Vaccination against SARS-CoV-2 in Haemodialysis Patients: Spike’s Ab Response and the Influence of BMI and Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants’ Inclusion
2.2. Sample Collection
2.3. Anti-Spike IgG Level Quantification
2.4. Measure of Body Mass Index
2.5. Statistical Analysis
3. Results
3.1. Humoral Response of Vaccinated Patients after 3 and 6 Months
3.2. Humoral Response of COVID-19-Recovered Patients (Control Group)
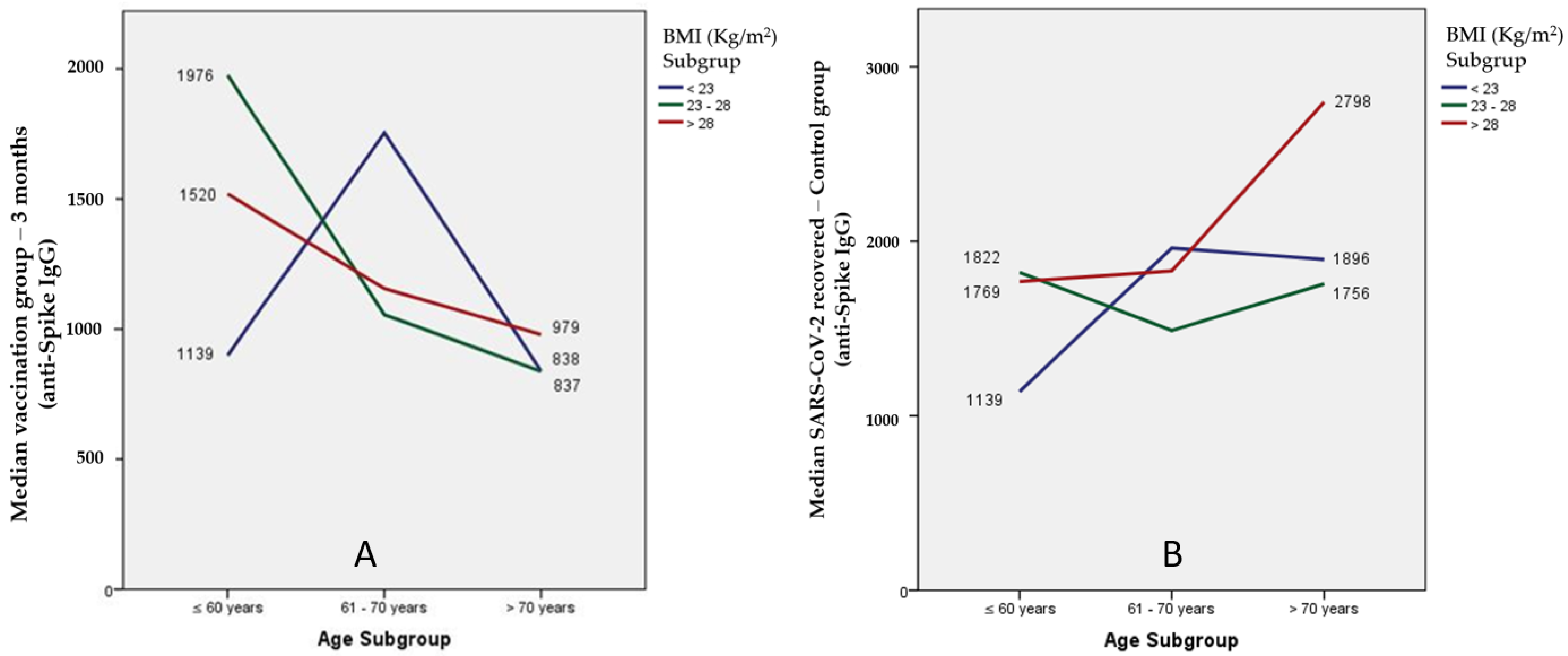
3.3. Anti-Spike Antibody Response between Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; de Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef]
- Jager, K.J.; Kramer, A.; Chesnaye, N.C.; Couchoud, C.; Sánchez-Álvarez, J.E.; Garneata, L.; Collart, F.; Hemmelder, M.H.; Ambühl, P.; Kerschbaum, J.; et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipien ts across Europe. Kidney Int. 2020, 98, 1540–1548. [Google Scholar] [CrossRef]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Miskulin, D.C.; Manley, H.J.; Stewart, C.; Ladik, V.; Hosford, J.; Lacson, E.C.; Johnson, D.S.; et al. COVID-19 Among US Dialysis Patients: Risk Factors and Outcomes From a National Dialysis Provider. Am. J. Kidney Dis. 2021, 77, 748–756.e1. [Google Scholar] [CrossRef]
- Lano, G.; Braconnier, A.; Bataille, S.; Cavaille, G.; Moussi-Frances, J.; Gondouin, B.; Bindi, P.; Nakhla, M.; Mansour, J.; Halin, P.; et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin. Kidney J. 2020, 13, 878–888. [Google Scholar] [CrossRef]
- Francis, A.; Baigent, C.; Ikizler, T.A.; Cockwell, P.; Jha, V. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: A call to action. Kidney Int. 2021, 99, 791–793. [Google Scholar] [CrossRef]
- Fazendeiro Matos, J.; Peralta, R.; Felix, C.; Pinto, B.; Ponce, P. Vacinação Contra a COVID-19 numa Rede de Clínicas de Hemodiálise em Portugal: Uma Experiência Promissora. Acta Med. Port. 2022, 35, 336–342. [Google Scholar] [CrossRef]
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and Cellular Response Following Vaccination with the BNT162b2 mRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front. Immunol. 2021, 4, 727850. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.D.M.; Marcos, M.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.L.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Krueger, K.M.; Halasa, N.; Ison, M.G. SARS-CoV-2 Vaccine in Dialysis Patients: Time for a Boost? Am. J. Kidney Dis. 2022, 79, 162–163. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Krammer, F.; Weir, J.P.; Engelhardt, O.; Katz, J.M.; Cox, R.J. Meeting report and review: Immunological assays and correlates of protection for next-generation influenza vaccines. Influenza Other Respir. Viruses 2020, 14, 237–243. [Google Scholar] [CrossRef]
- Betjes, M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef]
- Bartlett, J.E.; Kotrlik, J.W.K.J.W.; Higgins, C. Organizational research: Determining appropriate sample size in survey research appropriate sample size in survey research. Inf. Technol. Learn. Perform. J. 2001, 19, 43–50. [Google Scholar]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral response to the pfizer bnt162b2 vaccine in patients undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef]
- Agur, T.; Ben-Dor, N.; Goldman, S.; Lichtenberg, S.; Herman-Edelstein, M.; Yahav, D.; Rozen-Zvi, B.; Zingerman, B. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—A prospective cohort study. Nephrol. Dial. Transplant. 2021, 36, 1347–1349. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.-L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.-M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: Time for a boost. Kidney Int. 2021, 100, 1334–1335. [Google Scholar] [CrossRef]
- Labriola, L.; Scohy, A.; Seghers, F.; Perlot, Q.; De Greef, J.; Desmet, C.; Romain, C.; Morelle, J.; Yombi, J.-C.; Kabamba, B.; et al. A Longitudinal, 3-Month Serologic Assessment of SARS-CoV-2 Infections in a Belgian Hemodialysis Facility. Clin. J. Am. Soc. Nephrol. 2021, 16, 613–614. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.-M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Speer, C.; Göth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kälble, F.; Kreysing, M.; Reichel, P.; et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with bnt162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef]
- Melin, J.; Svensson, M.K.; Albinsson, B.; Winqvist, O.; Pauksens, K. Humoral and cellular response to SARS-CoV-2 BNT162b2 mRNA vaccine in hemodialysis patients. BMC Immunol. 2021, 22, 70. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 ( Pfizer-BioNTech ) in Patients on Hemodialysis. Vaccines 2021, 9, 360. [Google Scholar] [CrossRef]
- Vergara, A.; Bosch, M.M.D.; Toapanta, N. The Impact of Age on Mortality in Chronic Haemodialysis Population with COVID-19. J. Clin. Med. 2021, 10, 3022. [Google Scholar] [CrossRef]
- Forbes, S.; Davari, M.; Gnanasampanthan, S.; Roth, N.; Young, G.; Rajakariar, R.; Cove-Smith, A.; Yaqoob, M.M.; Cutino-Moguel, T.; Mahalingasivam, V.; et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol. Dial. Transpl. 2021, 36, 1292–1297. [Google Scholar] [CrossRef]
- Alfano, G.; Fontana, F.; Morisi, N.; Giaroni, F.; Mori, G.; Guaraldi, G.; Magistroni, R.; Cappelli, G.; Mussini, C.; Bacca, E.; et al. One-year persistence of neutralizing anti-SARS-CoV-2 antibodies in dialysis patients recovered from COVID-19. Hemodial. Int. 2021, 25, E53–E56. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.H.P. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2021, 79, 185–192. [Google Scholar] [CrossRef]
- Weigert, A.; Bergman, M.L.; Gonçalves, L.A.; Godinho, I.; Duarte, N.; Abrantes, R.; Borges, P.; Brennand, A.; Malheiro, V.; Matoso, P.; et al. Longitudinal Analysis of Antibody Responses to the mRNA BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Front. Med. 2021, 24, 796676. [Google Scholar] [CrossRef]
- Bonelli, M.M.; Mrak, D.; Perkmann, T.; Haslacher, H.; Aletaha, D. CoV-2 vaccination in rituximab-treated patients: Evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021, 80, 1355–1356. [Google Scholar] [CrossRef]
- Frantzen, L.; Cavaillé, G.; Thibeaut, S.; El-Haik, Y. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in a haemodialysis cohort. Nephrol. Dial. Transplant. 2021, 36, 1756–1757. [Google Scholar] [CrossRef] [PubMed]

| Vaccinated Group (n = 321) | COVID-19-Recovered Group (n = 183) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), median (IQR) | 67 [56–78] | 70 [59–80] | p = 0.042 |
| BMI (Kg/m2), median (IQR) | 24.8 [22–28.20] | 24.8 [21.50–29.30] | p = 0.646 |
| Dialysis vintage (months), median (IQR) | 62 [27.50–116] | 57 [30–101] | p = 0.942 |
| Male, n (%) | 190 (59.20) | 107 (58.50) | |
| Vascular access | |||
| AVF n (%) | 245 (66.0) | 126 (33.9) | |
| AVG n (%) | 30 (60) | 20 (40) | |
| CVC n (%) | 46 (55.42) | 37 (44.58) | |
| Causes of ESRD (n, %) | |||
| Diabetic nephropaty | 49 (37.12) | 83 (62.88) | |
| Hypertension/vascular disease | 27 (41.54) | 38 (58.46) | |
| Polycystic kidney disease | 13 (37.14) | 22 (62.86) | |
| Glomerulonephritis | 22 (27.50) | 58 (72.50) | |
| Chronic pyelonephritis | 19 (44.19) | 24 (55.81) | |
| Other causes | 53 (36.31) | 96 (64.43) |
| Variables | Vaccinated Group t0 | Vaccinated Group t1 | COVID-19 Recovered Group | p-Value |
|---|---|---|---|---|
| Anti-Spike IgG (AU/mL) median (IQR) | 1120 [493–2805] | 455 [189–967] | <0.001 a | |
| Anti-Spike IgG (AU/mL) median (IQR) | 1120 [493–2805] | 1836 [749–5168] | <0.001 b | |
| Anti-Spike IgG (AU/mL) median (IQR) | 455 [189–967] | 1836 [749–5168] | <0.001 c |
| Variables | Anti-Spike IgG (AU/mL) 3rd Month | Anti-Spike IgG (AU/mL) 6th Month | COVID-19 Recovered Anti-Spike IgG (Au/mL) 6th Month |
|---|---|---|---|
| Age Groups | |||
| ≤60 years, median (IQR) | 1495 [686–3232] | 554 [304–1224] | 1769 [283–3394] |
| 61–70 years, median (IQR) | 1122 [482–2834] | 416 [189–836] | 1821 [990–4563] |
| >70 years, median (IQR) | 874 [351–2053] | 363 [128–852] | 2096 [890–9849] |
| Body mass index | |||
| <23 Kg/m2, median (IQR) | 898 [342–2577] | 363 [147–836] | 1831 [794–6545] |
| 23–28 Kg/m2, median (IQR) | 1155 [544–2838] | 493 [227–986] | 1791 [515–3822] |
| >28 Kg/m2, median (IQR) | 1229 [609–2853] | 460 [196–1136] | 2485 [818–5117] |
| Variables | Anti-Spike IgG 3rd Month (AU/mL) | p-Value | Anti-Spike IgG 6th Month (AU/mL) | p-Value | COVID-19 Recovered (AU/mL) | p-Value |
|---|---|---|---|---|---|---|
| Age Groups | ||||||
| <70 years, (n) median (IQR) | (190) 1380 (641–3021) | 0.002 | 712 (285–1774) | 0.003 | (95) 1807 (598–3945) | 0.038 |
| ≥70 years, (n) median (IQR) | (131) 838 (345–2053) | 684 (231–1949) | (88) 2096 (882–9872) | |||
| Body mass index | ||||||
| <30 Kg/m2, (n) median (IQR) | (268) 1083 (493–2813) | 451 (196–981) | 144–1817 (760–5330) | |||
| ≥30 Kg/m2, (n) median (IQR) | (53) 1210 (459–2607) | 0.975 | 469 (166–835) | 0.889 | 39–2621 (711–5166) | 0.689 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce, P.; Peralta, R.; Felix, C.; Pinto, C.; Pinto, B.; Matos, J.F. Vaccination against SARS-CoV-2 in Haemodialysis Patients: Spike’s Ab Response and the Influence of BMI and Age. Int. J. Environ. Res. Public Health 2022, 19, 10091. https://doi.org/10.3390/ijerph191610091
Ponce P, Peralta R, Felix C, Pinto C, Pinto B, Matos JF. Vaccination against SARS-CoV-2 in Haemodialysis Patients: Spike’s Ab Response and the Influence of BMI and Age. International Journal of Environmental Research and Public Health. 2022; 19(16):10091. https://doi.org/10.3390/ijerph191610091
Chicago/Turabian StylePonce, Pedro, Ricardo Peralta, Carla Felix, Carla Pinto, Bruno Pinto, and João Fazendeiro Matos. 2022. "Vaccination against SARS-CoV-2 in Haemodialysis Patients: Spike’s Ab Response and the Influence of BMI and Age" International Journal of Environmental Research and Public Health 19, no. 16: 10091. https://doi.org/10.3390/ijerph191610091
APA StylePonce, P., Peralta, R., Felix, C., Pinto, C., Pinto, B., & Matos, J. F. (2022). Vaccination against SARS-CoV-2 in Haemodialysis Patients: Spike’s Ab Response and the Influence of BMI and Age. International Journal of Environmental Research and Public Health, 19(16), 10091. https://doi.org/10.3390/ijerph191610091







