Occupational Exposure Assessment to Antineoplastic Drugs in Nine Italian Hospital Centers over a 5-Year Survey Program
Abstract
1. Introduction
2. Materials and Methods
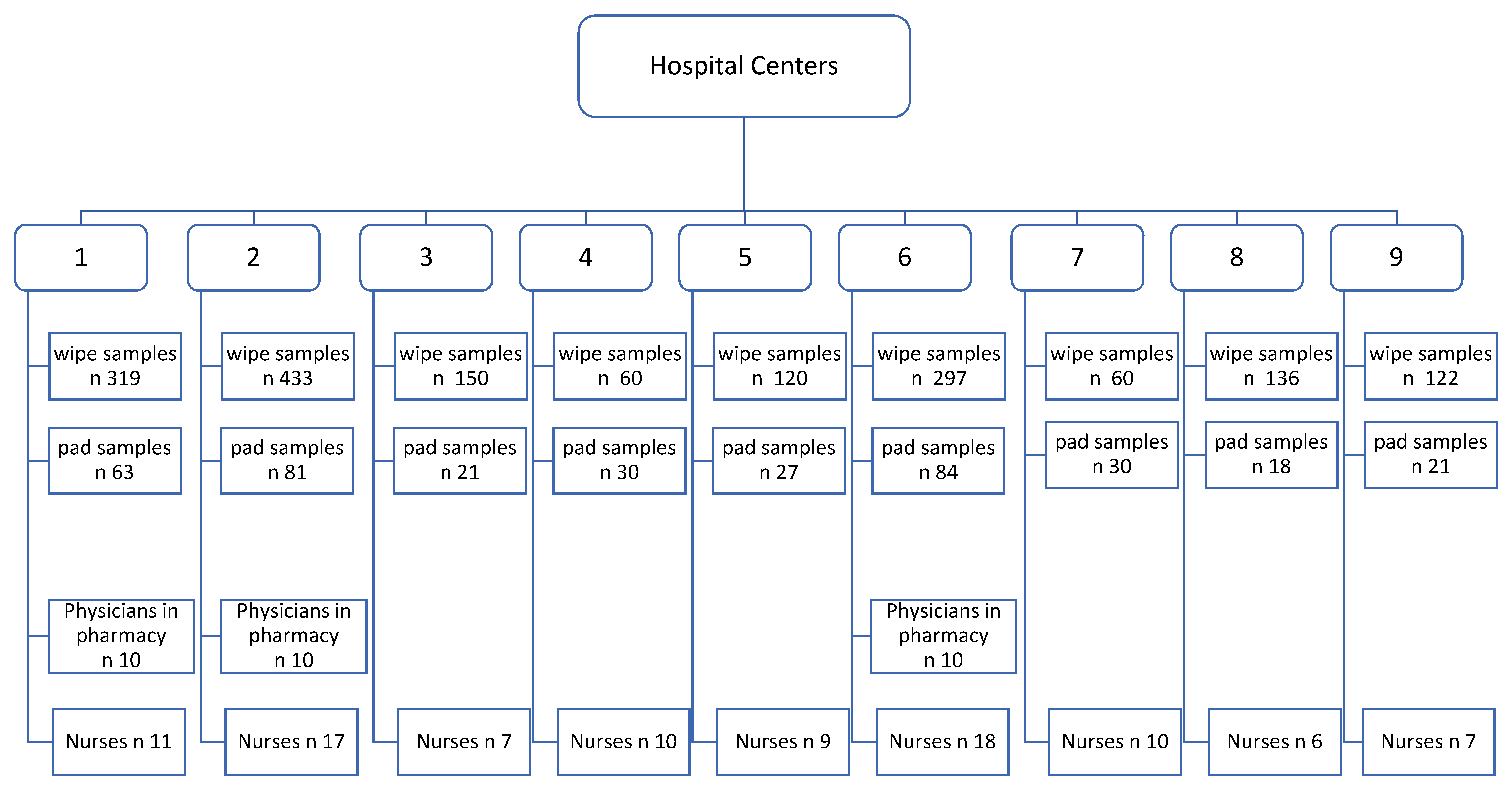
2.1. Study Design
2.2. Sampling Techniques
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. Frequency of Positive Data
3.1.1. Wipe Samples
3.1.2. Pad Samples
3.2. Pharmacy Areas
3.2.1. Wipe Samples
3.2.2. Pad Samples
3.3. Patient Care Units
3.3.1. Wipe Samples
3.3.2. Pad Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.H.; MacKenzie, B.A.; DeBord, D.G.; Trout, D.B.; O’Callaghan, J.P. NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016; DHHS (NIOSH) Publication Number 2016-161 (Supersedes 2014-138); U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2016.
- Dugheri, S.; Bonari, A.; Pompilio, I.; Boccalon, P.; Mucci, N.; Arcangeli, G. A New Approach to Assessing Occupational Exposure to Antineoplastic Drugs in Hospital Environments. Arh. Hig. Rada Toksikol. 2018, 69, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Palamini, M.; Gagné, S.; Caron, N.; Bussières, J.-F. Cross-Sectional Evaluation of Surface Contamination with 9 Antineoplastic Drugs in 93 Canadian Healthcare Centers: 2019 Results. J. Oncol. Pharm. Pract. 2020, 26, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Jeronimo, M.; Astrakianakis, G.; Apte, C.; Hon, C.-Y. Wipe Sampling Method and Evaluation of Environmental Variables for Assessing Surface Contamination of 10 Antineoplastic Drugs by Liquid Chromatography/Tandem Mass Spectrometry. Ann. Work Expo. Health 2017, 61, 1003–1014. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Mutation Research. Updated 18 April 2018. Available online: http://Monographs.Iarc.Fr/ENG/Classification/Index.Php (accessed on 6 June 2018).
- Simon, N.; Vasseur, M.; Pinturaud, M.; Soichot, M.; Richeval, C.; Humbert, L.; Lebecque, M.; Sidikou, O.; Barthelemy, C.; Bonnabry, P.; et al. Effectiveness of a Closed-System Transfer Device in Reducing Surface Contamination in a New Antineoplastic Drug-Compounding Unit: A Prospective, Controlled, Parallel Study. PLoS ONE 2016, 11, e0159052. [Google Scholar] [CrossRef]
- Simon, N.; Guichard, N.; Odou, P.; Decaudin, B.; Bonnabry, P.; Fleury-Souverain, S. Efficiency of Four Solutions in Removing 23 Conventional Antineoplastic Drugs from Contaminated Surfaces. PLoS ONE 2020, 15, e0235131. [Google Scholar] [CrossRef]
- Guichard, N.; Fekete, S.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Computer-Assisted UHPLC–MS Method Development and Optimization for the Determination of 24 Antineoplastic Drugs Used in Hospital Pharmacy. J. Pharm. Biomed. Anal. 2019, 164, 395–401. [Google Scholar] [CrossRef]
- Koller, M.; Böhlandt, A.; Haberl, C.; Nowak, D.; Schierl, R. Environmental and Biological Monitoring on an Oncology Ward during a Complete Working Week. Toxicol. Lett. 2018, 298, 158–163. [Google Scholar] [CrossRef]
- Soubieux, A.; Palamini, M.; Tanguay, C.; Bussières, J.-F. Evaluation of Decontamination Strategies for Cyclophosphamide. J. Oncol. Pharm. Pract. 2020, 26, 413–422. [Google Scholar] [CrossRef]
- Hilliquin, D.; Tanguay, C.; Bussières, J.-F. External Contamination of Commercial Containers by Antineoplastic Agents: A Literature Review. Eur. J. Hosp. Pharm. 2020, 27, 313–314. [Google Scholar] [CrossRef]
- Yu, E. Occupational Exposure in Health Care Personnel to Antineoplastic Drugs and Initiation of Safe Handling in Hong Kong: A Literature Review. J. Infus. Nurs. 2020, 43, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Odou, P.; Décaudin, B.; Bonnabry, P.; Fleury Souverain, S. Occupational Exposure to Conventional Antineoplastic Drugs: Can It Be Further Limited? Eur. J. Hosp. Pharm. 2020, 27, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Kiffmeyer, T.K.; Tuerk, J.; Hahn, M.; Stuetzer, H.; Hadtstein, C.; Heinemann, A.; Eickmann, U. Application and Assessment of a Regular Environmental Monitoring of the Antineoplastic Drug Contamination Level in Pharmacies—The MEWIP Project. Ann. Occup. Hyg. 2012, 57, 444–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sottani, C.; Grignani, E.; Oddone, E.; Dezza, B.; Negri, S.; Villani, S.; Cottica, D. Monitoring Surface Contamination by Antineoplastic Drugs in Italian Hospitals: Performance-Based Hygienic Guidance Values (HGVs) Project. Ann. Work Expo. Health 2017, 61, 994–1002. [Google Scholar] [CrossRef]
- Sessink, P.J. Environmental Contamination with Cytostatic Drugs: Past, Present and Future. Saf. Consid. Oncol. Pharm. 2011, 3, 3–5. [Google Scholar]
- Hedmer, M.; Wohlfart, G. Hygienic Guidance Values for Wipe Sampling of Antineoplastic Drugs in Swedish Hospitals. J. Environ. Monit. 2012, 14, 1968. [Google Scholar] [CrossRef]
- Schierl, R.; Böhlandt, A.; Nowak, D. Guidance Values for Surface Monitoring of Antineoplastic Drugs in German Pharmacies. Ann. Occup. Hyg. 2009, 53, 703–711. [Google Scholar] [CrossRef]
- Astrakianakis, G.; Jeronimo, M.; Griffiths, A.; Colombo, M.; Kramer, D.; Demers, P.A.; Hon, C.Y. The Application of Novel Field Measurement and Field Evaluation Protocols for Assessing Health Care Workers’ Exposure Risk to Antineoplastic Drugs. J. Occup. Environ. 2020, 17, 373–382. [Google Scholar] [CrossRef]
- Chauchat, L.; Tanguay, C.; Caron, N.; Gagné, S.; Labrèche, F.; Bussières, J. Surface Contamination with Ten Antineoplastic Drugs in 83 Canadian Centers. J. Oncol. Pharm. Pract. 2019, 25, 1089–1098. [Google Scholar] [CrossRef]
- Jeronimo, M.; Arnold, S.; Astrakianakis, G.; Lyden, G.; Stewart, Q.; Petersen, A.; Chambers, C.; Johnson, D.M.; Zimdars, E.; Kaup, H.; et al. Spatial and Temporal Variability in Antineoplastic Drug Surface Contamination in Cancer Care Centers in Alberta and Minnesota. Ann. Work Expo. Health 2021, 65, 760–774. [Google Scholar] [CrossRef]
- Chabut, C.; Tanguay, C.; Gagné, S.; Caron, N.; Bussières, J.-F. Surface Contamination with Nine Antineoplastic Drugs in 109 Canadian Centers; 10 Years of a Monitoring Program. J. Oncol. Pharm. Pract. 2022, 28, 343–352. [Google Scholar] [CrossRef] [PubMed]
- GURI: Provvedimento di Linee-Guida per la Sicurezza e la Salute dei Lavoratori Esposti a Chemioterapici Antiblastici in Ambiente Sanitario (Repertorio Atti No. 736). Available online: https://www.fnopi.it/wp-content/uploads/PROV050899.pdf (accessed on 20 June 2022).
- Negri, S.; Oddone, E.; Morandi, F.; Sottani, C.; Gardinali, F.; Lillo, A.; Pastoris, O.; Dacrema, V.; Losurdo, A.; Grignani, E.; et al. Validation of Cleaning Procedures Used in an Italian Hospital Pharmacy for Antineoplastic Drug Decontamination: A New Tool for Industrial Hygiene. Med. Lav. 2019, 110, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Spezia, S.; Bocca, B.; Forte, G.; Gatti, A.; Mincione, G.; Ronchi, A.; Bavazzano, P.; Alimonti, A.; Minoia, C. Comparison of Inductively Coupled Plasma Mass Spectrometry Techniques in the Determination of Platinum in Urine: Quadrupole vs. Sector Field. Rapid Commun. Mass Spectrom. 2005, 19, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Kibby, T. A review of surface wipe sampling compared to biologic monitoring for occupational exposure to antineoplastic drugs. J. Occup. Environ. Hyg. 2017, 14, 159–174. [Google Scholar] [CrossRef]
- Connor, T.H.; Celano, P.; Frame, J.N.; Zon, R.T. Summary of the Workshop on the Safe Handling of Hazardous Drugs Cohosted by the National Institute for Occupational Safety and Health and the American Society of Clinical Oncology. J. Oncol. Pract. 2017, 13, 199–205. [Google Scholar] [CrossRef]
- Labrèche, F.; Ouellet, C.; Roberge, B.; Caron, N.J.; Yennek, A.; Bussières, J.-F. Occupational Exposure to Antineoplastic Drugs: What about Hospital Sanitation Personnel? Int. Arch. Occup. Environ. Health 2021, 94, 1877–1888. [Google Scholar] [CrossRef]

| Years of the Study (2016–2021) | Hospital Center | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antineoplastic Drugs | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Qualitative Classification of Center size | Large | Large | Large | Medium | Small | Medium | Small | Small | Large | |
| Total number of beds | 545 | 567 | 223 | 77 | 32 | 76 | 42 | 48 | 244 | |
| Presence of Pharmacy Unit | yes | Yes | no | no | no | yes | no | no | no | |
| Number of Technicians Involved in Compounding | 7 | 6 | 8 | |||||||
| Presence of Patient Care Units | yes | Yes | yes | yes | yes | yes | yes | yes | yes | |
| Presence of Inpatient Beds | yes | Yes | yes | |||||||
| Presence of Outpatient Seats | yes | Yes | yes | yes | yes | yes | yes | yes | yes | |
| Average Amount (mg) of each Drug Prepared on the Sampling Day | CP 1 | 18,184 | 16,160 | 6840 | ||||||
| 5–FU 2 | 155,953 | 130,934 | 46,334 | |||||||
| GEM 3 | 66,332 | 58,377 | 18,858 | |||||||
| Pt 4 | 9358 | 8053 | 4688 | |||||||
| Number of Technicians Involved in Administration | 46 | 79 | 17 | 9 | 10 | 58 | 6 | 2 | 13 | |
| Average Number of Patients Treated per Day | 49 | 66 | 30 | 13 | 24 | 67 | 36 | 4 | 23 | |
| Closed System Transfer Device Used in the Last 6 Years | yes | Yes | yes | yes | yes | yes | yes | yes | yes | |
| Percent of Data Above LOD % | Total Number of Data (Positive Data) | ||||||
|---|---|---|---|---|---|---|---|
| Years of Study | CP 1 | 5–FU 2 | GEM 3 | Pt 4 | % > LOD 5 | ||
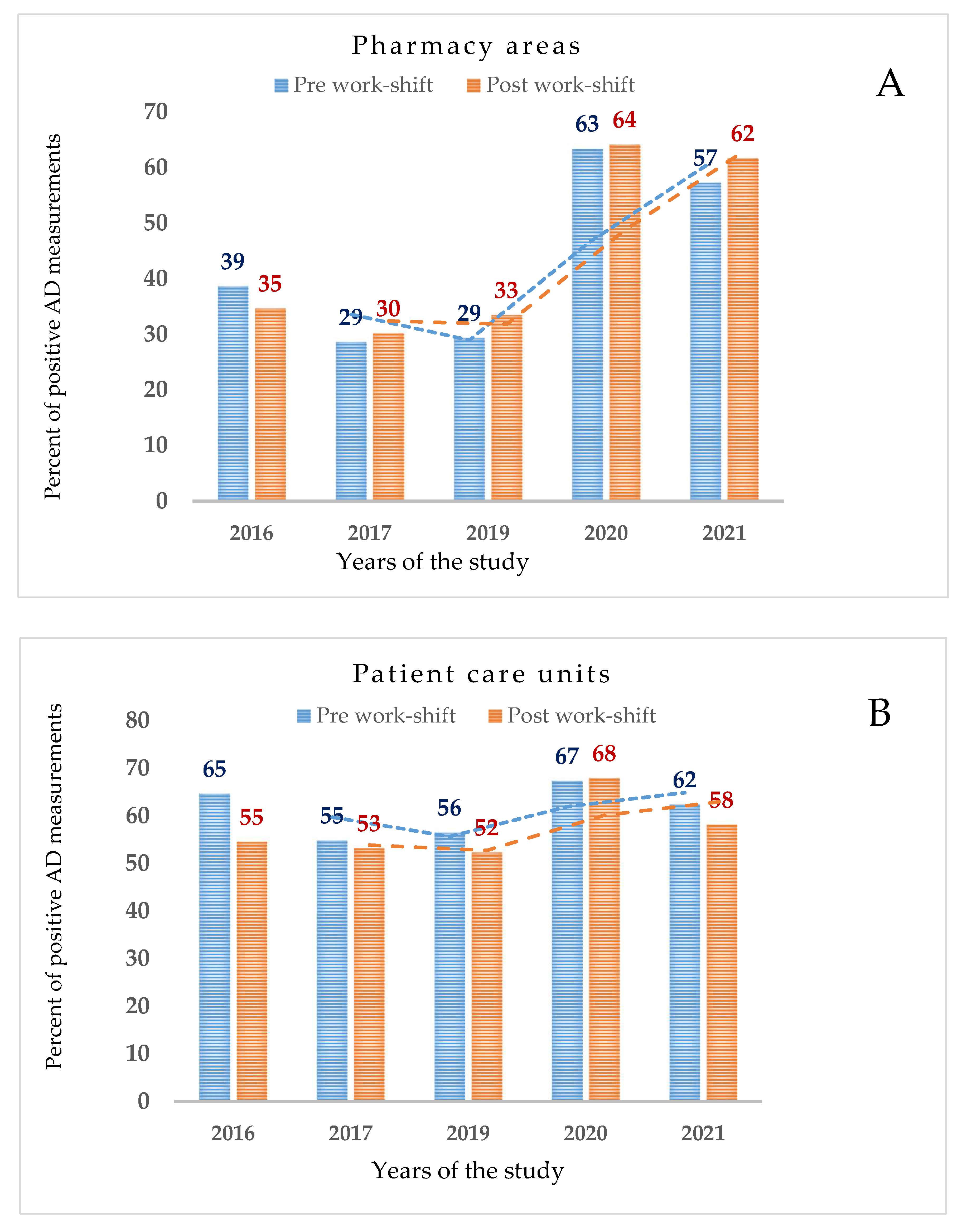
| Pharmacy Areas, n = 489 | 2016 | 42 | 22 | 36 | 47 | 352 (129) | 37 |
| 2017 | 24 | 20 | 35 | 39 | 384 (113) | 29 | |
| 2019 | 44 | 24 | 32 | 26 | 420 (132) | 31 | |
| 2020 | 75 | 54 | 83 | 43 | 420 (268) | 64 | |
| 2021 | 67 | 22 | 80 | 68 | 380 (226) | 59 | |
| 2016–2021 | 51 | 29 | 54 | 44 | |||
| Subtotal of data (positive data) | % > LOD 5 | ||||||
| 2016–2021 | 489 (249) | 489 (141) | 489 (263) | 489 (215) | 1956 (868) | 44 | |
| Percent of data above LOD % | Total number of data (positive data) | ||||||
| Years of study | CP1 | 5–FU 2 | GEM 3 | Pt 4 | % > LOD 5 | ||
| Patient Care Units, n = 1208 | 2016 | 68 | 25 | 67 | 78 | 928 (553) | 60 |
| 2017 | 44 | 31 | 69 | 72 | 944 (510) | 54 | |
| 2019 | 64 | 42 | 62 | 50 | 988 (538) | 54 | |
| 2020 | 77 | 55 | 78 | 62 | 844 (571) | 68 | |
| 2021 | 68 | 19 | 77 | 77 | 1128 (681) | 60 | |
| 2016–2021 | 64 | 33 | 77 | 68 | |||
| Subtotal of data (positive data) | % > LOD 5 | ||||||
| 2016–2021 | 1208 (776) | 1208 (401) | 1208 (852) | 1208 (824) | 4832 (2853) | 59 | |
| Percent of Data above LOD % | Total Number of Data (Positive Data) | ||||||
|---|---|---|---|---|---|---|---|
| Years of Study | CP 1 | 5–FU 2 | GEM 3 | Pt 4 | % > LOD 5 | ||
| Pharmacy Areas, n = 93 | 2016 | 6 | 28 | 28 | 22 | 72 (15) | 21 |
| 2017 | 11 | 33 | 39 | 6 | 72 (16) | 22 | |
| 2019 | 38 | 48 | 33 | 19 | 84 (29) | 35 | |
| 2020 | 11 | 11 | 33 | 6 | 72 /11) | 15 | |
| 2021 | <LOD | 28 | 56 | 22 | 72 (19) | 26 | |
| 2016–2021 | 14 | 30 | 38 | 15 | |||
| Subtotal data (positive data) | % > LOD | ||||||
| 2016–2021 | 93 (13) | 93 (28) | 93 (35) | 93 (14) | 372 (90) | 24 | |
| Percent of Data above LOD % | Total Number of Data (Positive data) | ||||||
| Years of study | CP 1 | 5–FU 2 | GEM 3 | Pt 4 | % > LOD 5 | ||
| Patient Care Units, n = 282 | 2016 | 16 | 7 | 17 | 7 | 300 (35) | 12 |
| 2017 | 7 | 10 | 25 | 9 | 276 (35) | 13 | |
| 2019 | 4 | 9 | 18 | <LOD | 180 (14) | 8 | |
| 2020 | 12 | 6 | 36 | 3 | 132 (19) | 14 | |
| 2021 | 7 | 3 | 10 | 3 | 240 (14) | 6 | |
| 2016–2021 | 10 | 7 | 20 | 5 | |||
| Subtotal data (positive data) | % > LOD 5 | ||||||
| 2016–2021 | 282(27) | 282 (20) | 282 (56) | 282 (14) | 1128 (117) | 10 | |
| Surface Concentration for Each Drug in Pharmacy Areas | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP 1 (ng/cm2) | 5–FU 2 (ng/cm2) | GEM 3 (ng/cm2) | Pt 4 (ng/cm2) | ||||||||||
| Sampling Location | 75th | 90th | Max | 75th | 90th | Max | 75th | 90th | Max | 75th | 90th | Max | |
| BSC work surface * n = 672 | 0.001 | 0.002 | 0.010 | 0.278 | 1.375 | 9.270 | 0.005 | 0.014 | 0.677 | 0.006 | 0.017 | 0.177 | |
| Checking counter n=152 | 0.002 | 0.004 | 0.012 | 0.013 | 0.019 | 0.022 | 0.002 | 0.016 | 0.019 | 0.002 | 0.005 | 0.008 | |
| Handles | Passthrough handle (inside cleanroom) n = 104 | 0.019 | 0.118 | 0.300 | 0.553 | 1.097 | 1.460 | 0.062 | 0.100 | 2.700 | 0.075 | 0.118 | 0.431 |
| Door handle (inside) n = 120 | 0.007 | 0.007 | 0.010 | 0.333 | 0.333 | 0.333 | 0.006 | 0.007 | 0.007 | 0.007 | 0.007 | 0.018 | |
| Fridge handle (inside) n = 48 | 0.021 | 0.031 | 0.050 | 0.508 | 0.742 | 0.898 | 0.008 | 0.013 | 0.017 | 0.015 | 0.019 | 0.022 | |
| Floors | Floor in front of BSC n = 160 | 0.056 | 0.167 | 0.423 | 0.065 | 0.125 | 5.974 | 0.028 | 0.050 | 0.107 | 0.004 | 0.066 | 0.129 |
| Floor in cleanroom n = 96 | 0.011 | 0.014 | 0.022 | 0.030 | 0.210 | 0.250 | 0.005 | 0.020 | 0.045 | 0.005 | 0.005 | 0.005 | |
| Floor in front of cleanroom n = 32 | <LOD | <LOD | <LOD | 0.081 | 0.095 | 0.104 | <LOD | <LOD | <LOD | 0.001 | 0.001 | 0.001 | |
| Floor in anteroom n = 120 | 0.028 | 0.039 | 0.092 | 0.062 | 0.083 | 0.083 | 0.009 | 0.027 | 0.038 | 0.006 | 0.020 | 0.062 | |
| Floor in front pass-through n = 24 | 0.001 | 0.001 | 0.001 | <LOD | <LOD | <LOD | 0.012 | 0.018 | 0.022 | 0.002 | 0.002 | 0.002 | |
| Trays | Trays used for drug delivery n = 152 | 0.020 | 0.032 | 0.045 | 0.084 | 0.289 | 1.735 | 0.002 | 0.025 | 0.193 | 0.012 | 0.039 | 0.043 |
| Trays/countertops used at the nurse’s station for documentation n = 96 | 0.003 | 0.007 | 0.021 | 0.012 | 0.026 | 0.084 | 0.001 | 0.003 | 0.010 | 0.0001 | 0.001 | 0.007 | |
| Infusion bag surface n = 44 | 0.001 | 0.001 | 0.001 | 0.052 | 0.132 | 0.132 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Mouse PC n = 32 | 0.007 | 0.008 | 0.008 | 0.160 | 0.200 | 0.227 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | |
| Barcode surface n = 32 | 0.005 | 0.015 | 0.080 | 0.088 | 0.460 | 0.550 | 0.022 | 0.057 | 0.708 | 0.004 | 0.052 | 0.523 | |
| Subtotal n = 489 (1956 data) | 0.007 | 0.026 | 0.423 | 0.107 | 0.346 | 9.270 | 0.007 | 0.028 | 2.700 | 0.006 | 0.026 | 0.523 | |
| Surface Concentration for Each Drug in Patient Care Units | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP 1 (ng/cm2) | 5–FU 2 (ng/cm2) | GEM 3 (ng/cm2) | Pt 4 (ng/cm2) | ||||||||||
| Sampling Location | 75th | 90th | Max | 75th | 90th | Max | 75th | 90th | Max | 75th | 90th | Max | |
| Counters | Countertop used for validation n = 76 | <LOD | 0.001 | 0.001 | 0.013 | 0.029 | 0.040 | 0.004 | 0.015 | 0.020 | 0.001 | 0.005 | 0.011 |
| Countertop used for deposition of ready-to-use bags n = 664 | 0.002 | 0.007 | 0.201 | 0.066 | 0.260 | 112.500 | 0.004 | 0.013 | 0.117 | 0.003 | 0.005 | 0.058 | |
| Bedside table used by patients n = 256 | 0.005 | 0.021 | 0.097 | 0.198 | 0.356 | 0.440 | 0.017 | 0.038 | 0.296 | 0.007 | 0.013 | 0.039 | |
| Pole for infusion bags n = 704 | 0.038 | 0.742 | 27.023 | 0.532 | 1.464 | 5.560 | 0.049 | 0.184 | 4.753 | 0.020 | 0.071 | 0.986 | |
| Bed bell surface used by patient n = 16 | 0.233 | 0.256 | 0.271 | <LOD | <LOD | >LOD | 0.020 | 0.021 | 0.022 | 0.001 | 0.001 | 0.001 | |
| Armrest of patient treatment chair n = 104 | 0.024 | 0.109 | 0.187 | 0.319 | 0.892 | 2.678 | 0.004 | 0.077 | 0.351 | 0.027 | 0.943 | 1.411 | |
| Touch-screen of the perfusion pump n = 244 | 0.011 | 0.018 | 0.125 | 0.099 | 0.243 | 2.269 | 0.027 | 0.087 | 0.543 | 0.006 | 0.031 | 0.173 | |
| Trays used for drug delivery n = 88 | 0.003 | 0.036 | 0.621 | 5.560 | 6.666 | 7.403 | 0.001 | 0.002 | 0.013 | < LOD | < LOD | < LOD | |
| Tablet touch-screen n = 144 | 0.003 | 0.004 | 0.004 | 0.134 | 0.327 | 0.574 | 0.008 | 0.023 | 0.069 | 0.003 | 0.004 | 0.005 | |
| Barcode surface n = 324 | 0.014 | 0.080 | 0.257 | 0.119 | 0.634 | 6.825 | 0.013 | 0.036 | 0.430 | 0.001 | 0.002 | 0.004 | |
| Mouse PC n = 52 | 0.056 | 0.110 | 0.146 | 0.672 | 1.533 | 2.108 | 0.046 | 0.049 | 0.051 | 0.002 | 0.002 | 0.002 | |
| Floors | Floor in front of pole n = 580 | 0.012 | 0.056 | 0.924 | 0.141 | 0.264 | 3.016 | 0.028 | 0.085 | 0.424 | 0.025 | 0.062 | 0.246 |
| Floor in restroom n = 496 | 0.017 | 0.068 | 1.208 | 0.202 | 1.463 | 9.154 | 0.239 | 0.721 | 12.998 | 0.314 | 1.153 | 4.913 | |
| Floor in front of restroom n = 168 | 0.018 | 0.099 | 0.634 | 0.135 | 0.273 | 1.454 | 0.088 | 0.162 | 1.345 | 0.196 | 0.454 | 1.948 | |
| Floor in patient room n = 224 | 0.004 | 0.007 | 0.015 | 0.096 | 0.200 | 0.361 | 0.017 | 0.047 | 0.627 | 0.018 | 0.040 | 0.118 | |
| Floor in nursing room n = 172 | 0.003 | 0.008 | 0.054 | 0.029 | 0.137 | 0.348 | 0.007 | 0.012 | 0.103 | 0.004 | 0.033 | 0.066 | |
| Floor in storage room n = 188 | 0.003 | 0.011 | 0.205 | 0.247 | 0.300 | 0.322 | 0.037 | 0.092 | 1.107 | 0.040 | 0.082 | 0.264 | |
| Handles | Handle in restroom (inside) n = 152 | 0.095 | 0.280 | 9.908 | 0.417 | 0.417 | 0.417 | 0.039 | 0.214 | 0.508 | 0.017 | 0.037 | 0.206 |
| Fridge handle (inside) at the nurse station n = 88 | 0.008 | 0.017 | 0.028 | 0.088 | 0.088 | 0.088 | 0.008 | 0.019 | 0.023 | 0.002 | 0.005 | 0.006 | |
| Toilet surface in restroom n = 48 | 0.005 | 0.006 | 0.006 | 0.031 | 0.042 | 0.050 | 0.016 | 0.323 | 0.536 | 0.096 | 0.107 | 0.121 | |
| Subtotal n = 1208 (4832 data) | 0.012 | 0.070 | 27.023 | 0.191 | 0.443 | 112.500 | 0.034 | 0.146 | 12.998 | 0.033 | 0.141 | 4.913 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sottani, C.; Grignani, E.; Cornacchia, M.; Negri, S.; Cuna, F.S.R.d.; Cottica, D.; Bruzzese, D.; Severi, P.; Strocchi, D.; Verna, G.; et al. Occupational Exposure Assessment to Antineoplastic Drugs in Nine Italian Hospital Centers over a 5-Year Survey Program. Int. J. Environ. Res. Public Health 2022, 19, 8601. https://doi.org/10.3390/ijerph19148601
Sottani C, Grignani E, Cornacchia M, Negri S, Cuna FSRd, Cottica D, Bruzzese D, Severi P, Strocchi D, Verna G, et al. Occupational Exposure Assessment to Antineoplastic Drugs in Nine Italian Hospital Centers over a 5-Year Survey Program. International Journal of Environmental Research and Public Health. 2022; 19(14):8601. https://doi.org/10.3390/ijerph19148601
Chicago/Turabian StyleSottani, Cristina, Elena Grignani, Marco Cornacchia, Sara Negri, Francesco Saverio Robustelli della Cuna, Danilo Cottica, Dario Bruzzese, Paolo Severi, Daniele Strocchi, Giovanni Verna, and et al. 2022. "Occupational Exposure Assessment to Antineoplastic Drugs in Nine Italian Hospital Centers over a 5-Year Survey Program" International Journal of Environmental Research and Public Health 19, no. 14: 8601. https://doi.org/10.3390/ijerph19148601
APA StyleSottani, C., Grignani, E., Cornacchia, M., Negri, S., Cuna, F. S. R. d., Cottica, D., Bruzzese, D., Severi, P., Strocchi, D., Verna, G., Leso, V., & Iavicoli, I. (2022). Occupational Exposure Assessment to Antineoplastic Drugs in Nine Italian Hospital Centers over a 5-Year Survey Program. International Journal of Environmental Research and Public Health, 19(14), 8601. https://doi.org/10.3390/ijerph19148601












