Demographic Control Measure Implications of Tuberculosis Infection for Migrant Workers across Taiwan Regions
Abstract
:1. Introduction
2. Background
3. Materials and Methods
3.1. Study Design
3.2. Setting, Sample, and Data Collection
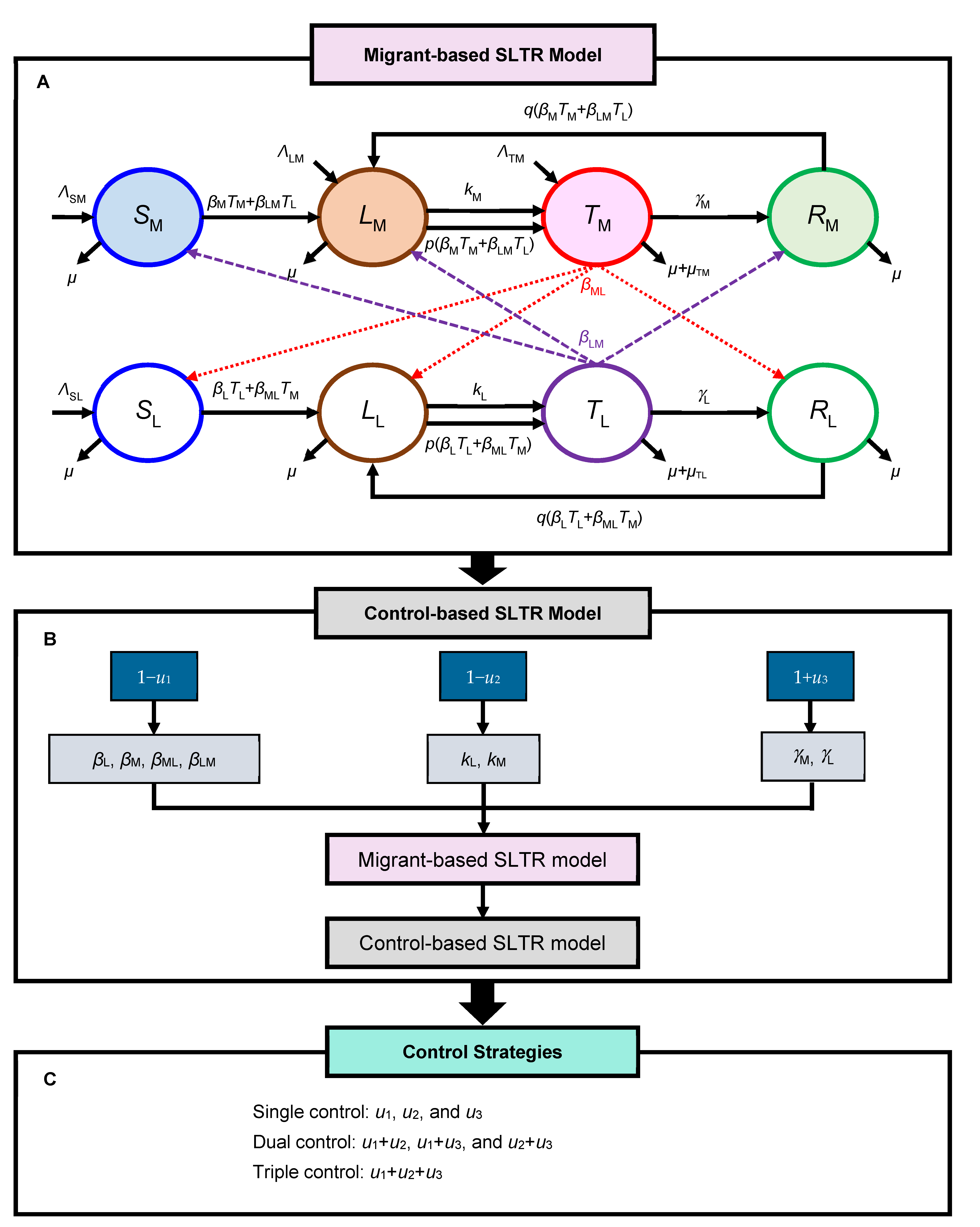
3.3. Migrant-BASED SLTR Model
3.4. Control-Based SLTR Model
3.5. Model Parameterization
3.6. Data and Sensitivity Analyses
4. Results
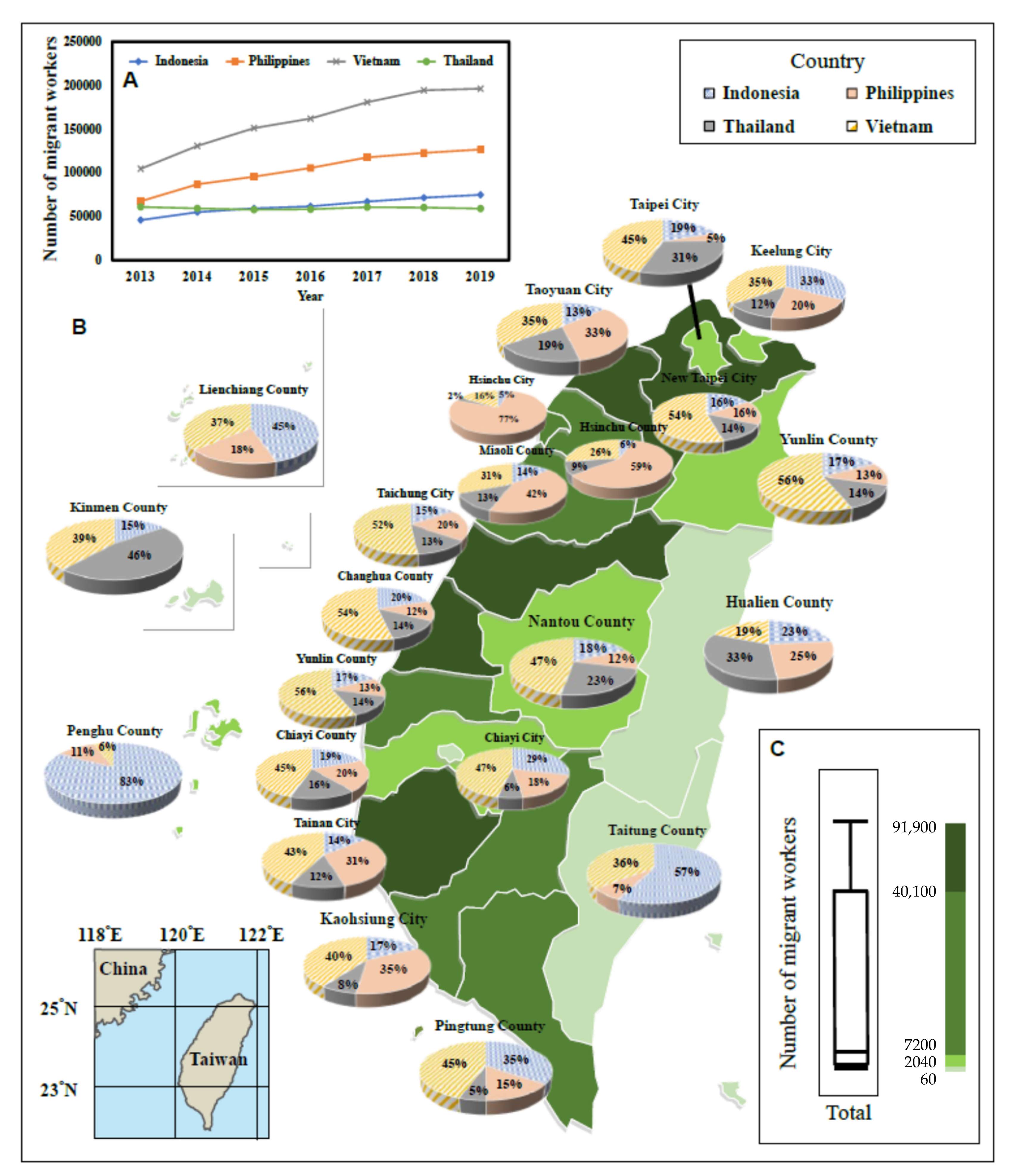
4.1. Data Interpretations
4.2. Sensitivity Performance
4.3. Single Control Effects
4.4. Dual and Triple Combination Control Strategies Effects
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 4 February 2022).
- Lu, C.-W.; Lee, Y.-H.; Pan, Y.-H.; Chang, H.-H.; Wu, Y.-C.; Sheng, W.-H.; Huang, K.-C. Tuberculosis among migrant workers in Taiwan. Global Health 2019, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, P.E.; Soini, H.; Turtiainen, P.; Vasankari, T.; Ruutu, P.; Nuorti, J.P.; Lyytikäinen, O. Enhanced surveillance for tuberculosis among foreign-born persons, Finland, 2014–2016. BMC Public Health 2018, 18, 610. [Google Scholar] [CrossRef] [PubMed]
- Lönnroth, K.; Mor, Z.; Erkens, C.; Bruchfeld, J.; Nathavitharana, R.R.; Van Der Werf, M.J.; Lange, C. Tuberculosis in migrants in low-incidence countries: Epidemiology and intervention entry points. Int. J. Tuberc. Lung Dis. 2017, 21, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.W.; Yates, T.A.; Zenner, D.; White, P.J.; Abubakar, I.; Hayward, A.C. Pre-entry screening programmes for tuberculosis in migrants to low-incidence countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1240–1249. [Google Scholar] [CrossRef]
- Kawatsu, L.; Uchimura, K.; Izumi, K.; Ohkado, A.; Ishikawa, N. Profile of tuberculosis among the foreign-born population in Japan, 2007–2014. West. Pac. Surveill. Response J. 2016, 7, 7–16. [Google Scholar] [CrossRef]
- Greenaway, C.; Sandoe, A.; Vissandjee, B.; Kitai, I.; Gruner, D.; Wobeser, W.; Pottie, K.; Ueffing, E.; Menzies, D.; Schwartzman, K.; et al. Tuberculosis: Evidence review for newly arriving immigrants and refugees. Can. Med Assoc. J. 2011, 183, E939–E951. [Google Scholar] [CrossRef]
- Win, K.M.K.; Chee, C.B.E.; Shen, L.; Wang, Y.T.; Cutter, J. Tuberculosis among foreign-born persons, Singapore, 2000–2009. Emerg. Infect. Dis. 2011, 17, 517–519. [Google Scholar] [CrossRef]
- Bai, K.-J.; Chiang, C.-Y.; Lee, C.-N.; Chang, J.-H.; Wu, L.-C.; Yu, M.-C. Tuberculosis among foreign-born persons in Taiwan, 2002–2005. J. Formos. Med. Assoc. 2008, 107, 389–395. [Google Scholar] [CrossRef]
- Taiwan MOL (Ministry of Labor). Number of Migrant Workers; Taiwan MOL: Taipei, Taiwan, 2021. Available online: https://statdb.mol.gov.tw/evta/JspProxy.aspx?sys=210&kind=21&type=1&funid=wq14131&rdm=rarrbd9j (accessed on 5 January 2022).
- Taiwan CDC (Centers for Disease Control). Taiwan Tuberculosis Control Report 2018; Taiwan CDC: Taipei, Taiwan, 2018. Available online: https://www.cdc.gov.tw/InfectionReport/Info/uKmf00HvSmkNaX9lNY-raQ?infoId=OGFfElHCFaaPSKME03dAzA (accessed on 10 November 2021).
- Hsieh, C.-J.; Lin, L.-C.; Kuo, B.I.-T.; Chiang, C.; Su, W.-J.; Shih, J.-F. Exploring the efficacy of a case management model using DOTS in the adherence of patients with pulmonary tuberculosis. J. Clin. Nurs. 2008, 17, 869–875. [Google Scholar] [CrossRef]
- Chien, J.-Y.; Lai, C.-C.; Tan, C.-K.; Chien, S.-T.; Yu, C.-J.; Hsueh, P.-R. Decline in rates of acquired multidrug-resistant tuberculosis after implementation of the directly observed therapy, short course (DOTS) and DOTS-Plus programmes in Taiwan. J. Antimicrob. Chemother. 2013, 68, 1910–1916. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Infectious Diseases of Human: Dynamics and Control; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Waaler, H.; Geser, A.; Andersen, S. The use of mathematical models in the study of the epidemiology of tuberculosis. Am. J. Public Health Nations Health 1962, 52, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Revelle, C.S.; Lynn, W.R.; Feldmann, F. Mathematical models for the economic allocation of tuberculosis control activities in developing nations. Am. Rev. Respir. Dis. 1967, 96, 893–909. [Google Scholar] [PubMed]
- Blower, S.M.; Mclean, A.R.; Porco, T.C.; Small, P.M.; Hopewell, P.C.; Sanchez, M.A.; Moss, A.R. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1995, 1, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Whang, S.; Choi, S.; Jung, E. A dynamic model for tuberculosis transmission and optimal treatment strategies in South Korea. J. Theor. Biol. 2011, 279, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; de los Reyes, V.A.A.; Jung, E. Mathematical model and intervention strategies for mitigating tuberculosis in the Philippines. J. Theor. Biol. 2018, 443, 100–112. [Google Scholar] [CrossRef]
- Kim, S.; de Los Reyes, V.A.A.; Jung, E. Country-specific intervention strategies for top three TB burden countries using mathematical model. PLoS ONE 2020, 15, e0230964. [Google Scholar] [CrossRef] [PubMed]
- Ozcaglar, C.; Shabbeer, A.; Vandenberg, S.L.; Yener, B.; Bennett, K.P. Epidemiological models of Mycobacterium tuberculosis complex infections. Math. Biosci. 2012, 236, 77–96. [Google Scholar] [CrossRef]
- Jia, Z.W.; Tang, G.Y.; Jin, Z.; Dye, C.; Vlas, S.J.; Li, X.-W.; Feng, D.; Fang, L.-Q.; Zhao, W.-J.; Cao, W.-C. Modeling the impact of immigration on the epidemiology of tuberculosis. Theor. Popul. Biol. 2008, 73, 437–448. [Google Scholar] [CrossRef]
- Bosch, J.H.W.; Nagelkerke, N.J.D.; Broekmans, J.F.; Borgdorff, M.W. The impact of immigration on the elimination of tuberculosis in The Netherlands: A model-based approach. Int. J. Tuberc. Lung Dis. 2002, 6, 130–136. [Google Scholar]
- Shrestha, S.; Hill, A.N.; Marks, S.M.; Dowdy, D.W. Comparing drivers and dynamics of tuberculosis in California, Florida, New York, and Texas. Am. J. Respir. Crit. Care Med. 2017, 196, 1050–1059. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X. Influence of temporary migration on the transmission of infectious diseases in a migrants’ home village. J. Theor. Biol. 2012, 300, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, K.; Feng, Z.; Wu, J. Projection of tuberculosis incidence with increasing immigration trends. J. Theor. Biol. 2008, 254, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Labor Republic. Foreign Workers in Productive industries and Social Welfare by Area and Nationality. Available online: https://www.mol.gov.tw/statistics/ (accessed on 30 September 2019).
- Taiwan CDC (Centers for Disease Control). Surveillance Statistics Data of Tuberculosis in Taiwan; Taiwan CDC: Taipei, Taiwan, 2019. Available online: https://daily.cdc.gov.tw/stoptb/CareMagChart.aspx (accessed on 30 September 2019).
- Taiwan CDC (Centers for Disease Control). Taiwan Tuberculosis Control Report 2019; Taiwan CDC: Taipei, Taiwan, 2020. Available online: https://www.cdc.gov.tw/InfectionReport/Info/uKmf00HvSmkNaX9lNY-raQ?infoId=Wdm7FNziGCkyzFPuek6oZQ (accessed on 30 September 2019).
- Liu, L.; Wu, J.; Zhao, X.Q. The impact of migrant workers on the tuberculosis transmission: General models and a case study for China. Math. Biosci. Eng. 2012, 9, 785–807. [Google Scholar]
- Dale, K.D.; Trauer, J.M.; Dodd, P.J.; Houben, R.M.G.J.; Denholm, J.T. Estimating long-term tuberculosis reactivation rates in Australian migrants. Clin. Infect. Dis. 2020, 70, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.P.; Luh, D.L.; Chang, S.H.; Suo, J.; Chang, H.; Chen, H.-H. Tuberculin reactivity in adults after 50 years of universal bacille Calmette–Guérin vaccination in Taiwan. Trans. R Soc. Trop. Med. Hyg. 2005, 99, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Dye, C.; Garnett, G.P.; Sleeman, K.; Williams, B.G. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet 1998, 352, 1886–1891. [Google Scholar] [CrossRef]
- Houben, R.M.G.J.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Taiwan MOTI (Ministry of the Interior). Demographic Data; Taiwan MOTI: Taipei, Taiwan, 2018. Available online: https://www.ris.gov.tw/app/portal/346 (accessed on 30 September 2019).
- Chen, T.H.; Wen, T.H.; Fang, C.T.; Chan, P.-C. Assessing the infection risk of Tuberculosis (TB) contacts in different case-contact contexts. Taiwan J. Public Health 2017, 36, 107–121. [Google Scholar]
- Laws & Regulations Database of The Republic of China. Regulations Governing Management of the Health Examination of Employed Aliens. 2020. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0050018 (accessed on 5 November 2021).
- Kuan, M.M. Nationwide surveillance algorithms for tuberculosis among migrant workers from highly endemic countries following pre-entry screening in Taiwan. BMC Public Health 2018, 18, 1151. [Google Scholar] [CrossRef]
- Schwartz, N.G.; Price, S.F.; Pratt, R.H.; Langer, A.J. Tuberculosis—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 286–289. [Google Scholar] [CrossRef]
- Walter, N.D.; Painter, J.; Parker, M.; Lowenthal, P.; Flood, J.; Fu, Y.; Asis, R.; Randall Reves for the Tuberculosis Epidemiologic Studies Consortium. Persistent latent tuberculosis reactivation risk in United States migrants. Am. J. Respir. Crit. Care Med. 2014, 189, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lönnroth, K.; Migliori, G.B.; Abubakar, I.; D’Ambrosio, L.; de Vries, G.; Diel, R.; Douglas, P.; Falzon, D.; Gaudreau, M.-A.; Goletti, D.; et al. Towards tuberculosis elimination: An action framework for low-incidence countries. Eur. Respir. J. 2015, 45, 928–952. [Google Scholar] [CrossRef] [PubMed]
- LoBue, P.A.; Mermin, J.H. Latent tuberculosis infection: The final frontier of tuberculosis elimination in the USA. Lancet Infect. Dis. 2017, 17, e327–e333. [Google Scholar] [CrossRef]
- Taiwan MOHW (Ministry of Health and Welfare). 2020. Available online: https://www.cdc.gov.tw/File/Get/jforpWW6ZU2soOVvPv1nfw (accessed on 5 November 2021).
- Horsburgh, C.R., Jr.; O’Donnell, M.; Chamblee, S.; Moreland, J.L.; Johnson, J.; Marsh, B.J.; Narita, M.; Johnson, L.S.; von Reyn, C.F. Revisiting rates of reactivation tuberculosis: A population-based approach. Am. J. Respir. Crit. Care Med. 2010, 182, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.M.; Kammerer, J.S.; Winston, C.A.; Navin, T.R.; Horsburgh, C.R. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am. J. Epidemiol. 2014, 179, 216–225. [Google Scholar] [CrossRef]


| Symbol | Equation | |
|---|---|---|
| Migrant population (NM) | ||
| SM | (1) | |
| LM | (2) | |
| TM | (3) | |
| RM | (4) | |
| Local population (NL) | ||
| SL | (5) | |
| LL | (6) | |
| TL | (7) | |
| RL | (8) | |
| Migrant Population (NM) | |
| (9) | |
| (10) | |
| (11) | |
| (12) | |
| NM = SM + LM + TM + RM | (13) |
| Local population (NL) | |
| (14) | |
| (15) | |
| (16) | |
| (17) | |
| NL = SL + LL + TL + RL | (18) |
| Symbol | Description | Estimate | ||
|---|---|---|---|---|
| Taoyuan City | Taichung City | New Taipei City | ||
| Local subpopulation | ||||
| SL(0) d | Susceptible individuals in the local subpopulation | 2,211,473 | 2,791,803 | 3,978,357 |
| LL(0) c | Latently infected individuals in the local subpopulation | 8173 | 10,318 | 14,704 |
| TL(0) a | Infectious TB cases in the local subpopulation | 662 | 1007 | 1509 |
| RL(0) b | Recovered cases in the local subpopulation | 484 | 766 | 1077 |
| Migrant subpopulation | ||||
| SM(0) g | Susceptible individuals in the migrant subpopulation | 63,461 | 54,156 | 38,699 |
| LM(0) f | Latently infected individuals in the migrant subpopulation | 28,305 | 24,154 | 17,261 |
| TM(0) e | Infectious TB cases in the migrant subpopulation | 133 | 113 | 81 |
| RM(0) | Recovered cases in the migrant subpopulation | 0 | 0 | 0 |
| Symbol | Description | Taoyuan City | Taichung City | New Taipei City |
|---|---|---|---|---|
| ΛSM | Recruitment rate into SM (person·year−1) | 24,394 | 20,818 | 14,876 |
| ΛLM | Recruitment rate into LM (person·year−1) | 10,864 | 9271 | 6625 |
| ΛTM | Recruitment rate into TM (person·year−1) | 15 | 12 | 9 |
| ΛSL | Crude birth rate into SL (year−1) | 0.0102 | 0.0081 | 0.0072 |
| βL a | Transmission rate for the local subpopulation (person−1·year−1) | 5 × 10−7 | 5 × 10−7 | 5 × 10−7 |
| βM a | Transmission rate for the migrant subpopulation (person−1·year−1) | 5.9172 × 10−7 | 5.9172 × 10−7 | 5.9172 × 10−7 |
| βML a | Transmission rate for migrants in the local subpopulation (person−1·year−1) | 5 × 10−9 | 5 × 10−9 | 5 × 10−9 |
| βLM a | Transmission rate for locals in the migrant subpopulation (person−1·year−1) | 10−8 | 10−8 | 10−8 |
| kM b | Reactivation rate in LM (year−1) | 0.004 | 0.004 | 0.004 |
| kL b | Reactivation rate in LL (year−1) | 0.004 | 0.004 | 0.004 |
| c | Recovery rate of TM (year−1) | 0.731 | 0.761 | 0.714 |
| c | Recovery rate of TL (year−1) | 0.731 | 0.761 | 0.714 |
| µ | Background mortality rate (year−1) | 0.0578 | 0.0611 | 0.06 |
| c | TB-induced mortality rate in TM (year−1) | 0.187 | 0.174 | 0.195 |
| c | TB-induced mortality rate in TL (year−1) | 0.187 | 0.174 | 0.195 |
| p d | Partial immunity that decreases the probability of fast progression after reinfection for TL | 0.8 | 0.8 | 0.8 |
| q d | Partial immunity that decreases the probability of fast progression after reinfection for RL | 0.8 | 0.8 | 0.8 |
| Total Number of L + T (Individuals) | |||||||
|---|---|---|---|---|---|---|---|
| Year | Without Control | u1 = 10% | u1 = 90% | u2 = 10% | u2 = 90% | u3 = 80% | u3 = 95% |
| Taoyuan City | |||||||
| 2018 | 37,295 | 37,295 | 37,295 | 37,295 | 37,295 | 37,295 | 37,295 |
| 2019 | 59,767 | 58,104 | 45,999 | 58,879 | 51,913 | 56,642 | 56,159 |
| 2020 | 93,665 | 88,229 | 54,258 | 90,079 | 63,927 | 81,213 | 79,516 |
| 2021 | 142,652 | 129,680 | 62,012 | 133,700 | 75,017 | 111,404 | 107,606 |
| 2022 | 214,558 | 186,689 | 69,239 | 195,390 | 85,766 | 148,114 | 140,984 |
| 2023 | 326,190 | 267,077 | 75,937 | 286,527 | 96,386 | 192,655 | 180,420 |
| Taichung City | |||||||
| 2018 | 35,515 | 35,515 | 35,515 | 35,515 | 35,515 | 35,515 | 35,515 |
| 2019 | 61,642 | 59,356 | 43,002 | 60,537 | 51,948 | 57,303 | 56,640 |
| 2020 | 101,770 | 94,333 | 50,153 | 97,070 | 63,911 | 84,759 | 82,490 |
| 2021 | 163,894 | 145,302 | 56,883 | 151,335 | 74,338 | 120,001 | 114,859 |
| 2022 | 264,941 | 221,243 | 63,156 | 235,232 | 84,208 | 165,267 | 155,276 |
| 2023 | 450,456 | 341,343 | 68,956 | 377,625 | 93,855 | 223,944 | 205,858 |
| New Taipei City | |||||||
| 2018 | 33,575 | 33,575 | 33,575 | 33,575 | 33,575 | 33,575 | 33,575 |
| 2019 | 73,941 | 69,572 | 39,767 | 72,206 | 58,982 | 66,268 | 65,089 |
| 2020 | 140,817 | 125,403 | 45,653 | 132,080 | 74,457 | 108,809 | 104,579 |
| 2021 | 270,939 | 223,490 | 51,238 | 241,378 | 86,626 | 173,205 | 162,375 |
| 2022 | 591,115 | 419,955 | 56,497 | 482,552 | 97,725 | 276,781 | 251,047 |
| 2023 | 2,043,320 | 966,314 | 61,401 | 1,378,324 | 108,557 | 464,309 | 399,111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Wang, T.-Y.; Tsai, H.-C.; Chen, C.-Y.; Lu, T.-H.; Lin, Y.-J.; You, S.-H.; Yang, Y.-F.; Liao, C.-M. Demographic Control Measure Implications of Tuberculosis Infection for Migrant Workers across Taiwan Regions. Int. J. Environ. Res. Public Health 2022, 19, 9899. https://doi.org/10.3390/ijerph19169899
Chen S-C, Wang T-Y, Tsai H-C, Chen C-Y, Lu T-H, Lin Y-J, You S-H, Yang Y-F, Liao C-M. Demographic Control Measure Implications of Tuberculosis Infection for Migrant Workers across Taiwan Regions. International Journal of Environmental Research and Public Health. 2022; 19(16):9899. https://doi.org/10.3390/ijerph19169899
Chicago/Turabian StyleChen, Szu-Chieh, Tzu-Yun Wang, Hsin-Chieh Tsai, Chi-Yun Chen, Tien-Hsuan Lu, Yi-Jun Lin, Shu-Han You, Ying-Fei Yang, and Chung-Min Liao. 2022. "Demographic Control Measure Implications of Tuberculosis Infection for Migrant Workers across Taiwan Regions" International Journal of Environmental Research and Public Health 19, no. 16: 9899. https://doi.org/10.3390/ijerph19169899
APA StyleChen, S.-C., Wang, T.-Y., Tsai, H.-C., Chen, C.-Y., Lu, T.-H., Lin, Y.-J., You, S.-H., Yang, Y.-F., & Liao, C.-M. (2022). Demographic Control Measure Implications of Tuberculosis Infection for Migrant Workers across Taiwan Regions. International Journal of Environmental Research and Public Health, 19(16), 9899. https://doi.org/10.3390/ijerph19169899







