Abstract
Wastewater surveillance systems have become an important component of COVID-19 outbreak monitoring in high-income settings. However, its use in most low-income settings has not been well-studied. This study assessed the feasibility and utility of wastewater surveillance system to monitor SARS-CoV-2 RNA in Addis Ababa, Ethiopia. The study was conducted at nine Membrane Bio-reactor (MBR) wastewater processing plants. The samples were collected in two separate time series. Wastewater samples and known leftover RT-PCR tested nasopharyngeal swabs were processed using two extraction protocols with different sample conditions. SARS-CoV-2 wastewater RT-PCR testing was conducted using RIDA GENE SARS-CoV-2 RUO protocol for wastewater SARS-CoV-2 RNA testing. Wastewater SARS-CoV-2 RNA RT-PCR protocol adaptation, optimization, and detection were conducted in an Addis Ababa, Ethiopia context. Samples collected during the first time series, when the national COVID-19 case load was low, were all negative. Conversely, samples collected during the second time series were all positive, coinciding with the highest daily reported new cases of COVID-19 in Ethiopia. The wastewater-based SARS-CoV-2 surveillance approach is feasible for Addis Ababa. The COVID-19 wastewater based epidemiological approach can potentially fill the evidence gap in distribution and dynamics of COVID-19 in Ethiopia and other low-income settings.
1. Introduction
Despite the remarkable worldwide advancements in capability for early case detection, implementation of COVID-19 vaccination programs, and extensive invigoration of non-pharmacological preventive measures [1], the COVID-19 global pandemic is ongoing, and in many countries, the incidence of infections is on the rise again. Considering this fact, some scholars are speculating the potential coexistence of the world with COVID-19 disease as a new normal [2].
As of 29 June 2022, an estimate of the Johns Hopkins University depicts globally more than half a billion cumulative cases of COVID-19, more than 6 million deaths, and at the same time, more than 11 billion administered vaccine doses [3,4]. Ethiopia performed a cumulative of around 5 million COVID-19 tests as of 29 June 2022, corresponding with less than 5% of its total population. During the same period, the country reported 488,108 confirmed cases, 7535 COVID-19-related deaths, and about 42 million administered doses of the vaccine [3,5]. Due to a presumably low detection rate, the actual incidence of COVID-19 cases in Ethiopia may be much higher than the reported one. A recent sero-epidemiological survey among health care workers and communities in Addis Ababa and Jimma has indicated a sero-prevalence of more than 70%, indicating that cases remain largely undetected [6]. More than half of these cases and about 62% of COVID-19-related deaths in Ethiopia were reported from Addis Ababa, home to less than 5% of the national population [7]. Besides the understandable high burden of the cases and deaths in urban metropolitans, the observed COVID-19 burden in the capital may indicate a testing bias towards large cities with relatively better access to testing infrastructure. The true epidemiological picture of the pandemic is thus likely to be greatly different from what has officially been reported, as could be demonstrated by serological studies [6].
During early stages of the pandemic, the global COVID-19 control strategy has focused on non-pharmacological measures mainly targeting individuals [8]. Compliance to these recommended public health measures is highly dependent on the individual level of understanding, willingness, behavior, and available resources. In addition, epidemiological data, which has been used to estimate virus circulation, transmission and incidence, has been found to be highly dependent on the currently applied testing strategies and measures such as lock-downs or the closure of schools. The search for alternatives for estimations of virus circulation has brought about the investigation of urban wastewater, which allows an early detection of virus circulation in a given catchment area, and even a geographic mapping of transmission, based on the sampling at different wastewater collection points [9,10,11,12,13,14,15,16,17,18,19,20,21].
This COVID-19 wastewater-based epidemiology approach has created an opportunity to understand the distribution and dynamics of the disease in defined sewage catchment areas. The COVID-19 mitigation strategies can therefore be differentially targeted to communities where sewage samples can indicate outbreaks early on in a given catchment area. However, most of the published data on wastewater epidemiology was generated from higher income settings, such as Europe, Australia, the United States (US), China, India, Japan, and South Africa, the only country from Africa [9,10,11,12,13,14,15,16,17,18,19,20,21]. Arguably, evidence generated from high-income countries might not be directly applicable for low-resource settings due to differences in geo-climatic and cultural aspects, wastewater composition, and the applied technologies in the wastewater management system.
Wastewater-based epidemiology of infectious diseases is not a new concept. It has been utilized for enteric viruses as an early warning system [22]. With the emergence of the COVID-19 pandemic, it has now received large global attention for its simplicity and cost effectiveness [10,11,12,13,14,15,16,17,18,19,20,21,22]. Apart from the manuscripts mentioned above, there are also many articles including reviews addressing critical issues related to waste management, pollution, policy and regulations in China, Indonesia, Bangladesh, India and Malaysia during the era of the COVID-19 pandemic [23,24,25,26].
We believe that the capability for detection and monitoring of SARS-CoV-2 RNA from wastewater systems in low- and middle-income-countries (LMICs) such as Ethiopia would not only mend the evidence gaps about the true burden of COVID-19, but it would also generate the capacity to monitor other infectious diseases with enteric excretion in the future.
Thus, this study aims to assess the feasibility of the approach, build a wastewater-based epidemiology capacity, and to give first insights into data generated for the assessment and monitoring of SARS-CoV-2 RNA from wastewater management plants in Addis Ababa, Ethiopia.
2. Materials and Methods
2.1. Study Setting
Addis Ababa is the capital city of Ethiopia, with an estimated population of 5.2 million as of 2022 [27]. Administratively, it comprises 11 sub-cities. There are two central wastewater treatment ponds that are connected by a sewage network, serving a catchment population of about 10% of the total urban population. In addition, there are 14 decentralized wastewater processing plants at different condominium sites [28]. Addis Ababa Water and Sewerage Authority (AAWSA) is the governing body of the municipal wastewater system.
The rapid booming of the city with common housing projects (mostly condominium construction) in the past 15 years forced AAWSA to plant containerized Membrane Bioreactors as wastewater treatment plants. These plants are designed to transform dissolved and particulate constituents into less hazardous end products by combining conventional activated sludge processes with membrane separation. The principle and detailed operations of MBR units is described elsewhere [29].
The MBR units in Addis Ababa have been serving the wastewater processing need of fourteen residential complexes. The units have a treatment capacity of 20,000 cubic meter of wastewater per day [29]. For this study, we included nine MBR units representing 64% of the total units planted in Addis Ababa (Figure 1).
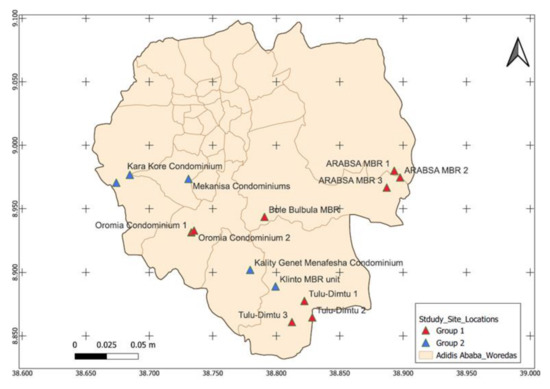
Figure 1.
Map of decentralized MBR units in Addis Ababa, where group 1 represents MBR units included in this study and group 2 represents MBR units not included in the current study. Woredas are geographical units in Addis Ababa. Source: this map is created by Chaile Mulu (Geospatial Epidemiologist at National Data Management Center, Ethiopian Public Health Institute).
2.2. Waste Water Sample Collection
Wastewater samples from participating MBR units were collected longitudinally and in two separate time series using an integrated sampling method. In the first series, the inlet wastewater samples were collected during a period of 25 October 2020 to 13 December 2020 every Sunday morning between 7:00 AM and 9:00 AM. The once weekly rhythm at the same time period was chosen in order to reduce time dependent variations in the use of water- and sewage-infrastructure by the population. On Sunday mornings in particular, most inhabitants in Addis Ababa are at home; thus, the sewage collected from residential areas is dominated by the wastewater generated in the private toilets. The average distance from private toilet to MBR units is three km, resulting in short flow times from the sink to the treatment plant. Accordingly, the sewage is not hermetically closed; thus, there is a possibility of wastewater to be mixed with rain water in sewage lines on the way to the MBR. The samples (300 mL) were collected as qualified spot samples in duplicates and transported to St. Paul’s Hospital Millennium Medical College (SPHMMC) using an ice-loaded cool box. At SPHMMC, the samples were transferred to −80 °C freezers immediately and later brought to the National Animal Health and Diagnostic Investigation Center (NAHDIC).
In the second time series, the wastewater samples collection was resumed for three weekly time points from 25 December 2021 to 9 January 2022 to acquire some information about the epidemiology of the fourth COVID-19 wave in Ethiopia and compare the inlet and aeration samples for viral nucleic acid recovery. The samples were collected using cool boxes with ice packs and processed freshly within 24 h at NAHDIC.
The NAHDIC is the referral and reference veterinary medicine laboratory in Ethiopia. It is located in Sebeta, 25 km southwest of Addis Ababa. It is the center of excellence for animal disease surveillance, investigation, diagnosis, and research. NAHDIC has implemented an ISO/IEC 1725 quality system [30]. It should be noted here that there was no sample collection for eleven months between the two time series data collection points. This is due to the fact that the main goal of this study is to assess the feasibility of a COVID-19 wastewater-based epidemiology in an Ethiopian (low resource settings) context. Despite the resource constraints, we did our best to collect data that covers low and high COVID-19 community transmission time points to generate representative data. Furthermore, it is not the intent of this study to understand the dynamics of SARS-CoV-2 RNA concentration in wastewater throughout the two year period of the COVID-19 pandemic in Ethiopia. Thus, to balance the limited resources we have while generating scientifically valid representative data, the time points between January–November 2021 were not included.
Wastewater Sample Processing and RNA Concentration
Fresh wastewater samples were processed within 24 h of collection. Frozen samples were thawed on ice and subsequently processed. First, the samples were sieved using gauze to separate sludge from liquid. 50 mL of sieved wastewater samples were transferred to Corning falcon tubes and centrifuged at 2500× g for 20 min at 4 °C using a Thermo Scientific JOUAN CR4i centrifuge [9]. Thirty-eight mL of the supernatant was transferred to ultracentrifuge tubes and placed in a Beckman Coulter Avanti JXN-30 ultracentrifuge using the JA-25.50 rotor. The tubes were centrifuged at a speed of 27,000× g at 4 °C for one hour [9]. The supernatants were discarded carefully using a 30 mL automatic biuret. The pellets were re-suspended in 500 μL nuclease free water provided in the QIAGEN kit and immediately transferred to the molecular laboratory for RNA extraction.
2.3. RNA Extraction
RNA extraction was executed using an Allprep powerVial DNA/RNA extraction kit (QIAGEN, Hilden, Germany) and QIAamp Viral RNA Mini kit (QIAGEN, Hilden, Germany) following the manufacturer’s instruction [31,32].
2.4. Master Mix and RT-PCR Test
The master mix was prepared following BGI and RIDA GENE SARS-CoV-2 RUO (r-biopharm, Darmstadt, Germany) RT-PCR testing protocols depending on the experiment types. As per BGI (Shenzhen, China) protocol [33], a master mix for a single RT-PCR reaction was prepared by mixing 18.5 μL of reaction mix (including reagent for amplification, probes, primers targeted SARS-CoV-2 ORF1ab gene, ICR) with 1.5 μL of enzyme mix [33]. The 20 μL of master mix was dispensed in each reaction well including the no template (negative) control and the positive control (standard). Finally, 10 μL of no template control, wastewater eluate and positive control were added in the respective designated reaction wells [33]. The reaction wells were sealed and briefly centrifuged before they were placed for amplification into an ABI 7500 RT-PCR system. The ABI 7500 RT-PCR was programmed to run one cycle at 50 °C for 20 min, one cycle at 95 °C for 10 min, for 40 cycles (95 °C for 15 s and 60 °C for 30 s) [33].
RIDA GENE SARS-CoV-2 RUO (r-biopharm, Darmstadt, Germany) master mix was prepared by mixing 19.3 μL of reaction mix (including primer for target E-gene), 0.7 μL of Taq-Polymerase, and 1 μL of ICR [34]. Twenty-one μL of the master mix was dispensed in each reaction wells including no template and positive control wells. Five μL of each no template control, wastewater eluate, and positive control was dispensed in each respective reaction well with master mix [34].
The micro-well plate was sealed and loaded on Applied Biosystems Real-Time PCR Instruments (ABI 7500) with the following PCR profile; Reverse transcription 10 min, 58 °C, initial denaturation 1 min, 95 °C, cycles 45 (PCR denaturation 15 s, 95 °C, annealing/extension 30 s, 60 °C), and temperature transition rate/ramp rate: maximum.
2.5. RT-PCR Signal Detection
The detection channel was set as Fluorescein amides (FAM) for SARS-CoV-2 RNA and Victoria (VIC) for ICR (Internal Control Reaction) for both BGI and RIDA master mix tests, as suggested by the manufacturer of the assays. The auto cycle threshold (Ct) and baseline functions of the ABI 7500 Fast Real-Time PCR System software version 1.4, Singapore were used to analyze the data.
2.6. Quality Control and Interpretation of the PCR Result
All RT-PCR readings of this study were quality assured and interpreted using the criteria described in Table 1. The contents of the table are summarized from the RT-PCR testing result interpretation recommendations of the BGI (Shenzhen, Guangdong, China) and r-biopharm (Darmstadt, Germany) [33,34] RT-PCR testing protocols.

Table 1.
Quality control indicators and interpretation of the PCR readings.
3. Results
3.1. Optimization of SARS-CoV-2 Wastewater RT-PCR Testing
Adaptation, customization, and optimization of wastewater SARS-CoV-2 RNA RT-PCR testing to the local context was conducted. For this purpose, a total of six different trials were performed using two different extractions and SARS-CoV-2 RNA RT-PCR testing protocols at different sampling conditions. The experiment conditions and observed results were presented in Table 2. Detailed description of the experiments was attached as an appendix at the end of the manuscript (Appendix A).

Table 2.
Detailed descriptions of RNA extraction and PCR testing performed to optimize wastewater COVID-19 RNA detection in Addis Ababa.
3.2. Stored and Fresh Wastewater Processing Result
The 72 inlet wastewater samples collected from nine MBR processing plants were RT-PCR tested. The samples were collected over 8 weeks from 25 October 2020 to 13 December 2020 and stored in −80 °C freezer. SARS-CoV-2 RNA was not detected from any of these stored wastewater samples. To give more insight, we present the number of daily COVID-19 new cases and the positivity rate during 8 weeks of this research data collection period (Figure 2). Between October–December 2020, the average daily COVID 19 new cases and positivity rate was 478 and 9%, respectively (Figure 2).
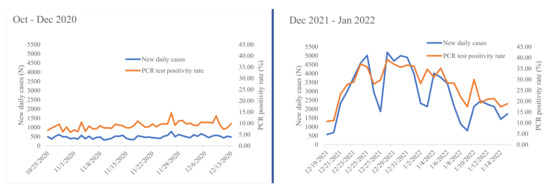
Figure 2.
Daily new COVID-19 cases and RT-PCR test positive rate of Ethiopia as reported by the Ministry of Health during our study periods of 19 October to 13 December 2020 and 19 December 2021 to 15 January 2022 (data source: Ministry of Health-Ethiopia daily COVID-19 testing period).
Most (88.9%) of the inlet fresh wastewater samples collected from 25 December 2021 to 9 January 2022 and processed within 24 h of collection were positive for SARS-CoV-2 RNA (Table 3). Furthermore, the positivity was maintained for three consecutive weeks. According to Ethiopian Ministry of Health data, the average number of daily new COVID-19 cases and positivity rates during this second wastewater collection time period have increased to 2874 and 27%, respectively (Figure 2). On the other hand, all fresh wastewater samples collected from the aeration tank during the aforementioned data collection dates at similar conditions were negative for SARS-CoV-2 RNA (Table 3).

Table 3.
SARS-CoV-2 PCR test result of fresh wastewater inlet and aeration samples collected from 9 MBR units at Addis Ababa between 25 December 2021 to 9 January 2022.
4. Discussion
In this study, an optimization of the COVID-19 wastewater PCR protocol to fit with the local context was performed successfully. The major challenge during optimization was inhibition. The identification of the types of inhibitors present in the studied Ethiopian wastewater system was beyond the scope of this study. However, wastewater PCR inhibition is usually associated with presence of debris, fulmic acids, metal ions, polyphenol, and high activity of RNase enzymes [35,36]. These chemical compounds can interfere with PCR testing through different mechanisms including degradation of the target nucleic acids [36]. Nonetheless, our finding indicates that wastewater-based surveillance can be used to monitor infectious disease outbreak in settings where the traditional disease surveillance system is difficult due to limited resources for laboratory testing.
All wastewater samples collected at an early stage of the pandemic and stored in −80 °C freezers for more than one year were negative. We can only speculate about the reasons. First of all, during the early stage of the pandemic in Ethiopia, the concentration of SARS-CoV-2 RNA in wastewater might have been just simply below the detection limit of PCR testing. This is directly linked with the number of COVID-19 infected individuals shedding the virus in the sewage catchment area during the data collection period. At that time, the maximum numbers of cases detected weekly in Ethiopia were 4206 [5]. Given this, the Ministry of Health report includes all cases detected in the country; the number of cases detected from Addis Ababa only was obviously lower than the reported weekly number. Thus, the chance of detecting SARS-CoV-2 RNA from wastewater is very low. The usage of gauze to filter the sludge and slow thawing of the stored samples may also contribute for the loss of viral particles or RNA. In addition, the inherent risk of RNA degradation during storage especially in low resource settings with repeated power interruption could not be ignored, even though we are not aware of any such incidence with regard to our used storage facility.
In this study, we have detected SARS-CoV-2 RNA for three consecutive weeks during the second time series of data collection. At this time period of data collection, the number of weekly COVID-19 cases detected from Ethiopia was more than three times higher than the first time series cases (Figure 2). According to Minster of Health data, the highest ever number of weekly detected case in the country was reported between 26 December 2021 and 1 January 2022. This coincided with the second week of our second wastewater data collection period. During this week, a total of 28,590 weekly cases were detected from Ethiopia [5]. Considering 55% of the total detected cases in Ethiopia are from Addis Ababa [7], a calculated estimate of 15,725 cases were reported from Addis Ababa. Recent published evidence estimated that the minimum number of SARS-CoV-2 infected cases needed to detect the viral RNA in wastewater ranged from a 253 to 409/10,000 population [37]. However, the highest 15,725 cases detected from Addis Ababa is only equivalent to 30 cases per 10,000 population considering the population size of Addis Ababa as 5.2 million [27]. This rate is at least eight times lower than the minimum SARS-CoV-2 detection threshold limit reported previously [37]. This data indicates that there are a significant number of COVID-19 cases in the community without being detected by implemented testing strategy of the country. Determining the minimum number of COVID-19-infected cases expected in the community to detect SARS-CoV-2 RNA from wastewater management plants in Addis Ababa was not in the scope of this study. However, generating such evidence would help to forecast the minimum number of SARS-CoV-2 infections in the community.
Several studies have reported that COVID-19 wastewater-based epidemiological approaches provide indirect information about the burden of COVID-19 in a defined community [10,11,12,13,14,15,16,17,18,19,20]. The approach provides a proxy indicator for the concentration of RNA shed by infected individuals at different stages of the disease, including even pre-symptomatic and asymptomatic cases [38]. Accordingly, an increase or a decrease in wastewater viral concentration can be inferred to the number of new or resolved SARS-CoV-2 infections in the community. This may allow tailoring prevention strategies to fit the local context considering chronological dynamics and spatial distribution. In addition, the periodic wastewater monitoring of COVID-19 supplemented with SARS-CoV-2 RNA sequencing data at defined communities allows for the timely detection of the emergence or surge of variants of concern before their detection in direct patient samples.
In resource-limited settings, the capacity to detect and monitor SARS-CoV-2 in wastewater gives a better insight about the distribution and dynamics of the pandemic at lower cost. Due to resource and capacity limitations, the reported number of conducted COVID-19 tests per 1000 population has been extremely low in Ethiopia. To substantiate this with figures from comparable settings, the current estimates (as of 23 June 2022) of the COVID-19 testing density for South Sudan, Ethiopia, Uganda, and Kenya are 36.05, 42.63, 58.34, and 67.80 per 1000 population, respectively. Just for comparison, the current (as of 23 June 2022) estimates of the COVID-19 testing rate for South Africa, Italy, UK, USA and Germany are 427.00, 3725.12, 7371.74, 2741.00, and 1560.04 per 1000 population, respectively [4]. Thus, the effective utilization of the innovative COVID-19 wastewater-based epidemiological approach may minimize the observed huge COVID-19-related evidence gap between high- and low-income-countries without compromising the limited resources in low- and middle-income-countries.
In the Ethiopian context, the COVID-19 wastewater-based epidemiological approach is more feasible in urban settings, especially in metropolitan cities such as the capital, Addis Ababa. In this city, there are 14 Membrane Bioreactor (MBR) units (Figure 1). It is estimated that a single MBR unit is capable of managing the wastewater produced by 15,000 residents or more [29]. Considering this fact, wastewater SARS-CoV-2 monitoring of the 14 MBR units in Addis Ababa could provide indirect epidemiological information about at least 210,000 residents. The condominium sites are separate compounds equipped with dedicated MBR. This makes the setting more convenient for early warning systems and localized implementation of mitigation strategies in cost efficient way to substantiate the magnitude of cost effectiveness, and to give a more detailed insight, we have analyzed recent publication from Ethiopia. The unit cost of detecting one RT-PCR positive COVID-19 case and the unit cost to detect one RT-PCR positive COVID-19 case through contact tracing is stated to be USD 37.70 and USD 54.00, respectively [39]. The same study has indicated that the cost for COVID-19 RT-PCR testing was around USD 3.91 and an additional USD 1.31 was needed for sample collection [39]. In this study, we observed that the cost to test SARS-CoV-2 RNA from one wastewater processing plant by RT-PCR was around USD 300.00, including all consumables. Considering the 14 MBR wastewater processing plants in Addis Ababa serving 210,000 residents, an estimated USD 1,100,400.00 (210,000 × USD 5.24) would be needed to do a onetime COVID-19 RT-PCR community mass testing. Comparatively, the cost of wastewater SARS-CoV-2 wastewater RT-PCR testing from 14 MBR plants in Addis Ababa would only be USD 4200.00. Nonetheless, rural areas and most other towns in Ethiopia to date are generally not served by such wastewater management systems. As a consequence, this system cannot be implemented in most parts of the country. As outbreaks such as COVID-19 frequently affect densely populated urban settings, and due to the fact that an increasing share of the population in LMICs does indeed reside in urban settings, this system remains of paramount relevance to Ethiopia and similar settings.
The entire set of samples collected from the aeration reactor tank was negative for SARS-CoV-2 RT-PCR testing. This finding indicates that the viral RNA could be suffering from degradation during the aeration process. The purpose of wastewater aeration process is to augment microbial growth to allow for the aerobic biodegradation of organic materials [29]. It normally takes 3–4 days at room temperature with continuous air (oxygen) supply. Considering this, the harsh aeration reactor condition coupled with high average atmospheric temperatures (>25 °C) can potentially degrade any RNA target.
This study has some limitations. As resource limitations restricted us to only three times weekly point observations of SARS-CoV-2 RNA, we could not reasonably estimate the weekly wastewater SARS-CoV-2 RNA viral loads and apply statistical tests to estimate the strength of association with weekly RT-PCR-detected cases.
5. Conclusions
The detection of SARS-CoV-2 RNA from municipal wastewater in resource-limited countries such as Ethiopia will enable one to survey and monitor the COVID-19 pandemic at a low cost. The COVID-19 wastewater-based epidemiological approach is well applicable to a metropolitan setting such as Addis Ababa, where new residential sites are equipped with dedicated MBR plants. In these settings, wastewater is much more concentrated and polluted with bleach solution and detergents than in areas which offer frequent running water, such as in mid- and high-income settings. The COVID-19 wastewater-based epidemiological approach can potentially fill the evidence gap in the distribution and dynamics of COVID-19 in Addis Ababa. This capacity should be utilized in Ethiopia and elsewhere in LMICs for an evidence-based policy or interventional decisions. The wastewater sample should be collected at the inflow or sewage pipes with short sink-to sample times. Due to large amounts of inhibitory substances in the sewage, good extraction conditions with effective inhibitor removal are required to obtain proper results.
Author Contributions
S.A. conceived the study, conducted the experiments, analyzed the results, prepared the first draft and finalized the draft based on comments and feedback from other authors. E.K.G., G.F. and A.W. conceived the study, supported the methodology and reviewed the draft for intellectual contents. A.G., A.A., B.T.A., W.T., G.B.H., M.B.M., T.R.C., R.B., D.N. and D.S. participated in data acquisition, reviewed the draft and intellectually contributed to the final version. All authors have read and agreed to this version of the manuscript.
Funding
This study was support by the One Health target network funds of the CIHLMU Center for International Health at Ludwig-Maximilians-Universität Munich, Germany, which is in turn funded by the German Ministry for Economic Cooperation and Development through the Exceed Program of the German Academic Exchange Program.
Institutional Review Board Statement
The study was ethically cleared by the SPHMMC ethical review board under ref number PM23/18 dated on 10 July 2020 and received permission from Addis Ababa Water and Sewage Authority to conduct the study. There is no direct human subject involvement in this study. However, stored nasopharyngeal left-over swabs were used for optimization of the RT-PCR.
Informed Consent Statement
The study was ethically cleared by SPHMMC ethical review board and granted permission from Addis Ababa Water and Sewage Authority. Informed consent is not applicable for wastewater samples as there is no human subject involved. However, for known COVID 19 RT-PCR positive and negative leftover sample controls, patient consent was waived due to the fact that the samples were retrieved from bio bank storage without any personal or other information linked with the samples.
Data Availability Statement
All data related with this research are presented in this manuscript. Furthermore, the Ethiopia Ministry of Health daily COVID-19 report data is available in this link https://covid19.who.int/WHO-COVID-19-global-data.csv (accessed on 12 March 2022).
Acknowledgments
We acknowledge AAWSA, NAHDIC, and SPHMMC for their support during data acquisition and facility support. We acknowledge Chalie Mulu (NDMC/EPHI) for creating the map of Addis Ababa and Teresa Perez (CIHLMU OH-Network Fund coordinator) for the unreserved support during the project time.
Conflicts of Interest
All authors declared that there is no conflict of interest concerning this manuscript.
Abbreviations
| AAWSA | Addis Ababa Water and Sewage Authority |
| ABI 7500 | Applied Biosystems Real-Time PCR Instrument 7500 |
| BOD | Biochemical Oxygen Demand |
| BSL | Bio-Safety Level |
| CIHLMU | Center for International Health at Ludwig-Maximilians-Universität |
| COVID-19 | Corona Virus Disease 2019 |
| FAM | Fluorescein AMides |
| ICR | Internal Control Reaction |
| LMIC | Low- and Middle-Income-Country |
| MBR | Membrane Bio-Reactors |
| NAHDIC | National Animal Health Diagnostic and Investigation Center |
| NTC | No Template Control |
| RT-PCR | Real-Time Reverse-Transcription Polymerase Chain Reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Corona Virus 2 |
| SPHMMC | St. Paul’s Hospital Millennium Medical College |
| VIC | Victoria |
Appendix A. Detail Description of the Wastewater SARS-CoV-2 RNA RT-PCR Tests Performed to Adapt the Technology to the Local Context
For the first trial, 12 wastewater samples collected and stored at −80 freezer for more than one year were used. The samples were placed at 2–8 °C for 24 h to facilitate slow thawing. They were processed and ultra-centrifuged as described in the methodology above. The pellets were suspended using 500 μL of QIAGEN nuclease free water. A hundred and fifty microliters of each sample was extracted using the QIAamp Viral RNA Mini kit (QIAGEN, Hilden, Germany) as per the manufacturer’s instruction [31]. SARS-CoV-2 RT-PCR testing was conducted induplicate using BGI Group (Shenzhen, China) and RIDA GENE SARS-CoV-2 RUO (r-biopharm, Darmstadt, Germany) master mixes. Unfortunately, only the positive control FAM signal was detected from both PCR tests without any internal control (VIC) signal (Table 2, Section 1). Thus, both RT-PCR tests on the target samples have to be considered as failed (Refer to the Quality Control and interpretations of RT-PCR reactions described in Table 1).
We followed the manufacturer’s recommendation for failed tests and re-extracted the samples under similar condition and repeated the RT-PCR testing using the RIDA GENE SARS-CoV-2 RUO master mix. Again, the RT-PCR testing failed, observing similar result as the previous experiment (Table 2 Section 2). Failure to observe the ICR (VIC) signal is usually associated with extraction problems and/or PCR inhibition. To further explore this, we run known PCR positive and negative patient swab samples, and three PCR positive eluates under similar conditions. Despite repeated efforts using BGI or RIDA master mixes, all tests failed or gave invalid results (Table 2, Section 3 and Section 4).
This called for the use of the Allprep powerVial DNA/RNA extraction kit (QIAGEN) which is a special extraction kit manufactured specifically for the recovery of RNA from wastewater, stool and other environmental samples. Its features enhanced the removal of inhibitory substances. The kits were imported from Germany.
With the Allprep powerVial DNA/RNA extraction kit (QIAGEN), we conducted the extraction of eight wastewater samples, one known PCR positive patient swab sample, and one known PCR negative swab sample. This time, the RT-PCR test passed the control requirements with valid individual test results (Table 1 and Table 2, Section 5). From this experiment, we can retrospectively deduct that in the previous RT-PCR testing attempts, nonspecific inhibitors or RNAse activities might have been the reason for the missing VIC signals.
To further verify the PCR inhibition, we conducted an experiment using 10 different samples composed of one 1:10 diluted (20 μL of positive control mixed with 180 μL of wastewater sample), one 1:2 diluted (100 μL of known PCR positive patient swab sample mixed with 100 μL of wastewater sample), seven wastewater pellet samples (200 μL each) and one 200 μL known PCR positive undiluted patient sample. These samples were extracted as per the Allprep powerVial DNA/RNA extraction kit (QIAGEN) recommendation, omitting the addition of the PM1/β-ME (Beta Mercapto Ethanol) mix buffer, which is important for removing any inhibitors. RT-PCR testing was conducted using the RIDA master mix. There was no signal detected from all reaction tubes except the undiluted and diluted positive control wells (Table 2, Section 5). This experiment confirms the presence of PCR inhibitors in the Addis Ababa wastewater system (Table 2).
References
- Aliabadi, H.A.M.; Eivazzadeh-Keihan, R.; Parikhani, A.B.; Mehraban, S.F.; Maleki, A.; Fereshteh, S.; Bazaz, M.; Zolriasatein, A.; Bozorgnia, B.; Rahmati, S.; et al. COVID-19: A systematic review and update on prevention, diagnosis, and treatment. MedComm 2022, 3, e115. [Google Scholar] [CrossRef]
- Mangindaan, D.; Adib, A.; Febrianta, H.; Hutabarat, D.J.C. Systematic Literature Review and Bibliometric Study of Waste Management in Indonesia in the COVID-19 Pandemic Era. Sustainability 2022, 14, 2556. [Google Scholar] [CrossRef]
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2022. Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 29 June 2022).
- Coronavirus (COVID-19) Testing. 2020. Available online: https://ourworldindata.org/coronavirus-testing#world-map-total#tests-performed-relative-to-the-size-of-population (accessed on 29 June 2022).
- Ethiopian Federal Health Minster COVID-19 Daily Report. 2022. Available online: https://covid19.who.int/region/afro/country/et (accessed on 29 June 2022).
- Gudina, E.K.; Ali, S.; Girma, E.; Gize, A.; Tegene, B.; Hundie, G.B.; Sime, W.T.; Ambachew, R.; Gebreyohanns, A.; Bekele, M.; et al. Sero epidemiology and model-based prediction of SARS-CoV-2 in Ethiopia: Longitudinal cohort study among front-line hospital workers and communities. Lancet Glob. Health 2021, 9, e1517–e1527. [Google Scholar] [CrossRef]
- Ethiopian Health Data COVID-19 Ethiopian Case Tracker Dashboard. Available online: https://ethiopianhealthdata.org/dashboard/covid19-ethiopia (accessed on 4 March 2022).
- World Health Organization Advice for the Public to Prevent COVID-19. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 9 March 2022).
- Rubio-Acero, R.; Beyerl, J.; Muenchhoff, M.; Roth, M.S.; Castelletti, N.; Paunovic, I.; Radon, K.; Springer, B.; Nagel, C.; Boehm, B.; et al. Spatially resolved qualified sewage spot sampling to track SARS-CoV-2 dynamics in Munich—One year of experience. Sci. Total Environ. 2021, 797, 149031. [Google Scholar] [CrossRef]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Mancini, P.; Ferraro, G.B.; Veneri, C.; Bonadonna, L.; Lucentini, L.; Suffredini, E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020, 736, 139652. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef]
- Nemudryi, A.; Nemudraia, A.; Wiegand, T.; Surya, K.; Buyukyoruk, M.; Cicha, C.; Vanderwood, K.K.; Wilkinson, R.; Wiedenheft, B. Temporal Detection and Phylogenetic Assessment of SARS-CoV-2 in Municipal Wastewater. Cell Rep. Med. 2020, 1, 100098. [Google Scholar] [CrossRef]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Maresca, M.; Longobardi, C.; Mancon, A.; Romeri, F.; et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Sherchan, S.P.; Shahin, S.; Ward, L.M.; Tandukar, S.; Aw, T.G.; Schmitz, B.; Ahmed, W.; Kitajima, M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Sci. Total Environ. 2020, 743, 140621. [Google Scholar] [CrossRef] [PubMed]
- Street, R.; Mathee, A.; Mangwana, N.; Dias, S.; Sharma, J.R.; Ramharack, P.; Louw, J.; Reddy, T.; Brocker, L.; Surujlal-Naicker, S.; et al. Spatial and Temporal Trends of SARS-CoV-2 RNA from Wastewater Treatment Plants over 6 Weeks in Cape Town, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 12085. [Google Scholar] [CrossRef]
- Wurtzer, S.; Marechal, V.; Mouchel, J.M.; Moulin, L. Time Course Quantitative Detection of SARS-CoV-2 in Parisian Wastewaters Correlates with COVID-19 Confirmed Cases. medRxiv 2020, 4, 10–13. [Google Scholar] [CrossRef]
- Kitajima, M.; Ahmed, W.; Bibby, K.; Carducci, A.; Gerba, C.P.; Hamilton, K.A.; Haramoto, E.; Rose, J.B. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci. Total Environ. 2020, 739, 139076. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xiao, A.; Zhang, J.; Gu, X.; Lee, W.L.; Kauffman, K.; Hanage, W.P.; Matus, M.; Ghaeli, N.; Endo, N.; et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Hovi, T.; Shulman, L.M.; Van Der Avoort, H.; Deshpande, J.; Roivainen, M.; De Gourville, E.M. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 2012, 140, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Bibi, S. Heavy Metal, Waste, COVID-19, and Rapid Industrialization in This Modern Era—Fit for Sustainable Future. Sustainability 2022, 14, 4746. [Google Scholar] [CrossRef]
- Shammi, M.; Rahman, M.; Ali, L.; Khan, A.S.M.; Siddique, A.B.; Ashadudzaman; Doza, B.; Alam, G.M.; Tareq, S.M. Application of short and rapid strategic environmental assessment (SEA) for biomedical waste management in Bangladesh. Case Stud. Chem. Environ. Eng. 2021, 5, 100177. [Google Scholar] [CrossRef]
- Kothari, R.; Sahab, S.; Singh, H.M.; Singh, R.P.; Singh, B.; Pathania, D.; Singh, A.; Yadav, S.; Allen, T.; Singh, S.; et al. COVID-19 and waste management in Indian scenario: Challenges and possible solutions. Environ. Sci. Pollut. Res. 2021, 28, 52702–52723. [Google Scholar] [CrossRef]
- Barasarathi, J.; Agamuthu, P. Clinical waste management under COVID-19 scenario in Malaysia. Waste Manag. Res. 2021, 39 (Suppl. 1), 18–26. [Google Scholar]
- World Population Review, Addis Ababa Population 2022. Available online: https://worldpopulationreview.com/world-cities/addis-ababa-population (accessed on 2 March 2022).
- Cirolia, L.R.; Hailu, T.; King, J.; Cruz, N.F.; Beall, J. Infrastructure governance in the post-networked city: State-led, high-tech sanitation in Addis Ababa’s condominium housing. EPC Politics Space 2021, 39, 1606–1624. [Google Scholar] [CrossRef]
- Mahlet, M. Performance Evaluation and Model-Based Optimization of Membrane Bioreactors: The Case of Addis Ababa Package Treatment Plant. AAU M.Sc Thesis. 2017. Available online: http://213.55.95.56/bitstream/handle/123456789/24825/Mahlet%20Melaku.pdf?sequence=1&isAllowed=y (accessed on 6 March 2022).
- Belaineh, R. Study on LSD Vaccine Efficacy Using Kenyan SGPV and Neethling (Poster). Available online: http://hpc.ilri.cgiar.org/beca/training/IMBB/posters/alemu.pdf (accessed on 2 March 2022).
- QIAamp® Viral RNA Mini Handbook July 2022. Available online: https://www.qiagen.com/us/resources/download.aspx?id=c80685c0-4103-49ea-aa72-8989420e3018&lang=en (accessed on 30 November 2021).
- AllPrep® PowerViral® DNA/RNA Kit Handbook For the Isolation of Viral or Bacterial Total Nucleic Acids from Waste Water and Stool Samples. 2018. Available online: https://www.qiagen.com/cn/resources/download.aspx?id=41a1323f-581c-4e65-8d66-2491be4c625e&lang=en (accessed on 28 December 2021).
- Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2, BGI Genomics Co. Ltd. (“BGI”). 2021. Available online: https://www.bgi.com/wp-content/uploads/sites/2/2021/04/EUA200034-S003.Instructions-for-Use.03292021.pdf (accessed on 30 November 2021).
- RIDA®GENE SARS-CoV-2, r-Biopharm. 2020. Available online: https://clinical.r-biopharm.com/wp-content/uploads/2020/06/pg6815_ridagene_sars-cov-2_2020-07-27_en_final.pdf (accessed on 28 December 2021).
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Simpson, S.L.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Blackall, L.L.; Bofill-Mas, S.; Bosch, A.; Brandão, J.; Choi, P.M.; et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2021, 805, 149877. [Google Scholar] [CrossRef]
- Hong, P.-Y.; Rachmadi, A.T.; Mantilla-Calderon, D.; Alkahtani, M.; Bashawri, Y.M.; Al Qarni, H.; O’Reilly, K.M.; Zhou, J. Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: To what extent of the outbreak can surveillance of wastewater tell us? Environ. Res. 2021, 195, 110748. [Google Scholar] [CrossRef]
- Takeda, T.; Kitajima, M.; Huong, N.T.T.; Setiyawan, A.S.; Setiadi, T.; Hung, D.T.; Haramoto, E. Institutionalising wastewater surveillance systems to minimise the impact of COVID-19: Cases of Indonesia, Japan and Viet Nam. Water Sci. Technol. 2020, 83, 251–256. [Google Scholar] [CrossRef]
- Yigezu, A.; Zewdie, S.A.; Mirkuzie, A.H.; Abera, A.; Hailu, A.; Agachew, M.; Memirie, S.T. Cost-analysis of COVID-19 sample collection, diagnosis, and contact tracing in low resource setting: The case of Addis Ababa, Ethiopia. PLoS ONE 2022, 17, e0269458. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).