The Effects of the Exogenous Melatonin on Shift Work Sleep Disorder in Health Personnel: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Data Souces
2.3. Information Processing
- Population: Health personnel—persons who work in the provision of health services, either as individual practitioners or as employees of health institutions and programs, even if they do not have professional training, and whether or not they are subject to public regulation.“Health Personnel”[Mesh] OR “Health Personnel”[Title/Abstract] OR “Health Care Provider*”[Title/Abstract] OR “Healthcare Provider*”[Title/Abstract] OR “Healthcare Worker*”[Title/Abstract] OR “Health Care Professional*”[Title/Abstract] OR “Nurse*”[Title/Abstract] OR “Pharmacist*”[Title/Abstract] OR “Physician*”[Title/Abstract] OR “Health Care Personnel”[Title/Abstract] OR “Health Care Practitioner*”[Title/Abstract] OR “Health Care Worker*”[Title/Abstract] OR “Health Profession Personnel”[Title/Abstract] OR “Healthcare Personnel”[Title/Abstract] OR “Healthcare Practitioner*”[Title/Abstract] OR “Healthcare Professional*”[Title/Abstract]
- Intervention: Melatonin—a biogenic amine found in animals and plants; in mammals, melatonin is produced by the pineal gland. Melatonin secretion increases in darkness and decreases during light exposure. Melatonin is involved in the regulation of sleep, mood and reproduction. Melatonin is also an effective antioxidant.“Melatonin”[Mesh] OR “Melatonin”[Title/Abstract] OR “Melatonina”[Title/Abstract]
- Outcome: Sleep disorders, circadian rhythm–dyssomnias associated with disruption of the normal 24-h sleep wake cycle secondary to travel shift work, or other causes.“Sleep Disorders, Circadian Rhythm”[Mesh] OR “Sleep Wake Schedule Disorder*”[Title/Abstract] OR “Circadian Rhythm Sleep Disorder*”[Title/Abstract] OR “Disturbed Nyctohemeral Rhythm*”[Title/Abstract] OR “Sleep Wake Cycle Disorder*”[Title/Abstract] OR “Shift Work Sleep Disorder*”[Title/Abstract] OR “Non 24 Hour Sleep Wake Disorder*”[Title/Abstract] OR “Nonorganic Sleep Wake Cycle Disorder*”[Title/Abstract] OR “Advanced Sleep Phase Syndrome*”[Title/Abstract] OR “Delayed Sleep Phase Syndrome*”[Title/Abstract] OR “Circadian Rhythm Sleep-Wake Disorder*”[Title/Abstract] OR “Sleep Phase Disorder*”[Title/Abstract] OR “Sleep Phase Syndrome*”[Title/Abstract] OR “Sleep Wake Phase Disorder*”[Title/Abstract] OR “Sleep Wake Phase Syndrome*”[Title/Abstract] OR “Sleep Wake Schedule Disorder*”[Title/Abstract]
2.4. Final Selection of Articles
- Inclusion: adjust the objectives of the search as follows: there is a treatment with exogenous melatonin in a clinical trial published in peer-reviewed journals and written in English, Spanish, Portuguese, Italian or German.
- Exclusion: The main problem in those articles where the full text could not be found lies in the fact there was no association between intervention and the outcome under study (criterion of causality); other articles included a non-adult population (under 18 years of age).
2.5. Documentary Quality, Level of Evidence, Degree of Recommendation and Study of Biases
2.6. Data Extraction
2.7. Data Analysis
2.8. Ethical Aspects
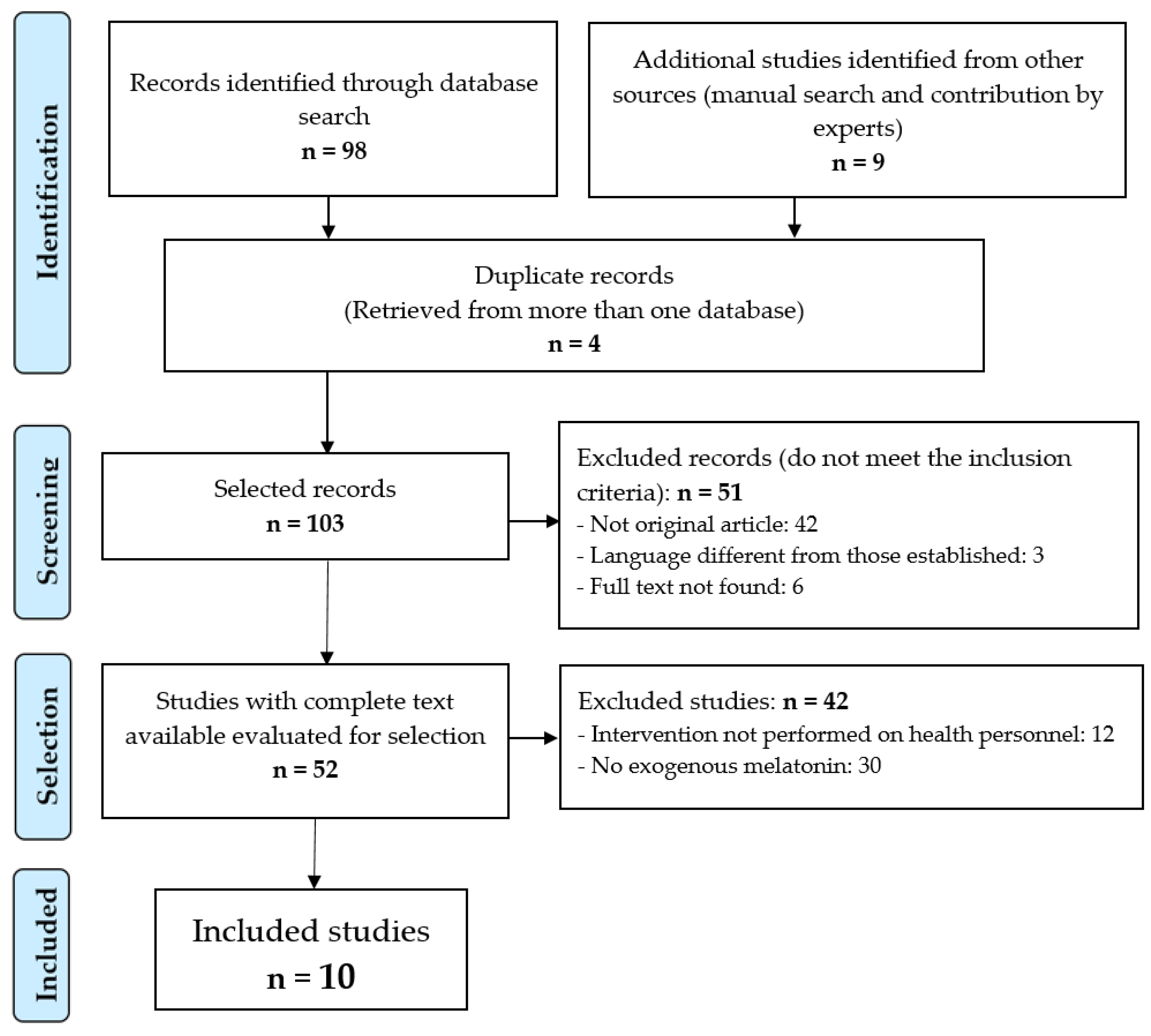
3. Results
3.1. Performed Interventions
3.2. Results of the Interventions
4. Discussion
Limitations of the Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, G. Shift Work and Occupational Medicine: An Overview. Occup. Med. Oxf. Engl. 2003, 53, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Loef, B.; van Baarle, D.; van der Beek, A.J.; van Kerkhof, L.W.; van de Langenberg, D.; Proper, K.I. Klokwerk + Study Protocol: An Observational Study to the Effects of Night-Shift Work on Body Weight and Infection Susceptibility and the Mechanisms Underlying These Health Effects. BMC Public Health 2016, 16, 692. [Google Scholar] [CrossRef] [PubMed]
- Liira, J.; Verbeek, J.H.; Costa, G.; Driscoll, T.R.; Sallinen, M.; Isotalo, L.K.; Ruotsalainen, J.H. Pharmacological Interventions for Sleepiness and Sleep Disturbances Caused by Shift Work. Sao Paulo Med. J. Rev. Paul. Med. 2015, 133, 67. [Google Scholar] [CrossRef]
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Herxheimer, A.; Petrie, K.J. Melatonin for the Prevention and Treatment of Jet Lag. Cochrane Database Syst. Rev. 2002, 2, CD001520. [Google Scholar] [CrossRef]
- Liira, J.; Verbeek, J.H.; Costa, G.; Driscoll, T.R.; Sallinen, M.; Isotalo, L.K.; Ruotsalainen, J.H. Pharmacological Interventions for Sleepiness and Sleep Disturbances Caused by Shift Work. Cochrane Database Syst. Rev. 2014, 8, CD009776. [Google Scholar] [CrossRef]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. En representación del Grupo de estudio de insomnio de la Sociedad Española de Sueño (SES) Melatonin in Sleep Disorders. Neurol. Barc. Spain 2018, in press. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic Reviews in Nutrition: Standardized Methodology. Br. J. Nutr. 2012, 107, S3–S7. [Google Scholar] [CrossRef]
- Cobos-Carbó, A.; Augustovski, F. CONSORT 2010 Declaration: Updated guideline for reporting parallel group randomised trials. Med. Clin. 2011, 137, 213–215. [Google Scholar] [CrossRef]
- Harbour, R.; Miller, J. A New System for Grading Recommendations in Evidence Based Guidelines. BMJ 2001, 323, 334–336. [Google Scholar] [CrossRef]
- Aguayo-Albasini, J.L.; Flores-Pastor, B.; Soria-Aledo, V. GRADE system: Classification of quality of evidence and strength of recommendation. Cir. Esp. 2014, 92, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Ley 14/2007, de 3 de Julio, de Investigación Biomédica. Available online: https://www.boe.es/eli/es/l/2007/07/03/14 (accessed on 18 January 2022).
- Marqueze, E.C.; Nogueira, L.F.R.; Vetter, C.; Skene, D.J.; Cipolla-Neto, J.; Moreno, C.R.C. Exogenous Melatonin Decreases Circadian Misalignment and Body Weight among Early Types. J. Pineal Res. 2021, 71, e12750. [Google Scholar] [CrossRef]
- Farahmand, S.; Vafaeian, M.; Vahidi, E.; Abdollahi, A.; Bagheri-Hariri, S.; Dehpour, A.R. Comparison of Exogenous Melatonin versus Placebo on Sleep Efficiency in Emergency Medicine Residents Working Night Shifts: A Randomized Trial. World J. Emerg. Med. 2018, 9, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sadeghniiat-Haghighi, K.; Bahrami, H.; Aminian, O.; Meysami, A.; Khajeh-Mehrizi, A. Melatonin Therapy in Shift Workers with Difficulty Falling Asleep: A Randomized, Double-Blind, Placebo-Controlled Crossover Field Study. Work 2016, 55, 225–230. [Google Scholar] [CrossRef]
- Sadeghniiat-Haghighi, K.; Aminian, O.; Pouryaghoub, G.; Yazdi, Z. Efficacy and Hypnotic Effects of Melatonin in Shift-Work Nurses: Double-Blind, Placebo-Controlled Crossover Trial. J. Circadian Rhythm. 2008, 6, 10. [Google Scholar] [CrossRef]
- Cavallo, A.; Ris, M.D.; Succop, P.; Jaskiewicz, J. Melatonin Treatment of Pediatric Residents for Adaptation to Night Shift Work. Ambul. Pediatr. Off. J. Ambul. Pediatr. Assoc. 2005, 5, 172–177. [Google Scholar] [CrossRef]
- Yoon, I.-Y.; Song, B.-G. Role of Morning Melatonin Administration and Attenuation of Sunlight Exposure in Improving Adaptation of Night-Shift Workers. Chronobiol. Int. 2002, 19, 903–913. [Google Scholar] [CrossRef]
- Jockovich, M.; Cosentino, D.; Cosentino, L.; Wears, R.L.; Seaberg, D.C. Effect of Exogenous Melatonin on Mood and Sleep Efficiency in Emergency Medicine Residents Working Night Shifts. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2000, 7, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.W.; Lawrence, L.M.; Wrenn, K.D.; Haynes, M.L.; Welch, L.W.; Schlack, H.M. Randomized Clinical Trial of Melatonin after Night-Shift Work: Efficacy and Neuropsychologic Effects. Ann. Emerg. Med. 1998, 32, 334–340. [Google Scholar] [CrossRef]
- Jorgensen, K.M.; Witting, M.D. Does Exogenous Melatonin Improve Day Sleep or Night Alertness in Emergency Physicians Working Night Shifts? Ann. Emerg. Med. 1998, 31, 699–704. [Google Scholar] [CrossRef]
- James, M.; Tremea, M.O.; Jones, J.S.; Krohmer, J.R. Can Melatonin Improve Adaptation to Night Shift? Am. J. Emerg. Med. 1998, 16, 367–370. [Google Scholar] [CrossRef]
- Hagger, M.S. What Makes a ‘Good’ Review Article? Some Reflections and Recommendations. Health Psychol. Rev. 2012, 6, 141–146. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Occupational Health for All: The Way to Health at Work. Available online: https://bit.ly/2Wtl0Gi (accessed on 26 February 2022).
- Muñoz-Cobo-Orosa, B.; Varela-Serrano, C.; Rodriguez-Ledott, M.; Sanz-Valero, J. Lesiones malignas de la piel en trabajadores del sector pesquero: Revisión sistemática. Arch. Prev. Riesgos Labor. 2021, 24, 47–61. [Google Scholar] [CrossRef]
- Troncoso-Piñeiro, P.; González de Giarratana, A.E.; Rivadulla-Lema, I.; Torres-Romero, M.G.; Sanz-Valero, J. Neoplasias En Trabajadores Expuestos al Aluminio y/o Sus Compuestos: Revisión Sistemática. Med. Segur. Trab. 2018, 64, 312–326. [Google Scholar]
- Melián-Fleitas, L.; Franco-Pérez, Á.; Caballero, P.; Sanz-Lorente, M.; Wanden-Berghe, C.; Sanz-Valero, J. Influence of Nutrition, Food and Diet-Related Interventions in the Workplace: A Meta-Analysis with Meta-Regression. Nutrients 2021, 13, 3945. [Google Scholar] [CrossRef]
- Gea Cabrera, A.; Caballero, P.; Wanden-Berghe, C.; Sanz-Lorente, M.; López-Pintor, E. Effectiveness of Workplace-Based Diet and Lifestyle Interventions on Risk Factors in Workers with Metabolic Syndrome: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2021, 13, 4560. [Google Scholar] [CrossRef]
- Begg, C.; Cho, M.; Eastwood, S.; Horton, R.; Moher, D.; Olkin, I.; Pitkin, R.; Rennie, D.; Schulz, K.F.; Simel, D.; et al. Improving the Quality of Reporting of Randomized Controlled Trials. The CONSORT Statement. JAMA 1996, 276, 637–639. [Google Scholar] [CrossRef]
- Turner, L.; Shamseer, L.; Altman, D.G.; Schulz, K.F.; Moher, D. Does Use of the CONSORT Statement Impact the Completeness of Reporting of Randomised Controlled Trials Published in Medical Journals? A Cochrane Review. Syst. Rev. 2012, 1, 60. [Google Scholar] [CrossRef] [PubMed]
- González-Castro, U. Cómo mejorar la calidad de la publicación de ensayos clínicos: La declaración CONSORT. Actas Dermosifiliogr. 2002, 93, 141–142. [Google Scholar] [CrossRef]
- Manterola, C.; Asenjo-Lobos, C.; Otzen, T. Hierarchy of evidence: Levels of evidence and grades of recommendation from current use. Rev. Chil. Infectol. Organo Of. Soc. Chil. Infectol. 2014, 31, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Teufer, B.; Ebenberger, A.; Affengruber, L.; Kien, C.; Klerings, I.; Szelag, M.; Grillich, L.; Griebler, U. Evidence-Based Occupational Health and Safety Interventions: A Comprehensive Overview of Reviews. BMJ Open 2019, 9, e032528. [Google Scholar] [CrossRef]
- Barriocanal-Gómez, P.; Del Pozo-Díez, C.M.; Kudryavtseva, O.; Portillo Chicano, I.; Sanz-Valero, J. Efectos Derivados de La Exposición Laboral En Las Mujeres Trabajadoras Embarazadas Expuestas a Sustancias Peligrosas: Revisión Sistemática. Arch. Prev. Riesgos Labor. 2021, 24, 263–296. [Google Scholar] [CrossRef]
- Sánchez-Moya, J.; Sanz-Valero, J.; Lopez-Pintor, E. Intervenciones Desde La Farmacia Comunitaria En Los Pacientes Adultos Que Reciben Atención de La Salud a Domicilio: Revisión Exploratoria. Hosp. Domic. 2020, 4, 209–227. [Google Scholar] [CrossRef]
- Domingo-Pueyo, A.; Sanz-Valero, J.; Wanden-Berghe, C. Disorders Induced by Direct Occupational Exposure to Noise: Systematic Review. Noise Health 2016, 18, 229–239. [Google Scholar] [CrossRef]
- Deschamps Perdomo, A.; Olivares Román, S.B.; De la Rosa Zabala, K.L.; Asunsolo del Barco, Á. Influencia de Los Turnos de Trabajo y Las Guardias Nocturnas En La Aparición Del Síndrome de Burnout En Médicos y Enfermeras. Med. Segur. Trab. 2011, 57, 224–241. [Google Scholar] [CrossRef]
- Vásquez-Trespalacios, E.M.; Jaramillo-Palacio, V.; Gaviria-Gallo, G.; Martínez-Valencia, A. Trabajo Durante La Noche y Alteraciones En La Melatonina En Trabajadoras Expuestas: Revisión de La Evidencia Reciente. CES Salud Pública 2015, 6, 181–189. [Google Scholar]
- Horrocks, N.; Pounder, R. (Eds.) Working the Night Shift: Preparation, Survival and Recovery—A Guide for Junior Doctors; Royal College of Physicians of London: London, UK, 2006. [Google Scholar]
- Boniol, M.; McIsaac, M.; Xu, L.; Wuliji, T.; Diallo, K.; Campbell, J. Gender Equity in the Health Workforce: Analysis of 104 Countries; World Health Organization: Geneve, Switzerland, 2019.
- Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQuAS). La Melatonina Exògena En Els Trastorns Del Son: Eficàcia i Seguretat; AQuAS: Barcelona, Spain, 2017. [Google Scholar]
- Álvarez Velásquez, S.; Sanz Valero, J. Ventajas de La Quimioterapia Domiciliaria En Los Enfermos Adultos Con Neoplasias: Revisión Sistemática. Hosp. Domic. 2020, 4, 25–41. [Google Scholar] [CrossRef]
- Buscemi, N.; Vandermeer, B.; Hooton, N.; Pandya, R.; Tjosvold, L.; Hartling, L.; Baker, G.; Klassen, T.P.; Vohra, S. The Efficacy and Safety of Exogenous Melatonin for Primary Sleep Disorders. A Meta-Analysis. J. Gen. Intern. Med. 2005, 20, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, A.; Honda, M.; Hayashino, Y. Ramelteon for the Treatment of Insomnia in Adults: A Systematic Review and Meta-Analysis. Sleep Med. 2014, 15, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Matheson, E.; Hainer, B.L. Insomnia: Pharmacologic Therapy. Am. Fam. Physician 2017, 96, 29–35. [Google Scholar]
- Buscemi, N.; Vandermeer, B.; Pandya, R.; Hooton, N.; Tjosvold, L.; Hartling, L.; Baker, G.; Vohra, S.; Klassen, T. Melatonin for Treatment of Sleep Disorders; U. S. Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004.
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary Factors and Fluctuating Levels of Melatonin. Food Nutr. Res. 2012, 56, e7252. [Google Scholar] [CrossRef] [PubMed]
- Fonzi, S.; Solinas, G.P.; Costelli, P.; Parodi, C.; Murialdo, G.; Bo, P.; Albergati, A.; Montalbetti, L.; Savoldi, F.; Polleri, A. Melatonin and Cortisol Circadian Secretion during Ethanol Withdrawal in Chronic Alcoholics. Chronobiologia 1994, 21, 109–112. [Google Scholar] [PubMed]
- Danel, T.; Touitou, Y. Alcohol Consumption Does Not Affect Melatonin Circadian Synchronization in Healthy Men. Alcohol Alcohol. 2006, 41, 386–390. [Google Scholar] [CrossRef][Green Version]
- Lemoine, P.; Nir, T.; Laudon, M.; Zisapel, N. Prolonged-Release Melatonin Improves Sleep Quality and Morning Alertness in Insomnia Patients Aged 55 Years and Older and Has No Withdrawal Effects. J. Sleep Res. 2007, 16, 372–380. [Google Scholar] [CrossRef]
- Shahrokhi, M.; Ghaeli, P.; Arya, P.; Shakiba, A.; Noormandi, A.; Soleimani, M.; Esfandbod, M. Comparing the Effects of Melatonin and Zolpidem on Sleep Quality, Depression, and Anxiety in PatientsWithColorectalCancerUndergoingChemotherapy. Basic Clin. Neurosci. 2021, 12, 105–114. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J.; Lao, K.S.J.; Wong, I.C.K. Adverse Events Associated with Melatonin for the Treatment of Primary or Secondary Sleep Disorders: A Systematic Review. CNS Drugs 2019, 33, 1167–1186. [Google Scholar] [CrossRef]
- Nave, R.; Iani, C.; Herer, P.; Gopher, D.; Lavie, P. Residual Effects of Daytime Administration of Melatonin on Performance Relevant to Flight. Behav. Brain Res. 2002, 131, 87–95. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.G.; Ford, I.; Crawford, G.; McConnachie, A.; Nir, T.; Laudon, M.; Zisapel, N. Nightly Treatment of Primary Insomnia with Prolonged Release Melatonin for 6 Months: A Randomized Placebo Controlled Trial on Age and Endogenous Melatonin as Predictors of Efficacy and Safety. BMC Med. 2010, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Abad, V.C.; Guilleminault, C. Insomnia in Elderly Patients: Recommendations for Pharmacological Management. Drugs Aging 2018, 35, 791–817. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.-N. Ramelteon in the Treatment of Chronic Insomnia: Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2012, 66, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Saletu, B.; Trinchard-Lugan, I. Sleep Laboratory Investigations on Hypnotic Properties of Melatonin. Psychopharmacology 1990, 100, 222–226. [Google Scholar] [CrossRef]
- Morera, A.L.; Henry, M.; Villaverde-Ruiz, M.L.; Gracia-Marco, R. Efficiency of melatonin in the treatment of insomnia. Actas Esp. Psiquiatr. 2000, 28, 325–329. [Google Scholar]
- Eckerberg, B.; Lowden, A.; Nagai, R.; Akerstedt, T. Melatonin Treatment Effects on Adolescent Students’ Sleep Timing and Sleepiness in a Placebo-Controlled Crossover Study. Chronobiol. Int. 2012, 29, 1239–1248. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A Review of Sleep Disorders and Melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- MacFarlane, J.G.; Cleghorn, J.M.; Brown, G.M.; Streiner, D.L. The Effects of Exogenous Melatonin on the Total Sleep Time and Daytime Alertness of Chronic Insomniacs: A Preliminary Study. Biol. Psychiatry 1991, 30, 371–376. [Google Scholar] [CrossRef]
- Riha, R.L. The Use and Misuse of Exogenous Melatonin in the Treatment of Sleep Disorders. Curr. Opin. Pulm. Med. 2018, 24, 543–548. [Google Scholar] [CrossRef]
- Sharkey, K.M.; Fogg, L.F.; Eastman, C.I. Effects of Melatonin Administration on Daytime Sleep after Simulated Night Shift Work. J. Sleep Res. 2001, 10, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Melatonin and Its Relevance to Jet Lag. Travel Med. Infect. Dis. 2009, 7, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Auger, R.R.; Burgess, H.J.; Emens, J.S.; Deriy, L.V.; Thomas, S.M.; Sharkey, K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015, 11, 1199–1236. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Studied Population | Country | Intervention Period | Intervention Type | Observed Result |
|---|---|---|---|---|---|
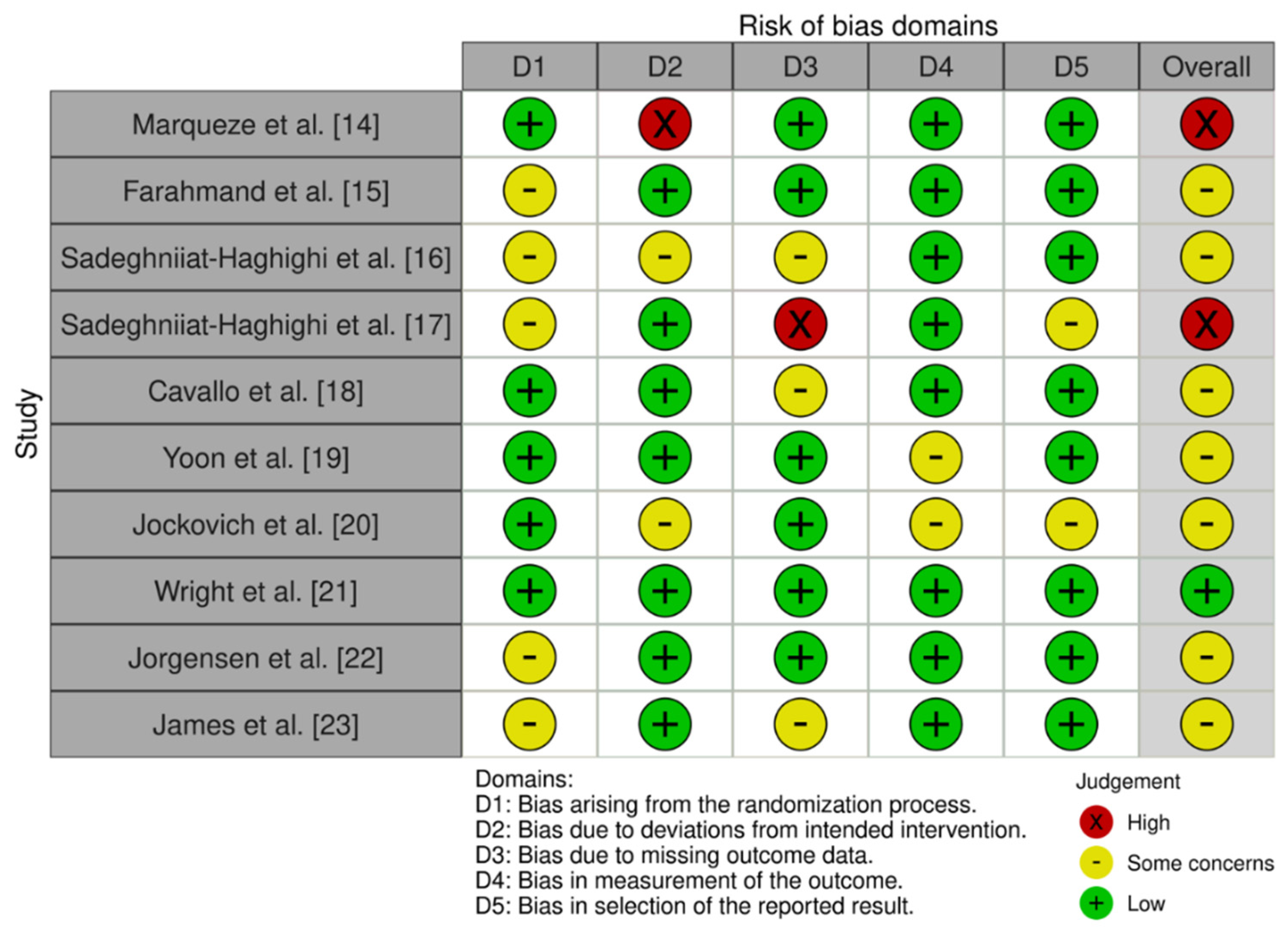
| Marqueze et al., 2021 [16] | Population type: Nurses N total: 27 H/M: 0/27 Age: 37.1 ± 5.9 years | Brazil | 24 weeks | Administration of 3 mg exogenous melatonin or placebo on nights when they were not working. Composite phase deviations (CPD) of mean sleep time based on actigraphy (efficiency and total sleep time) were calculated to measure circadian misalignment. | Significant 20% decrease in circadian misalignment (p < 0.001). As well as reduction in weight, waist and hip circumference. |
| Farahmand et al., 2018 [17] | Population type: emergency medicine residents N total: 24 (Gc:12-Gi: 12) H/M: 14/10 Age: 31.21 ± 5.23 years | Iran | 4 weeks (From 19 May to 19 June 2016) | Take 3 mg melatonin, versus placebo, 1 h before bedtime for 2 consecutive days. The measurement was made using the Karolinska Sleep Scale | Melatonin therapy meaningfully lessened daytime sleepiness in comparison with placebo from the second night onwards (p = 0.003). |
| Sadeghniiat-Haghighi et al., 2016 [18] | Population type: Shift workers with difficulty to get to sleep N total: 50 (Gc: 25–Gi:25) H/M: Not recorded Age: Not recorded | Iran | 3 nights’ treatment and 2 weeks washout period. | Take 3 mg melatonin, versus placebo, 30 min before bedtime. Total sleep time, sleep onset latency, sleep efficiency and awakening after sleep onset were analyzed. | Melatonin therapy improved sleep onset latency and decreased nocturnal awakenings, although there was no association when compared to the placebo group in relation to total sleep time and awakening after sleep onset (p > 0.05). Sleep onset latency and sleep efficiency improved significantly (p < 0.05). |
| Sadeghniiat-Haghighi et al., 2008 [19] | Population type: nurses with insomnia N total: 86 H/M: 0/86 Age: From 24 to 46 years | Iran | 1 night treatment with melatonin and washing out for 4 days. | Oral intake of 5 mg melatonin taken 30 min before night-time sleep. Insomnia, subjective sleep onset latency, number of awakenings and sleep duration were measured. | While the subjects were taking melatonin (p < 0.05), sleep onset latency lessened meaningfully. There was no association when analysing the number of awakenings and sleep duration. |
| Cavallo et al., 2005 [20] | Population type: 2º year paediatric residents N total: 45 H/M: 16/29 Age: 28.6 ± 1.9 years | USA | 2 Weeks | Taking melatonin (3 mg) vs. placebo before bedtime in the morning after the night shift. Standardized measures of sleep, mood and attention were assessed. | There were no significant differences in measures of sleep and mood. Significance was observed in the measure of attention (p = 0.03). |
| Yoon et al., 2002 [21] | Population type: Night shift nurses N total: 12 H/M: 0/12 Age: From 23 to 27 years | Korea | Follow-up for 9 days. | Three groups were set: placebo, melatonin, and melatonin with sunglasses. Melatonin (6 mg) was administered before bedtime for 2 days. Alertness, night-time sleep period and daytime sleep and mood were observed. | Total sleep period and total sleep times increased meaningfully with melatonin treatments (p < 0.05). Mood improved slightly. There was no significance between the melatonin treatment groups (with or without sunglasses). |
| Jockovich et al., 2000 [22] | Population type: emergency medicine residents N total: 19 H/M: 15/4 Age: 28.2 years | USA | 3 consecutive days after each night shift. | Melatonin (1 mg) administration or placebo, 30 to 60 min before the daytime sleep session, for 3 consecutive days after each night shift. It was evaluated by Actigraph 1000 (efficiency and total sleep time). The mood profile and Stanford Sleepiness Scale were utilized to quantify mood and sleepiness. | There was no difference in sleep efficiency, duration, or latency (p > 0.05) between the melatonin group and placebo. Neither there was significance in mood profile and sleepiness (p > 0.05). |
| Wright et al., 1998 [23] | Population type: doctors N total: 15 H/M: 12/3 Age: From 32 to 45 years | USA | 36 days (4 days for intervention, 28 days for washout and 4 days for intervention) | Melatonin (5 mg) administration or placebo for 3 consecutive nights after the night shift with crossover to the opposite agent after a subsequent block of night shifts. The primary outcome measure was the overall assessment of recovery as measured by a visual analogue scale. Secondary outcome measures included sleep quality, duration and fatigue. Furthermore, the Profile of Mood States and neuropsychological tests were used. | No beneficial effect of melatonin was found for sleep quality, fatigue or cognitive function in emergency physicians after the night shift (p < 0.05). The obtained results suggest that exogenous melatonin has limited value in the recovery of doctors after the night shift. |
| Jorgensen et al., 1998 [24] | Population type: resident doctors N total: 18 H/M: 16/2 Age: From 25 to 40 years | USA | 5, 4, 3 and 2-night series | Administration of 10 mg sublingual melatonin or placebo every morning after the evening urgency. During daytime sleep periods, subjective sleep data were recorded. During night shifts, alertness was assessed using the Stanford Sleepiness Scale. | Melatonin improved daytime sleep and night-time alertness (p = 0.3); however, in neither case was the improvement statistically meaningful. Exogenous melatonin had a slight benefit in terms of improved alertness (p < 0.05). |
| James et al., 1998 [25] | Population type: night-shifts paramedics N total: 22 H/M: 17/5 Age: From 20 to 41 años | USA | A total of 4 consecutive night shifts (2 melatonin, 2 placebo) | Administration of melatonin 6 mg one capsule orally 30 min before each consecutive day’s sleep. Assessment of sleep quality, post-treatment mood and workload ratings were measured daily using a Visual Analogue Scale (VAS). | No clinical benefits were observed in staff working rotating night shifts. Melatonin was associated with meaningful fewer interim awakenings during daytime sleep compared with placebo (p < 0.05). For the rest of the studied variables, no significant differences were found (p > 0.05). |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | Total | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marqueze et al., 2021 [16] | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 20.5 | 13.71 |
| Farahmand et al., 2018 [17] | 1 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0 | 0 | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 18 | 12.04 |
| Sadeghniiat-Haghighi et al., 2016 [18] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 15 | 10.03 |
| Sadeghniiat-Haghighi et al., 2008 [19] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 14.5 | 9.69 |
| Cavallo et al., 2005 [20] | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 0 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 19.5 | 13.04 |
| Yoon et al., 2002 [21] | 0.5 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 12 | 8.02 |
| Jockovich et al., 2000 [22] | 0.5 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 9.5 | 6.35 |
| Wright et al., 1998 [23] | 0.5 | 1 | 0.5 | 1 | 1 | 0.5 | 0 | 0 | 0 | 1 | 0.5 | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 16 | 10.70 |
| Jorgensen et al., 1998 [24] | 0.5 | 0.5 | 1 | 1 | 1 | 0 | 0 | 0.5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.5 | 0 | 0 | 1 | 9 | 6.02 |
| James et al., 1998 [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 0 | 0.5 | 1 | 0 | 0 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 15.5 | 10.36 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Marqueze et al. [16] | No | No | Yes | Yes | No | No | No |
| Farahmand et al. [17] | No | No | No | Yes | Yes | No | No |
| Sadeghniiat-Haghighi et al. [18] | No | No | Yes | Yes | Yes | No | No |
| Sadeghniiat-Haghighi et al. [19] | No | No | No | No | Yes | No | No |
| Cavallo et al. [20] | No | No | No | No | Yes | Yes | Yes |
| Yoon et al. [21] | No | No | No | No | Yes | Yes | No |
| Jockovich et al. [22] | No | No | No | No | Unclear | Yes | Yes |
| Wright et al. [23] | No | Unclear | Unclear | Unclear | Yes | Yes | No |
| Jorgensen et al. [24] | No | No | No | No | Yes | No | Yes |
| James et al. [25] | No | No | Unclear | Yes | Yes | No | Yes |
| |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriedo-Diez, B.; Tosoratto-Venturi, J.L.; Cantón-Manzano, C.; Wanden-Berghe, C.; Sanz-Valero, J. The Effects of the Exogenous Melatonin on Shift Work Sleep Disorder in Health Personnel: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10199. https://doi.org/10.3390/ijerph191610199
Carriedo-Diez B, Tosoratto-Venturi JL, Cantón-Manzano C, Wanden-Berghe C, Sanz-Valero J. The Effects of the Exogenous Melatonin on Shift Work Sleep Disorder in Health Personnel: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(16):10199. https://doi.org/10.3390/ijerph191610199
Chicago/Turabian StyleCarriedo-Diez, Bárbara, Javier Lucas Tosoratto-Venturi, Carmen Cantón-Manzano, Carmina Wanden-Berghe, and Javier Sanz-Valero. 2022. "The Effects of the Exogenous Melatonin on Shift Work Sleep Disorder in Health Personnel: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 16: 10199. https://doi.org/10.3390/ijerph191610199
APA StyleCarriedo-Diez, B., Tosoratto-Venturi, J. L., Cantón-Manzano, C., Wanden-Berghe, C., & Sanz-Valero, J. (2022). The Effects of the Exogenous Melatonin on Shift Work Sleep Disorder in Health Personnel: A Systematic Review. International Journal of Environmental Research and Public Health, 19(16), 10199. https://doi.org/10.3390/ijerph191610199






