Evaluation of a Community-Led Program for Primordial and Primary Prevention of Rheumatic Fever in Remote Northern Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Inclusion Criteria
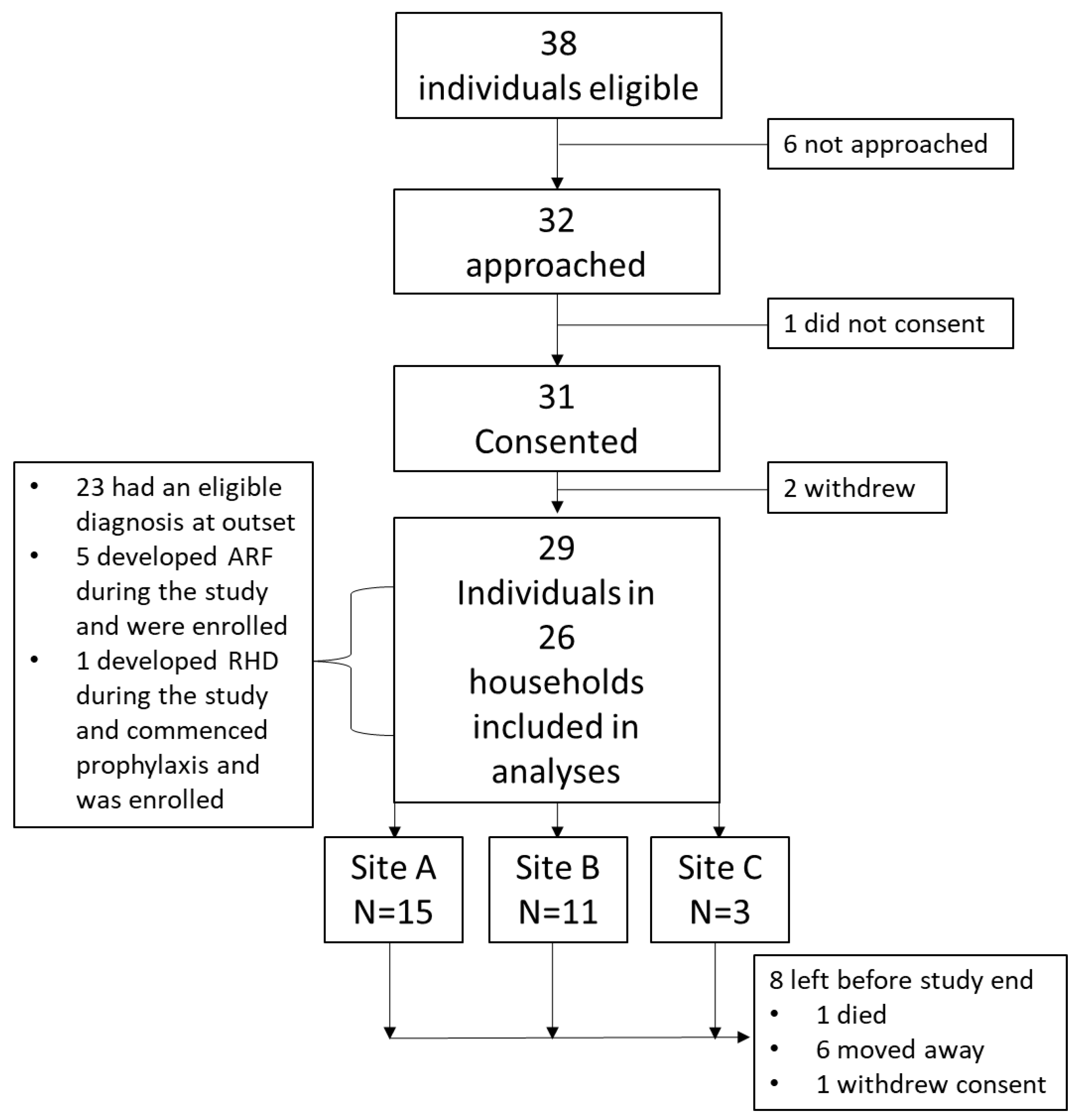
2.3. Consent
2.4. Outcome Measures
2.5. Data Collection
2.6. Data Reporting and Analyses
3. Results
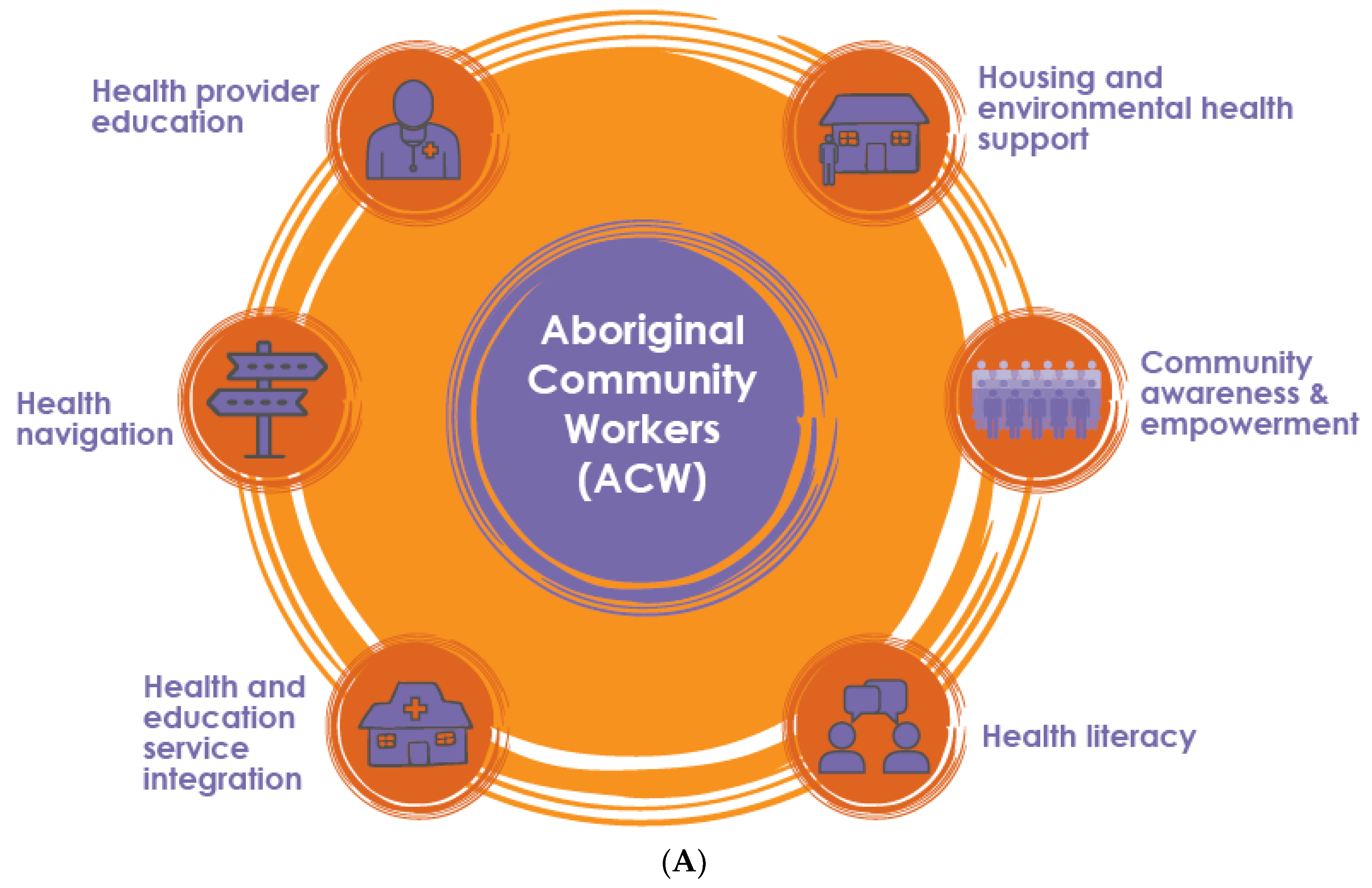
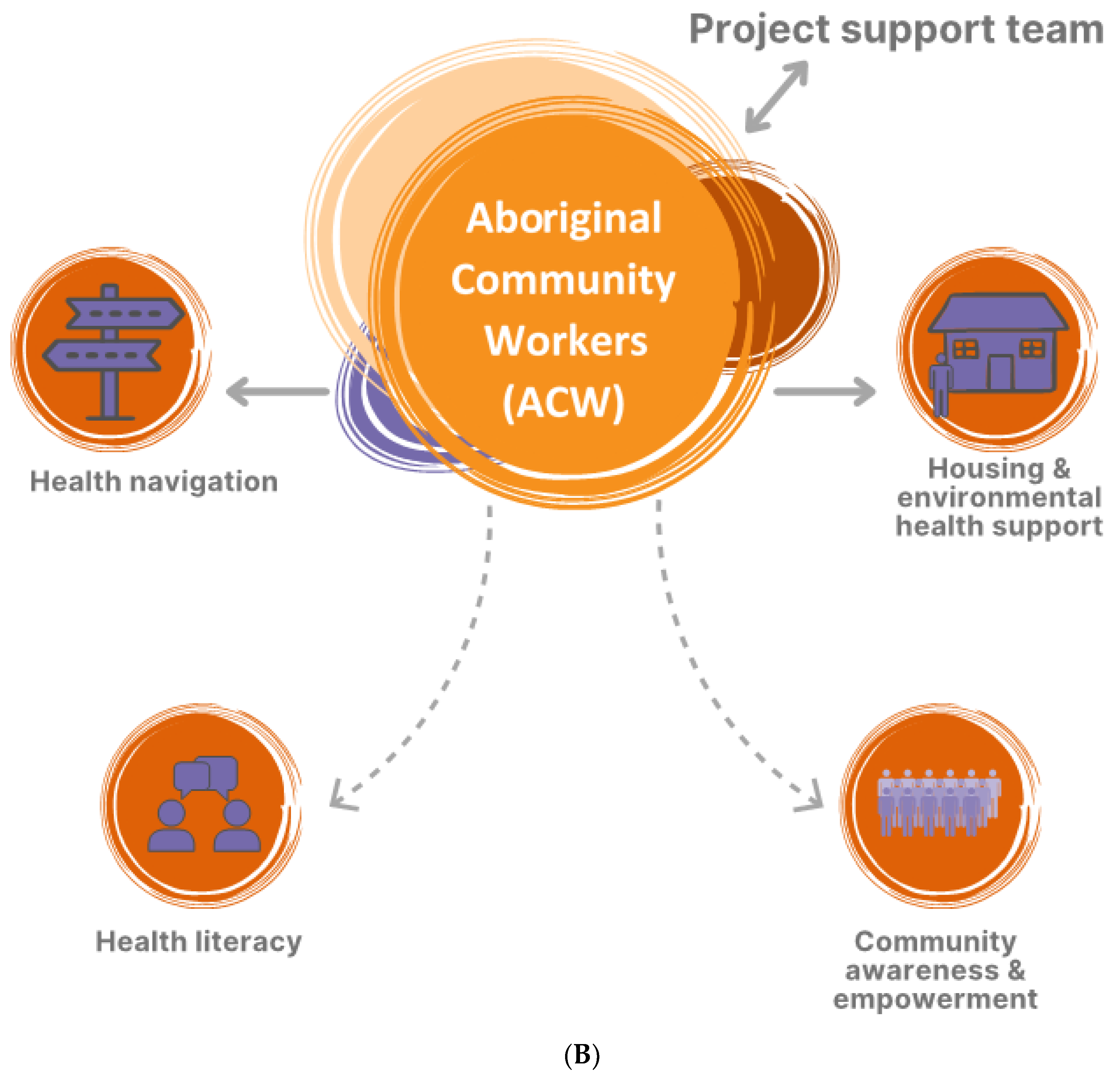
3.1. Implementation of Activities to Address ARF
3.2. Clinic-Documented Infections
3.3. Self-Reported Infections
3.4. ARF Diagnoses
3.5. RHD Severity
3.6. Household Occupancy and Health Hardware
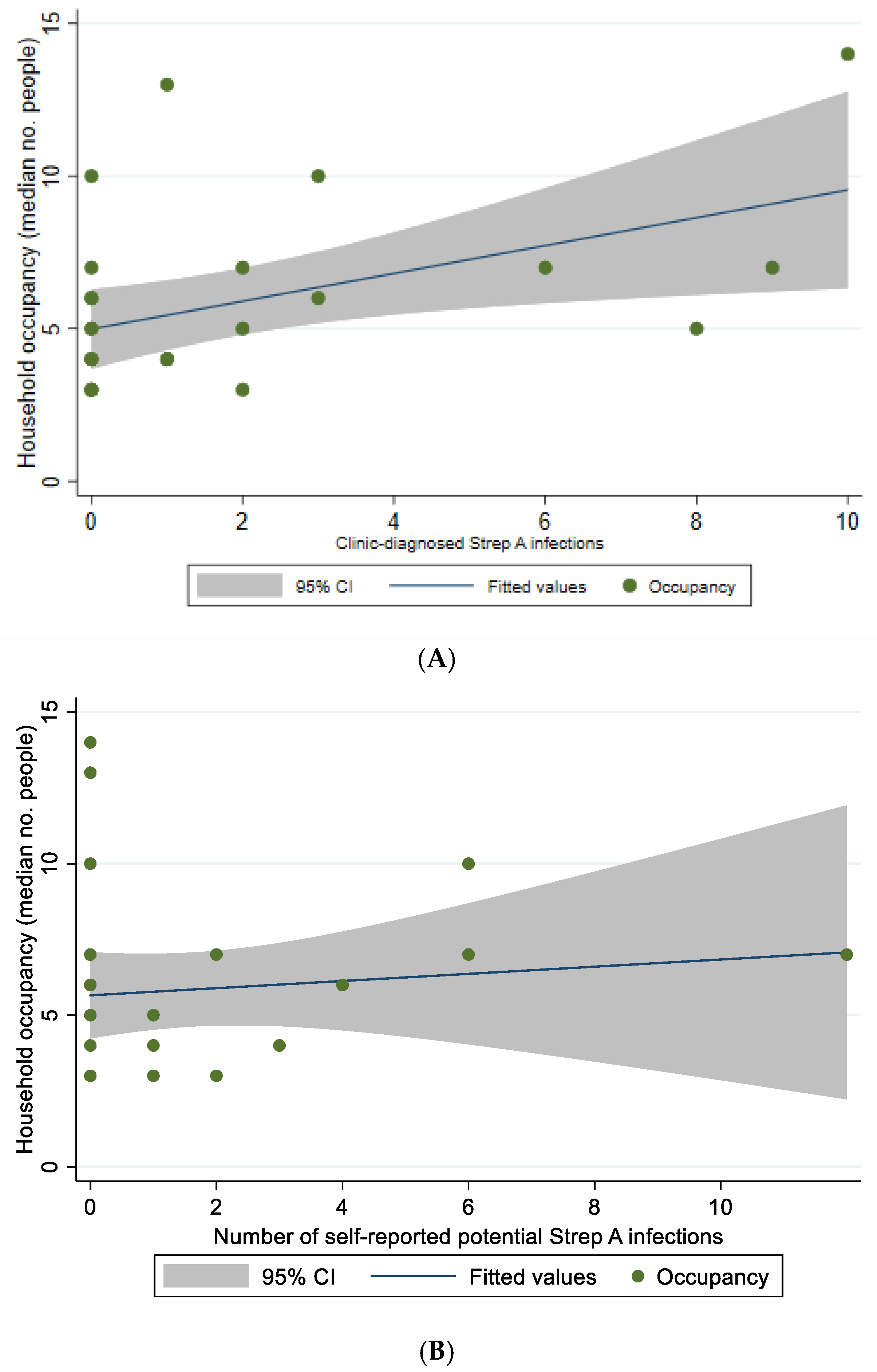
3.7. Association of Potential Streptococcal Infections with Household Occupancy and Health Hardware
3.8. Delivery of Penicillin Secondary Prophylaxis
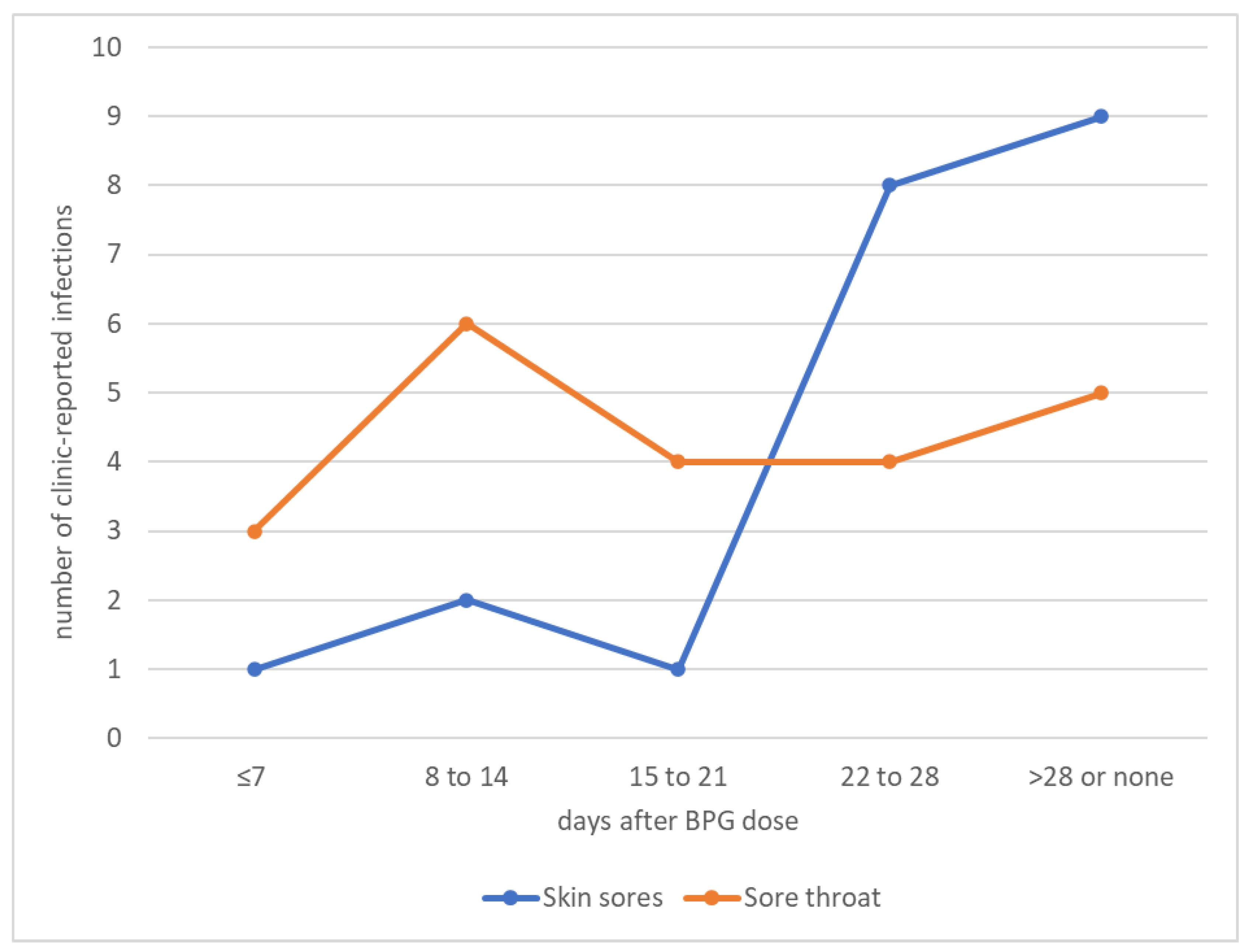
3.9. Association between Penicillin Adherence and Strep A Infections
3.10. Association between Strep A Infections and ARF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Australian Government Department of Health. Australia’s Long Term National Health Plan. 2019. Available online: https://www.health.gov.au/sites/default/files/australia-s-long-term-national-health-plan_0.pdf (accessed on 5 June 2022).
- Katzenellenbogen, J.M.; Bond-Smith, D.; Seth, R.J.; Dempsey, K.; Cannon, J.; Stacey, I.; Wade, V.; de Klerk, N.; Greenland, M.; Sanfilippo, F.M.; et al. Contemporary Incidence and Prevalence of Rheumatic Fever and Rheumatic Heart Disease in Australia Using Linked Data: The Case for Policy Change. J. Am. Heart Assoc. 2020, 9, e016851. [Google Scholar] [CrossRef] [PubMed]
- Katzenellenbogen, J.M.; Ralph, A.P.; Wyber, R.; Carapetis, J.R. Rheumatic heart disease: Infectious disease origin, chronic care approach. BMC Health Serv. Res. 2017, 17, 793. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.A.; Volmink, J.A.; Mayosi, B.M. Antibiotics for the primary prevention of acute rheumatic fever: A meta-analysis. BMC Cardiovasc. Disord. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- He, V.Y.; Condon, J.R.; Ralph, A.P.; Zhao, Y.; Roberts, K.; de Dassel, J.L.; Currie, B.J.; Fittock, M.; Edwards, K.N.; Carapetis, J.R. Long-Term Outcomes from Acute Rheumatic Fever and Rheumatic Heart Disease: A Data-Linkage and Survival Analysis Approach. Circulation 2016, 134, 222–232. [Google Scholar] [CrossRef]
- Health Policy Analysis. Evaluation of the Commonwealth Rheumatic Fever Strategy—Final Report. Canberra: Primary Healthcare Branch, Australian Commonwealth Department of Health. 2017. Available online: https://www.health.gov.au/sites/default/files/documents/2021/02/evaluation-of-the-rheumatic-fever-strategy.docx (accessed on 5 June 2022).
- Wyber, R.; Noonan, K.; Halkon, C.; Enkel, S.; Carapetis, J. The RHD Endgame Strategy: The blueprint to eliminate rheumatic heart disease in Australia by 2031. Eur. J. Public Health 2020, 30, ckaa165-059. [Google Scholar] [CrossRef]
- Stollerman, G.H. Rheumatic fever in the 21st century. Clin. Infect. Dis. 2001, 33, 806–814. [Google Scholar] [CrossRef]
- Ralph, A.P.; de Dassel, J.L.; Kirby, A.; Enkel, S.; Carapetis, J. Improving Delivery of Secondary Prophylaxis for Rheumatic Heart Disease in a High-Burden Setting: Outcome of a Stepped-Wedge, Community, Randomized Trial. J. Am. Heart Assoc. 2018, 7, e009308. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Acute Rheumatic Fever and Rheumatic Heart Disease in Australia, 2015–2019. 2021. Available online: https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/acute-rheumatic-fever-and-rheumatic-heart-disease/contents/summary (accessed on 5 June 2022).
- Coffey, P.M.; Ralph, A.P.; Krause, V.L. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006577. [Google Scholar] [CrossRef]
- Oliver, J.R.; Pierse, N.; Stefanogiannis, N.; Jackson, C.; Baker, M.G. Acute rheumatic fever and exposure to poor housing conditions in New Zealand: A descriptive study. J. Paediatr. Child Health 2017, 53, 358–364. [Google Scholar] [CrossRef]
- Bowen, A.C.; Tong, S.Y.; Andrews, R.M.; O’Meara, I.M.; McDonald, M.I.; Chatfield, M.D.; Currie, B.J.; Carapetis, J.R. Short-course oral co-trimoxazole versus intramuscular benzathine benzylpenicillin for impetigo in a highly endemic region: An open-label, randomised, controlled, non-inferiority trial. Lancet 2014, 384, 2132–2140. [Google Scholar] [CrossRef]
- Bowen, A.C.; Mahe, A.; Hay, R.J.; Andrews, R.M.; Steer, A.C.; Tong, S.Y.; Carapetis, J.R. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS ONE 2015, 10, e0136789. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Bennett, J.; Thomas, S.; Zhang, J.; Pierse, N.; Moreland, N.J.; Williamson, D.A.; Jack, S.; Baker, M. Preceding group A streptococcus skin and throat infections are individually associated with acute rheumatic fever: Evidence from New Zealand. BMJ Glob. Health 2021, 6, e007038. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Smeesters, P.R.; Steer, A.C. Streptococcal skin infection and rheumatic heart disease. Curr. Opin. Infect. Dis. 2012, 25, 145–153. [Google Scholar] [CrossRef] [PubMed]
- RHD Australia (ARF/RHD Writing Group). The 2020 Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease, 3rd ed.; Menzies School of Health Research: Darwin, Australia, 2020; Available online: https://www.rhdaustralia.org.au/arf-rhd-guideline (accessed on 5 June 2022).
- Denny, F.W.; Wannamaker, L.W.; Brink, W.R.; Rammelkamp, C.H., Jr.; Custer, E.A. Prevention of rheumatic fever; treatment of the preceding streptococcic infection. J. Am. Med. Assoc. 1950, 143, 151–153. [Google Scholar] [CrossRef]
- Haynes, E.; Marawili, M.; Marika, B.M.; Mitchell, A.G.; Phillips, J.; Bessarab, D.; Walker, R.; Cook, J.; Ralph, A.P. Community-based participatory action research on rheumatic heart disease in an Australian Aboriginal homeland: Evaluation of the ‘On track watch’ project. Eval. Progr. Plan. 2019, 74, 38–53. [Google Scholar] [CrossRef]
- Read, C.; Mitchell, A.G.; de Dassel, J.L.; Scrine, C.; Hendrickx, D.; Bailie, R.S.; Johnston, V.; Maguire, G.P.; Schultz, R.; Carapetis, J.R.; et al. Qualitative Evaluation of a Complex Intervention to Improve Rheumatic Heart Disease Secondary Prophylaxis. J. Am. Heart Assoc. 2018, 7, e009376. [Google Scholar] [CrossRef]
- Wyber, R.; Kelly, A.; Lee, A.M.; Mungatopi, V.; Kerrigan, V.; Babui, S.; Black, N.; Wade, V.; Fitzgerald, C.; Peiris, D.; et al. Formative evaluation of a community-Anderson Anderson based approach to reduce the incidence of Strep A infections and acute rheumatic fever. Aust. N. Z. J. Public Health 2021, 45, 449–454. [Google Scholar] [CrossRef]
- Kerrigan, V.; Kelly, A.; Lee, A.M.; Mungatopi, V.; Mitchell, A.G.; Wyber, R.; Ralph, A.P. A community-based program to reduce acute rheumatic fever and rheumatic heart disease in northern Australia. BMC Health Serv. Res. 2021, 21, 1127. [Google Scholar] [CrossRef]
- Australian Government Department of Health. Australian Statistical Geography Standard—Remoteness Area. 2021. Available online: https://www.health.gov.au/health-topics/health-workforce/health-workforce-classifications/australian-statistical-geography-standard-remoteness-area#how-do-i-find-locations-classified-under-the-asgsra (accessed on 18 November 2021).
- Davidson, L.; Knight, J.; Bowen, A.C. Skin infections in Australian Aboriginal children: A narrative review. Med. J. Aust. 2020, 212, 231–237. [Google Scholar] [CrossRef]
- Central Australian Rural Practitioners’ Association (CARPA) Editorial Committee. CARPA Standard Treatment Manual, 6th ed.; Flinders University and Charles Darwin University: Brinkin, Australia, 2014. [Google Scholar]
- Oliver, J.; Malliya Wadu, E.; Pierse, N.; Moreland, N.J.; Williamson, D.A.; Baker, M.G. Group A Streptococcus pharyngitis and pharyngeal carriage: A meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006335. [Google Scholar] [CrossRef]
- Bisno, A.L.; Gerber, M.A.; Gwaltney, J.M., Jr.; Kaplan, E.L.; Schwartz, R.H. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin. Infect. Dis. 2002, 35, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Vino, T.; Singh, G.R.; Davison, B.; Campbell, P.T.; Lydeamore, M.J.; Robinson, A.; McVernon, J.; Tong, S.Y.; Geard, N. Indigenous Australian household structure: A simple data collection tool and implications for close contact transmission of communicable diseases. PeerJ 2017, 5, e3958. [Google Scholar] [CrossRef] [PubMed]
- De Dassel, J.L.; de Klerk, N.; Carapetis, J.R.; Ralph, A.P. How Many Doses Make a Difference? An Analysis of Secondary Prevention of Rheumatic Fever and Rheumatic Heart Disease. J. Am. Heart Assoc. 2018, 7, e010223. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
- Health Navigator New Zealand. Maori Health—Support. 2021. Available online: https://www.healthnavigator.org.nz/videos/m/m%C4%81ori-health-videos/m%C4%81ori-health-support/ (accessed on 5 June 2022).
- Anderson, A.; Mills, C.; Eggleton, K. Whanau perceptions and experiences of acute rheumatic fever diagnosis for Maori in Northland, New Zealand. N. Z. Med. J. 2017, 130, 80–88. [Google Scholar]
- Mitchell, A.G.; Belton, S.; Johnston, V.; Wopurruwuy, G.; Ralph, A.P. “That heart sickness”: Young Aboriginal people’s understanding of rheumatic fever. Med. Anthropol. 2019, 38, 1–14. [Google Scholar] [CrossRef]
- Wakerman, J.; Humphreys, J.; Bourke, L.; Dunbar, T.; Jones, M.; Carey, T.A.; Guthridge, S.; Russell, D.; Lyle, D.; Zhao, Y.; et al. Assessing the Impact and Cost of Short-Term Health Workforce in Remote Indigenous Communities in Australia: A Mixed Methods Study Protocol. JMIR Res. Protoc. 2016, 5, e135. [Google Scholar] [CrossRef]
- Kerrigan, V.; Lee, A.M.; Ralph, A.P.; Lawton, P.D. Stay Strong: Aboriginal leaders deliver COVID-19 health messages. Health Promot. J. Aust. 2021, 32 (Suppl. 1), 203–204. [Google Scholar] [CrossRef]
- RHD Australia. Champions For Change Program. 2019. Available online: https://www.rhdaustralia.org.au/champions4change-program (accessed on 5 June 2022).
- Cannon, J.W.; Jack, S.; Wu, Y.; Zhang, J.; Baker, M.G.; Geelhoed, E.; Fraser, J.; Carapetis, J.R. An economic case for a vaccine to prevent group A streptococcus skin infections. Vaccine 2018, 36, 6968–6978. [Google Scholar] [CrossRef]
- Yeoh, D.K.; Anderson, A.; Cleland, G.; Bowen, A.C. Are scabies and impetigo “normalised”? A cross-sectional comparative study of hospitalised children in northern Australia assessing clinical recognition and treatment of skin infections. PLoS Negl. Trop. Dis. 2017, 11, e0005726. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.P.; Holt, D.C.; Islam, S.; Osowicki, J.; Carroll, D.E.; Tong, S.; Bowen, A. Potential for Molecular Testing for Group A Streptococcus to Improve Diagnosis and Management in a High-Risk Population: A Prospective Study. Open Forum Infect. Dis. 2019, 6, ofz097. [Google Scholar] [CrossRef] [PubMed]
- RHD Australia. iPhone and Android Apps. 2020. Available online: http://www.rhdaustralia.org.au/apps (accessed on 5 June 2022).
- Ralph, A.P.; Webb, R.; Moreland, N.J.; McGregor, R.; Bosco, A.; Broadhurst, D.; Lassmann, T.; Barnett, T.C.; Benothman, R.; Yan, J.; et al. Searching for a technology-driven acute rheumatic fever test: The START study protocol. BMJ Open 2021, 11, e053720. [Google Scholar] [CrossRef] [PubMed]
- Hand, R.M.; Salman, S.; Newall, N.; Vine, J.; Page-Sharp, M.; Bowen, A.; Gray, K.; Baker, A.; Kado, J.; Joseph, J.; et al. A population pharmacokinetic study of benzathine benzylpenicillin G administration in children and adolescents with rheumatic heart disease: New insights for improved secondary prophylaxis strategies. J. Antimicrob. Chemother. 2019, 74, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Kado, J.H.; Salman, S.; Henderson, R.; Hand, R.; Wyber, R.; Page-Sharp, M.; Batty, K.; Carapetis, J.; Manning, L. Subcutaneous administration of benzathine benzylpenicillin G has favourable pharmacokinetic characteristics for the prevention of rheumatic heart disease compared with intramuscular injection: A randomized, crossover, population pharmacokinetic study in healthy adult volunteers. J. Antimicrob. Chemother. 2020, 75, 2951–2959. [Google Scholar] [PubMed]

| N = 29 Participants (26 Households) * | |
|---|---|
| Sex | |
| Female | 16 (55%) |
| Male | 13 (45%) |
| Secondary prophylaxis prior to activity phase commencement | |
| Diagnosed before study commencement (1 February 2018): N (%) | 22 (76%) |
| Duration of secondary prophylaxis in those with prior diagnosis (median, range) | 5.2 years (0.2 to 24.2 years) |
| Diagnosed during the study (1 February 2018 onwards): N (%) | 7 (24%) |
| Median age at enrolment (range) | 14 years (7–76) |
| Site A (15 participants) | 13 years (7–50) |
| Site B (11 participants) | 15 years (7–39) |
| Site C (3 participants) | 32 years (14–76) |
| Disease severity at enrolment: N (%) | |
| Severe RHD | 4 (14%) |
| Moderate RHD | 1 (3%) |
| History of ARF or RHD requiring secondary prophylaxis | 24 (83%) |
| Inactive disease not requiring secondary prophylaxis * | 0 |
| Median number in household (range): N (%) | 5 (1–16) |
| Site A | 5 (3–16) |
| Site B | 5 (2–15) |
| Site C | 4 (3–12) |
| Primary participant sharing a mattress with ≥1 other: Number of surveys (%) | 338/1302 (26%) |
| Site A | 17/660 (3%) |
| Site B | 283/456 (62%) |
| Site C | 37/186 (20%) |
| Primary participant sharing a mattress with ≥2 others: N surveys (%) | 78/1302 (6%) |
| Site A | 0/660 (0%) |
| Site B | 78/456 (17%) |
| Site C | 0/186 (0%) |
| Soap unavailable, all sites: N surveys (%) | 3/1305 (0.3%) |
| Shower not working, all sites: N surveys (%) | 15/1304 (1%) |
| No hot water in shower, all sites: N surveys (%) | 19/1303 (1%) |
| Toilet not working, all sites: N surveys (%) | 8/1303 (0.5%) |
| No washing machine, all sites: N surveys (%) | 19/894 (2%) |
| Primary Participant | Contact Participant | Total | ||
|---|---|---|---|---|
| N | N = 29 | N = 26 | ||
| Potentially related to Strep A | ARF | 14 | 0 | 14 |
| ARF possible | 4 | 0 | 4 | |
| ARF probable | 2 | 0 | 2 | |
| ARF definite | 8 | 0 | 8 | |
| RHD * | 1 | 0 | 1 | |
| Acute post-streptococcal glomerulonephritis | 2 | 0 | 2 | |
| Skin sore | 21 | 13 | 34 | |
| Sore throat | 22 | 18 | 40 | |
| Joint pain possibly indicative of ARF | 4 | 3 | 7 | |
| Potentially related to environmental health conditions | Scabies | 8 | 7 | 15 |
| Skin boil | 14 | 18 | 32 | |
| Skin/soft tissue infection ** | 4 | 0 | 4 | |
| Ear infection | 17 | 11 | 28 | |
| Fever | 11 | 6 | 17 | |
| Fungal skin infection | 18 | 3 | 21 | |
| Lower respiratory tract infection | 13 | 19 | 32 | |
| Upper respiratory tract infection | 6 | 4 | 10 | |
| TOTAL | 169 | 102 | 271 |
| Baseline | Activity Yr1 | Activity Yr2 | Activity Yr3 | ||
|---|---|---|---|---|---|
| All diagnoses | |||||
| Counts | All ages | 49 | 59 | 36 | 16 |
| <15 years | 28 | 50 | 23 | 13 | |
| ≥15 years | 21 | 9 | 13 | 3 | |
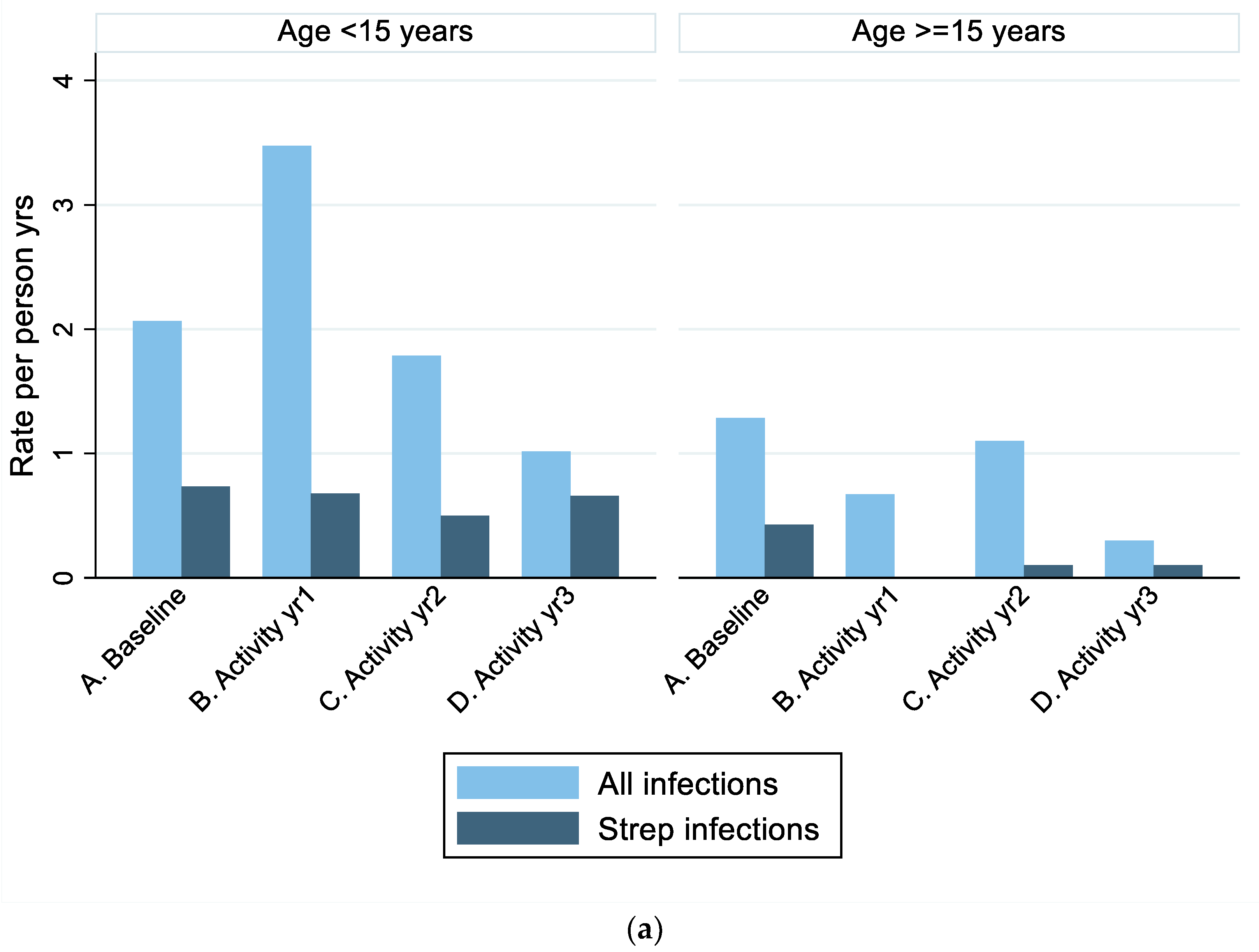
| Rates | All ages | 1.69 (1.10–2.28) | 2.12 (1.17–3.07) | 1.50 (0.75–2.25) | 0.72 (0.29–1.15) |
| <15 years | 2.07 (1.05–3.08) | 3.48 (1.96–4.99) | 1.78 (0.54–3.02) | 1.01 (0.33–1.70) | |
| ≥15 years | 1.28 (0.62–1.94) | 0.67 (0.11–1.23) | 1.10 (0.39–1.81) | 0.30 (−0.05–0.64) | |
| Skin sore | |||||
| Counts | All ages | 10 | 5 | 2 | 6 |
| <15 years | 10 | 5 | 2 | 5 | |
| ≥15 years | 0 | 0 | 0 | 1 | |
| Rates | All ages | 0.34 (0.70–0.62) | 0.17 (0.26–0.32) | 0.08 (−0.04–0.20) | 0.30 (0.06–0.54) |
| <15 years | 0.67 (0.17–1.16) | 0.33 (0.06–0.60) | 0.14 (−0.67–0.35) | 0.44 (0.05–0.84) | |
| ≥15 years | 0 | 0 | 0 | 0.1 (−0.12–0.32) | |
| Sore throat | |||||
| Counts | All ages | 7 | 5 | 6 | 4 |
| <15 years | 2 | 5 | 5 | 4 | |
| ≥15 years | 5 | 0 | 1 | 0 | |
| Rates | All ages | 0.21 (0.2–0.46) | 0.18 (−0.5–0.41) | 0.25 (−0.01–0.51) | 0.21 (−0.04–0.48) |
| <15 years | 0.13 (−0.06–0.33) | 0.34 (−0.11–0.80) | 0.35 (−0.07–0.79) | 0.37 (−0.08–0.82) | |
| ≥15 years | 0.36 (−0.07–0.79) | 0 | 0.10 (−0.13–0.33) | 0 |
| Baseline Phase | Activity Phase | ||||||
|---|---|---|---|---|---|---|---|
| Years 1–3 | p Value ‡ | ||||||
| 1 February 2017–31 January 2018 | 1 February 2018–31 January 2021 | Year 1 | Year 2 | Year 3 † | |||
| Participants contributing data | Number | 22 | 28 | 27 | 23 | 22 | |
| Benzathine penicillin G doses administered for ARF secondary prophylaxis | Number | 233 | 755 | 272 | 256 | 227 | |
| Proportion of scheduled doses * received | ≥80% | 17/22 (77%) | 45/72 (63%) | 16/27 (59%) | 16/23 (67%) | 13/22 (59%) | 0.201 |
| <80% | 5/22 (23%) | 27/72 (38%) | 11/27 (41%) | 7/23 (30%) | 9/22 (40%) | ||
| Days at risk | Number of days at risk per year: median (IQR) | 24 (9–80) | 60 (35–106) | 52 (28–94) | 66 (45–111) | 75 (49–106) | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralph, A.P.; Kelly, A.; Lee, A.-M.; Mungatopi, V.L.; Babui, S.R.; Budhathoki, N.K.; Wade, V.; Dassel, J.L.d.; Wyber, R. Evaluation of a Community-Led Program for Primordial and Primary Prevention of Rheumatic Fever in Remote Northern Australia. Int. J. Environ. Res. Public Health 2022, 19, 10215. https://doi.org/10.3390/ijerph191610215
Ralph AP, Kelly A, Lee A-M, Mungatopi VL, Babui SR, Budhathoki NK, Wade V, Dassel JLd, Wyber R. Evaluation of a Community-Led Program for Primordial and Primary Prevention of Rheumatic Fever in Remote Northern Australia. International Journal of Environmental Research and Public Health. 2022; 19(16):10215. https://doi.org/10.3390/ijerph191610215
Chicago/Turabian StyleRalph, Anna P., Angela Kelly, Anne-Marie Lee, Valerina L. Mungatopi, Segora R. Babui, Nanda Kaji Budhathoki, Vicki Wade, Jessica L. de Dassel, and Rosemary Wyber. 2022. "Evaluation of a Community-Led Program for Primordial and Primary Prevention of Rheumatic Fever in Remote Northern Australia" International Journal of Environmental Research and Public Health 19, no. 16: 10215. https://doi.org/10.3390/ijerph191610215
APA StyleRalph, A. P., Kelly, A., Lee, A.-M., Mungatopi, V. L., Babui, S. R., Budhathoki, N. K., Wade, V., Dassel, J. L. d., & Wyber, R. (2022). Evaluation of a Community-Led Program for Primordial and Primary Prevention of Rheumatic Fever in Remote Northern Australia. International Journal of Environmental Research and Public Health, 19(16), 10215. https://doi.org/10.3390/ijerph191610215




