 15,152 (19.8) | 2328 (21.6) |
| 2–4 | 48,617 (60.3) | 3778 (56.1) | 45,850 (59.9) | 6545 (60.6) |
| 5 (most deprived) | 15,672 (19.4) | 1795 (26.6) | 15,538 (20.3) | 1929 (17.9) |
| Medication, n (%) * | ||||
| Anti-cholesterol drug | 22,453 (27.9) | 3115 (46.2) | 21,750 (28.4) | 3818 (35.3) |
| Antihypertensive drug | 27,080 (33.6) | 3530 (52.4) | 26,108 (34.1) | 4502 (41.7) |
| Insulin | 1698 (2.1) | 484 (7.2) | 1906 (2.5) | 276 (2.6) |
| Family history, n (%) * | ||||
| Hypertension | 40,801 (49.4) | 2937 (43.6) | 38,709 (50.6) | 5029 (46.6) |
| DM | 22,375 (27.8) | 1757 (26.1) | 21,424 (28.0) | 2708 (25.1) |
| Cancer | 28,158 (34.9) | 2478 (36.8) | 26,366 (34.4) | 4270 (39.5) |
| No. of MetS components, n (%) w | ||||
| 3 | 53,232 (66.0) | 3964 (58.8) | 50,230 (65.6) | 6966 (64.5) |
| 4 | 22,022 (27.3) | 2061 (30.6) | 21,084 (27.5) | 2999 (27.8) |
| 5 | 5349 (6.6) | 714 (10.6) | 5226 (6.8) | 837 (7.7) |
| Healthy lifestyle factor, n (%) * | ||||
| Never/quit smoking | 72,234 (89.6) | 5541 (82.2) | 68,212 (89.1) | 9563 (88.5) |
| Moderate drinking | 57,421 (71.2) | 4662 (69.2) | 54,538 (71.3) | 7545 (69.8) |
| Good sleep | 40,625 (50.4) | 3001 (44.5) | 38,212 (49.9) | 5414 (50.1) |
| Healthy diet | 36,708 (45.4) | 2810 (41.7) | 34,631 (45.2) | 4887 (45.2) |
| Sufficient exercise | 54,578 (67.7) | 4191 (62.2) | 51,525 (67.3) | 7244 (67.1) |
| Social support | 43,990 (54.6) | 3233 (48.0) | 41,712 (54.5) | 5511 (51.0) |
| Less sedentary | 30,663 (38.0) | 2220 (32.9) | 28,957 (37.8) | 3926 (36.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.; Ni, J.-T.; Zhu, Z.-H.; Xu, H.-Q.; Tao, L.; Xie, T. Association of a Healthy Lifestyle with All-Cause, Cause-Specific Mortality and Incident Cancer among Individuals with Metabolic Syndrome: A Prospective Cohort Study in UK Biobank. Int. J. Environ. Res. Public Health 2022, 19, 9936. https://doi.org/10.3390/ijerph19169936
Wu E, Ni J-T, Zhu Z-H, Xu H-Q, Tao L, Xie T. Association of a Healthy Lifestyle with All-Cause, Cause-Specific Mortality and Incident Cancer among Individuals with Metabolic Syndrome: A Prospective Cohort Study in UK Biobank. International Journal of Environmental Research and Public Health. 2022; 19(16):9936. https://doi.org/10.3390/ijerph19169936
Chicago/Turabian StyleWu, E, Jun-Tao Ni, Zhao-Hui Zhu, Hong-Quan Xu, Lin Tao, and Tian Xie. 2022. "Association of a Healthy Lifestyle with All-Cause, Cause-Specific Mortality and Incident Cancer among Individuals with Metabolic Syndrome: A Prospective Cohort Study in UK Biobank" International Journal of Environmental Research and Public Health 19, no. 16: 9936. https://doi.org/10.3390/ijerph19169936
APA StyleWu, E., Ni, J.-T., Zhu, Z.-H., Xu, H.-Q., Tao, L., & Xie, T. (2022). Association of a Healthy Lifestyle with All-Cause, Cause-Specific Mortality and Incident Cancer among Individuals with Metabolic Syndrome: A Prospective Cohort Study in UK Biobank. International Journal of Environmental Research and Public Health, 19(16), 9936. https://doi.org/10.3390/ijerph19169936






