Environmental Influences on the Behavioural and Emotional Outcomes of Children: A Network Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Toenail Sample Collection and Laboratory Analysis
2.3. Questionnaires
2.4. Anthropometric Measurement
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Descriptive Summary
3.3. Network Analysis
3.4. Associations between CBCL, Mn Concentration and Other Factors
4. Discussion
4.1. Network Analysis
4.2. Manganese Concentrations
4.3. Manganese Concentrations and Behaviour Problems
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grandjean, P.; Landrigan, P. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Landrigan, P.J.; Lambertini, L.; Birnbaum, L.S. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ. Health Perspect. 2012, 120, a258–a260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boivin, M.J.; Kakooza, A.M.; Warf, B.C.; Davidson, L.L.; Grigorenko, E.L. Reducing neurodevelopmental disorders and disability through research and interventions. Nature 2015, 527, S155–S160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielski, T.; Weuve, J.; Bellinger, D.C.; Schwartz, J.; Lanphear, B.; Wright, R. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ. Health Perspect. 2012, 120, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, M.M.; El-Mazary, A.-A.M.; Maher, R.M.; Saber, M.M. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011, 37, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, L.E.; DiSilvestro, R.A. Zinc in attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2005, 15, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, L.A.; Rebello, T.; Buchsbaum, M.S.; Tucker, H.G.; Hodges, E.L. Abnormalities in hair trace elements as indicators of aberrant behavior. Compr. Psychiatry 1991, 32, 229–237. [Google Scholar] [CrossRef]
- Silvera, S.A.N.; Rohan, T.E. Trace elements and cancer risk: A review of the epidemiologic evidence. Cancer Causes Control 2007, 18, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Louria, D.B.; Joselow, M.M.; Browder, A.A. The human toxicity of certain trace elements. Ann. Intern. Med. 1972, 76, 307–319. [Google Scholar] [CrossRef]
- Karatela, S.; Ward, N.I. Trace elements and human obesity: An overview. Manip. J. Nurs. Health Sci. (MJNHS) 2016, 2, 50–59. [Google Scholar]
- Liu, J.; Hanlon, A.; Ma, C.; Zhao, S.R.; Cao, S.; Compher, C. Low blood zinc, iron, and other sociodemographic factors associated with behavior problems in preschoolers. Nutrients 2014, 6, 530–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galler, J.R.; Bryce, C.P.; Waber, D.P.; Medford, G.; Eaglesfield, G.D.; Fitzmaurice, G. Early malnutrition predicts parent reports of externalizing behaviors at ages 9–17. Nutr. Neurosci. 2011, 14, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raine, A.; Liu, J.; Venables, P.H.; A Mednick, S.; Dalais, C. Cohort profile: The mauritius child health project. Int. J. Epidemiol. 2010, 39, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamazaki, T.; Sawazaki, S.; Itomura, M.; Asaoka, E.; Nagao, Y.; Nishimura, N.; Yazawa, K.; Kuwamori, T.; Kobayashi, M. The effect of docosahexaenoic acid on aggression in young adults. A placebo-controlled double-blind study. J. Clin. Investig. 1996, 97, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Gesch, C.B.; Hammond, S.M.; Hampson, S.E.; Eves, A.; Crowder, M.J. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners: Randomised, placebo-controlled trial. Br. J. Psychiatry 2002, 181, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Gajos, J.M.; Beaver, K.M. The effect of omega-3 fatty acids on aggression: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 147–158. [Google Scholar] [CrossRef]
- Robinson, S.L.; Marín, C.; Oliveros, H.; Mora-Plazas, M.; Richards, B.J.; Lozoff, B.; Villamor, E. Iron deficiency, anemia, and low vitamin B-12 serostatus in middle childhood are associated with behavior problems in adolescent boys: Results from the bogotá school children cohort. J. Nutr. 2018, 148, 760–770. [Google Scholar] [CrossRef]
- Robinson, S.L.; Mora-Plazas, M.; Oliveros, H.; Marin, C.; Lozoff, B.; Villamor, E. Dietary patterns in middle childhood and behavior problems in adolescence. Eur. J. Clin. Nutr. 2021, 75, 1809–1818. [Google Scholar] [CrossRef]
- Campisi, S.C.; Zasowski, C.; Shah, S.; Shah, A.; Bradley-Ridout, G.; Korczak, D.J.; Szatmari, P. Assessing the evidence of micronutrients on depression among children and adolescents: An evidence gap map. Adv. Nutr. 2020, 11, 908–927. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Olung, N.F.; Akingbade, G.T.; Okoh, C.O.; Aschner, M. Environmental influence on neurodevelopmental disorders: Potential association of heavy metal exposure and autism. J. Trace Elem. Med. Biol. 2020, 62, 126638. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.W. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 3, pp. 133–164. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.L.; Araújo, C.F.; dos Santos, N.R.; Bandeira, M.J.; Anjos, A.L.S.; Carvalho, C.F.; Lima, C.S.; Abreu, J.N.S.; Mergler, D.; Menezes-Filho, J.A. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ. Res. 2018, 167, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; Factor-Litvak, P.; Gardner, J.M.; Graziano, J.H. Developmental impacts of heavy metals and undernutrition. Basic Clin. Pharmacol. Toxicol. 2008, 102, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Manganese; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012. [Google Scholar]
- Weiss, B. Vulnerability of children and the developing brain to neurotoxic hazards. Environ. Health Perspect. 2000, 108 (Suppl. 3), 375–381. [Google Scholar] [PubMed]
- Mergler, D.; Huel, G.; Bowler, R.; Iregren, A.; Belanger, S.; Baldwin, M.; Tardif, R.; Smargiassi, A.; Martin, L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994, 64, 151–180. [Google Scholar] [CrossRef]
- Riojas-Rodríguez, H.; Solís-Vivanco, R.; Schilmann, A.; Montes, S.; Rodríguez, S.; Ríos, C.; Rodríguez-Agudelo, Y. Intellectual function in mexican children living in a mining area and environmentally exposed to manganese. Environ. Health Perspect. 2010, 118, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Freire, C.; Amaya, E.; Gil, F.; Fernández, M.F.; Murcia, M.; Llop, S.; Andiarena, A.; Aurrekoetxea, J.; Bustamante, M.; Guxens, M.; et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The environment and childhood (INMA) project. Sci. Total Environ. 2018, 621, 340–351. [Google Scholar] [CrossRef]
- Henn, B.C.; Schnaas, L.; Ettinger, A.S.; Schwartz, J.; Lamadrid-Figueroa, H.; Hernández-Avila, M.; Amarasiriwardena, C.; Hu, H.; Bellinger, D.C.; Wright, R.; et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 2012, 120, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Sanders, A.P.; Henn, B.C.; Wright, R. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: A review of recent literature. Curr. Environ. Health Rep. 2015, 2, 284–294. [Google Scholar] [CrossRef] [Green Version]
- McDermott, S.; Wu, J.; Cai, B.; Lawson, A.; Aelion, C.M. Probability of intellectual disability is associated with soil concentrations of arsenic and lead. Chemosphere 2011, 84, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Karatela, S.; Coomarasamy, C.; Paterson, J.; Ward, N.I. Household smoking status and heavy metal concentrations in toenails of children. Int. J. Environ. Res. Public Health 2019, 16, 3871. [Google Scholar] [CrossRef] [Green Version]
- Karatela, S.; Coomarasamy, C.; Paterson, J.; Ward, N.I. Exposure to toenail heavy metals and child behavior problems in nine-year-old children: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 4120. [Google Scholar] [CrossRef]
- Karatela, S.; Ward, N.I.; Zeng, I.S.; Paterson, J. Status and interrelationship of toenail elements in Pacific children. J. Trace Elements Med. Biol. 2018, 46, 10–16. [Google Scholar] [CrossRef]
- Statistics New Zealand. 2018 Census ethnic group summaries. In Pacific Peoples Ethinic Group; Statistics: Wellington, New Zealand, 2022. [Google Scholar]
- Paterson, J.; Percival, T.; Schluter, P.; Sundborn, G.; Abbott, M.; Carter, S.; Cowley-Malcolm, E.; Borrows, J.; Gao, W.; The PIF Study Group. Cohort profile: The pacific islands families (PIF) study. Int. J. Epidemiol. 2008, 37, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA school-Age Forms & Profiles: Child Behavior Checklist for Ages 6–18, Teacher’s Report Form, Youth Self-Report: An Integrated System of Multi-Informant Assessment; University of Vermont, Research Center for Children Youth & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Achenbach, T.M. Child behavior checklist. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer New York: New York, NY, USA, 2011; pp. 546–552. [Google Scholar]
- Hevey, D. Network analysis: A brief overview and tutorial. Health Psychol. Behav. Med. 2018, 6, 301–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, M.F.H.; Rhoden, C.R.; Amantéa, S.L.; Barbosa, F., Jr. Low concentrations of selenium and zinc in nails are associated with childhood asthma. Biol. Trace Element Res. 2011, 144, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Chartrand, M.S.; Aaseth, J. Manganese exposure and neurotoxic effects in children. Environ. Res. 2017, 155, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Bouabid, S.; Tinakoua, A.; Lakhdar-Ghazal, N.; Benazzouz, A. Manganese neurotoxicity: Behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J. Neurochem. 2016, 1, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.D.L.; Vasconcellos, M.; Montenegro, R.C.; O Bahia, M.; Costa, E.T.; Antunes, L.; Burbano, R.R. Genotoxic effects of aluminum, iron and manganese in human cells and experimental systems: A review of the literature. Hum. Exp. Toxicol. 2011, 30, 1435–1444. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Excess iron: Considerations related to development and early growth. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1600S–1605S. [Google Scholar] [CrossRef]
- Nielsen, F.H. Chapter 29—Manganese, molybdenum, boron, silicon, and other trace elements. In Present Knowledge in Nutrition, 11th ed.; Erdman, J.W., Jr., Macdonald, I.A., Zeisel, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 485–500. [Google Scholar]
- Nielsen, F.H.; Meacham, S.L. Growing evidence for human health benefits of boron. J. Evid. Based Complement. Altern. Med. 2011, 16, 169–180. [Google Scholar] [CrossRef]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The physiological role of boron on health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.; Goodman, B.A.; Farmer, V.C. The chemistry of aluminium, iron and manganese oxides in acid soils. In Soil Acidity; Ulrich, B., Sumner, M.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 97–124. [Google Scholar]
- Barrón, V.; Torrent, J. Iron, manganese and aluminium oxides and oxyhydroxides. In Minerals at the Nanoscale; Nieto, F., Livi, K.J.T., Oberti, R., Eds.; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 2013. [Google Scholar]
- Andrade, V.M.; Mateus, M.L.; Batoreu, M.C.; Aschner, M.; Dos Santos, A.P.M. Lead, arsenic, and manganese metal mixture exposures: Focus on biomarkers of effect. Biol. Trace Elem. Res. 2015, 166, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Brzóska, M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]
- Peraza, M.A.; Ayala-Fierro, F.; Barber, D.S.; Casarez, E.; Rael, L.T. Effects of micronutrients on metal toxicity. Environ. Health Perspect. 1998, 106 (Suppl. 1), 203–216. [Google Scholar]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. The influence of calcium content in diet on cumulation and toxicity of cadmium in the organism review. Arch. Toxicol. 1997, 72, 63–73. [Google Scholar]
- Matović, V.; Bulat, Z.; Đukić-Ćosić, D.; Soldatović, D. Zinc, copper or magnesium supplementation against cadmium toxicity: An experimental study. In Biometals: Molecular Structures, Binding Properties and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 1–32. [Google Scholar]
- Chan, S.; Gerson, B.; Subramaniam, S. The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin. Lab. Med. 1998, 18, 673–685. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Gerhardsson, L.; Mumtaz, M.M.; Ruiz, P.; Fowler, B.A. Chapter 14—Interactions and mixtures in metal toxicology. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 319–347. [Google Scholar]
- Berry, M.J.; Ralston, N.V.C. Mercury toxicity and the mitigating role of selenium. EcoHealth 2008, 5, 456–459. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Surette, C.; Cormier, P.; Foucher, D. Low level exposure to manganese from drinking water and cognition in school-age children. NeuroToxicology 2018, 64, 110–117. [Google Scholar] [CrossRef]
- Frisbie, S.H.; Mitchell, E.J.; Sarkar, B. Urgent need to reevaluate the latest World Health Organization guidelines for toxic inorganic substances in drinking water. Environ. Health 2015, 14, 63. [Google Scholar] [CrossRef] [Green Version]
- Di Ciaula, A.; Gentilini, P.; Diella, G.; Lopuzzo, M.; Ridolfi, R. Biomonitoring of metals in children living in an urban area and close to waste incinerators. Int. J. Environ. Res. Public Health 2020, 17, 1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, A.M.; Arora, M.; Harley, K.G.; Kogut, K.; Parra, K.; Hernández-Bonilla, D.; Gunier, R.B.; Bradman, A.; Smith, D.R.; Eskenazi, B. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ. Int. 2015, 84, 39–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.M.; Kippler, M.; Tofail, F.; Bölte, S.; Hamadani, J.; Vahter, M. Manganese in drinking water and cognitive abilities and behavior at 10 years of age: A prospective cohort study. Environ. Health Perspect. 2017, 125, 057003. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; Laforest, F.; Vandelac, L.; Bellinger, D.; Mergler, D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007, 115, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oulhote, Y.; Mergler, D.; Bouchard, M.F. Sex-and age-differences in blood manganese levels in the US general population: National health and nutrition examination survey 2011–2012. Environ. Health 2014, 13, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, P. A pilot study on the metal levels in the hair of hyperactive children. Med. Hypotheses 1983, 11, 309–318. [Google Scholar] [CrossRef]
- Ericson, J.E.; Crinella, F.M.; Clarke-Stewart, K.A.; Allhusen, V.D.; Chan, T.; Robertson, R.T. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 2007, 29, 181–187. [Google Scholar] [CrossRef]
- Olanow, C.W. Manganese-induced parkinsonism and Parkinson’s disease. Ann. N. Y. Acad. Sci. 2004, 1012, 209–223. [Google Scholar] [CrossRef]
- Pal, P.K.; Samii, A.; Calne, D.B. Manganese neurotoxicity: A review of clinical features, imaging and pathology. Neurotoxicology 1999, 20, 227–238. [Google Scholar]
- Mehrifar, Y.; Bahrami, M.; Sidabadi, E.; Pirami, H. The effects of occupational exposure to manganese fume on neurobehavioral and neurocognitive functions: An analytical cross-sectional study among welders. EXCLI J. 2020, 19, 372–386. [Google Scholar]
- Liu, W.; Xin, Y.; Li, Q.; Shang, Y.; Ping, Z.; Min, J.; Cahill, C.M.; Rogers, J.T.; Wang, F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ. Health 2020, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J. Manganese and birth outcome. Nutr. Rev. 2009, 67, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Wasserman, G.A.; Liu, X.; Ahmed, E.; Parvez, F.; Slavkovich, V.; Levy, D.; Mey, J.; van Geen, A.; Graziano, J.H.; et al. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology 2012, 33, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Oulhote, Y.; Mergler, D.; Barbeau, B.; Bellinger, D.C.; Bouffard, T.; Brodeur, M.; Saint-Amour, D.; Legrand, M.; Sauvé, S.; Bouchard, M.F. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ. Health Perspect. 2014, 122, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Filho, J.A.; de Carvalho-Vivas, C.F.; Viana, G.F.; Ferreira, J.R.; Nunes, L.S.; Mergler, D.; Abreu, N. Elevated manganese exposure and school-aged children’s behavior: A gender-stratified analysis. Neurotoxicology 2014, 45, 293–300. [Google Scholar] [CrossRef]
- De Carvalho, C.F.; Oulhote, Y.; Martorelli, M.; de Carvalho, C.O.; Menezes-Filho, J.A.; Argollo, N.; Abreu, N. Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. NeuroToxicology 2018, 69, 253–259. [Google Scholar] [CrossRef]
- Laohaudomchok, W.; Lin, X.; Herrick, R.F.; Fang, S.C.; Cavallari, J.M.; Christiani, D.C.; Weisskopf, M.G. Toenail, blood, and urine as biomarkers of manganese exposure. J. Occup. Environ. Med. 2011, 53, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-González, E.; García-Esquinas, E.; de Larrea-Baz, N.F.; Salcedo-Bellido, I.; Navas-Acien, A.; Lope, V.; Gómez-Ariza, J.L.; Pastor, R.; Pollán, M.; Pérez-Gómez, B. Toenails as biomarker of exposure to essential trace metals: A review. Environ. Res. 2019, 179, 108787. [Google Scholar] [CrossRef]
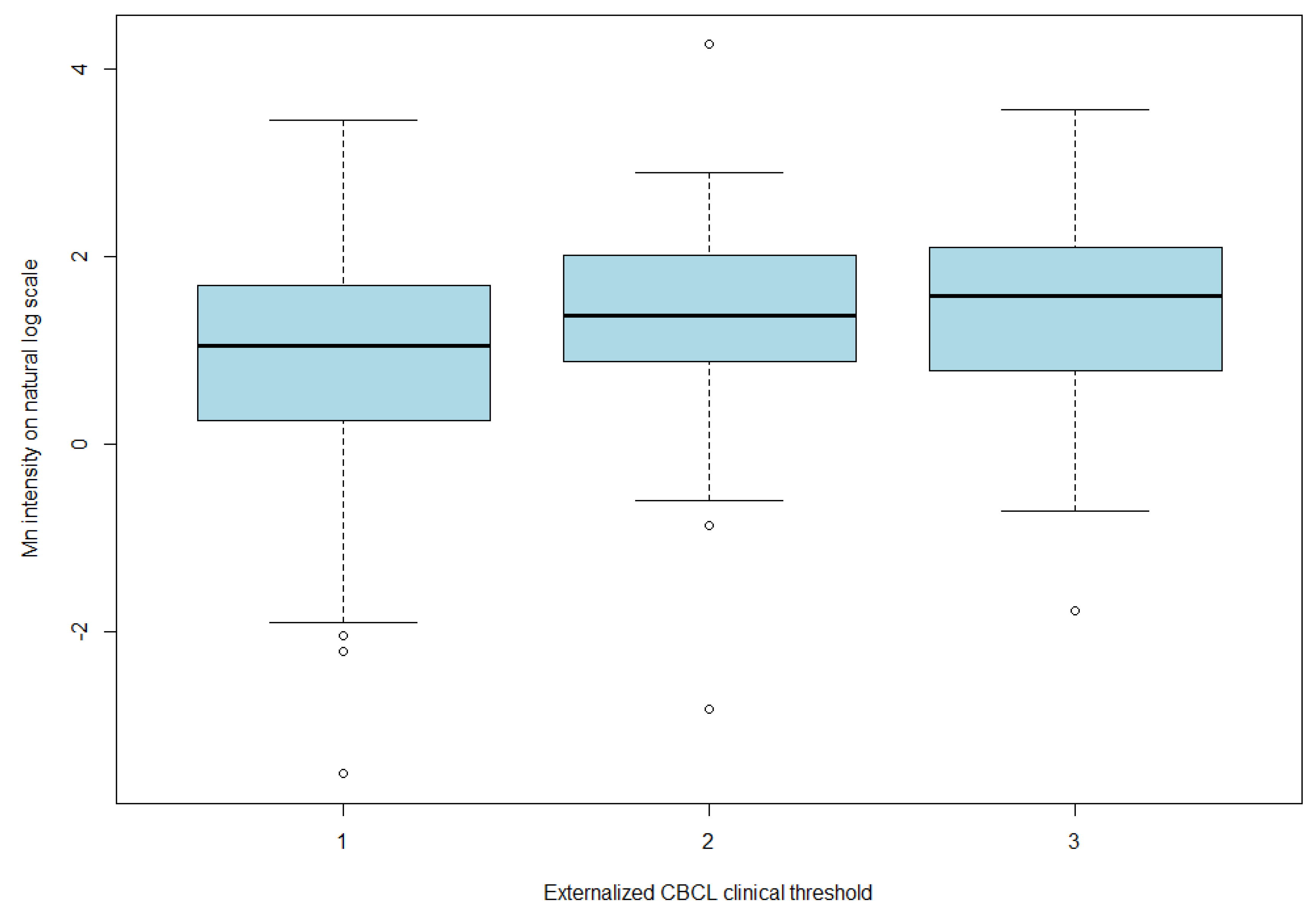

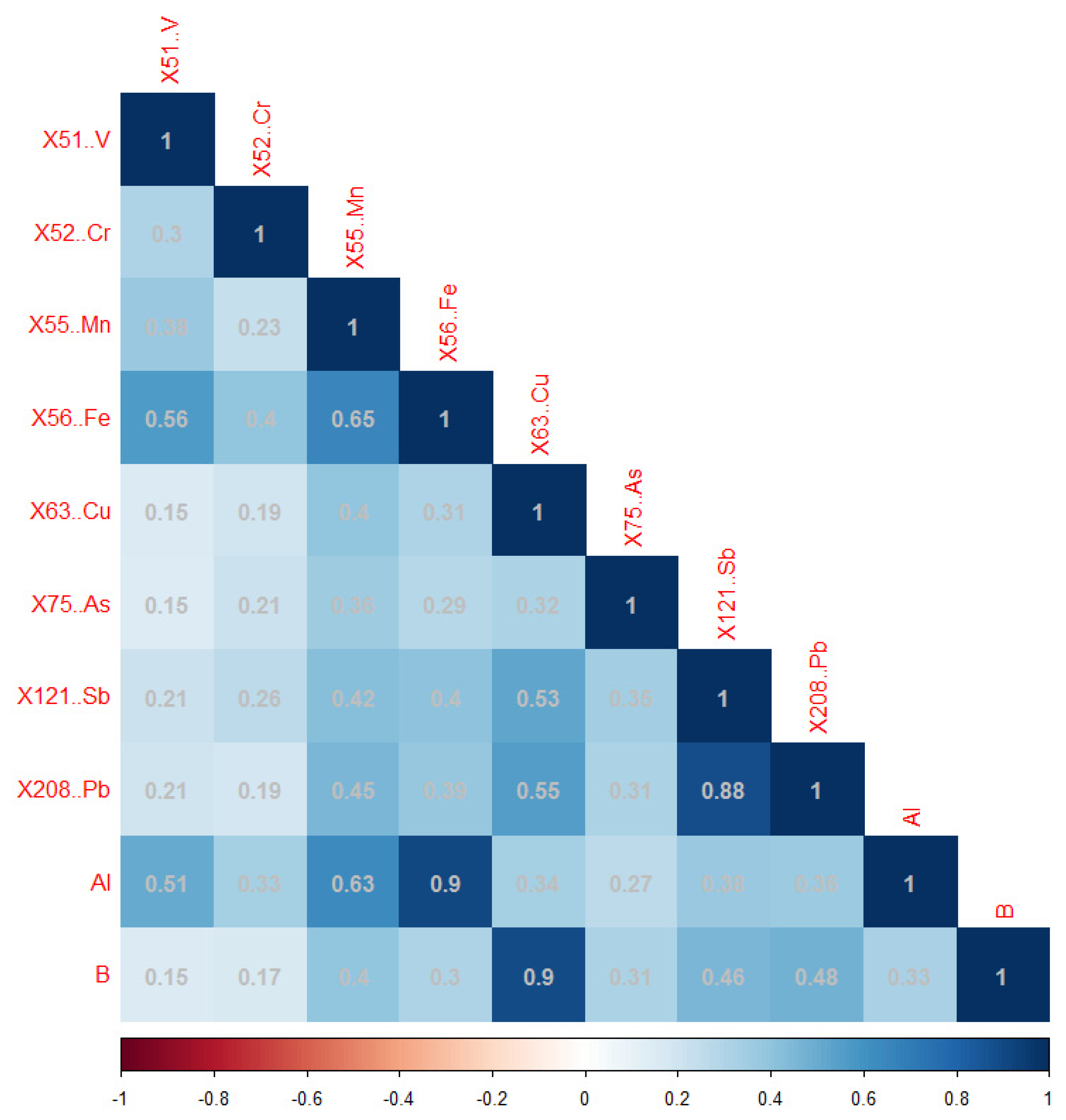
| (A) | |||||||||
| Externalized CBCL Quartile | Children (n) | Mean | Std Dev | Geo-mean | Min | Max | Median | 25th Percentile | 75th Percentile |
| 1 | 69 | 3.9 | 4.4 | 2.5 | 0.1 | 31.3 | 2.8 | 1.4 | 5.4 |
| 2 | 85 | 3.7 | 3.7 | 2.2 | 0.0 | 16.7 | 2.6 | 1.1 | 4.7 |
| 3 | 51 | 4.8 | 4.0 | 3.3 | 0.4 | 18.1 | 3.5 | 1.5 | 6.9 |
| 4 | 68 | 7.2 | 10.2 | 4.0 | 0.1 | 70.3 | 4.0 | 2.3 | 8.2 |
| (B) | |||||||||
| Externalising Clinical Range | Children (n) | Mean | Std Dev | Geo-mean | Min | Max | Median | 25th Percentile | 75th Percentile |
| 1 | 190 | 3.9 | 3.9 | 2.5 | 0.03 | 31.3 | 2.9 | 1.3 | 5.4 |
| 2 | 47 | 6.4 | 10.3 | 3.6 | 0.1 | 70.3 | 3.9 | 2.3 | 8.0 |
| 3 | 40 | 7.2 | 7.9 | 4.3 | 0.2 | 35.1 | 4.9 | 2.2 | 8.1 |
| Quartile Groups: | |||||||||
| Fe: | |||||||||
| Externalized CBCL Quartile | Children (n) | Mean | Std Dev | Geo-mean | Min | Max | Median | 25th Percentile | 75th Percentile |
| 1 | 69 | 81.4 | 57.2 | 65.3 | 13.7 | 246.4 | 62.9 | 39.1 | 106.2 |
| 2 | 85 | 114.8 | 215.8 | 70.5 | 16.0 | 1868.7 | 60.2 | 40.4 | 116.3 |
| 3 | 51 | 117.2 | 84.9 | 93.5 | 22.7 | 403.0 | 95.5 | 55.4 | 148.7 |
| 4 | 68 | 107.0 | 115.9 | 79.0 | 17.5 | 823.8 | 76.3 | 50.9 | 114.3 |
| Cu: | |||||||||
| Externalized CBCL Quartile | Children (n) | Mean | Std Dev | Geo-mean | Mini | Maxi | Median | 25th Percentile | 75th Percentile |
| 1 | 69 | 18.6 | 9.0 | 17.04 | 5.4 | 64.6 | 17.0 | 13.0 | 21.1 |
| 2 | 85 | 18.0 | 9.9 | 16.47 | 7.8 | 78.0 | 15.5 | 13.0 | 20.5 |
| 3 | 51 | 18.4 | 6.7 | 17.36 | 9.1 | 41.3 | 17.3 | 13.8 | 21.6 |
| 4 | 68 | 22.0 | 14.5 | 19.34 | 2.5 | 116.8 | 19.0 | 14.4 | 25.6 |
| B: | |||||||||
| Externalized CBCL Quartile | Children (n) | Mean | Std Dev | Geo-mean | Mini | Maxi | Median | 25th Percentile | 75th Percentile |
| 1 | 69 | 0.29 | 0.13 | 0.27 | 0.12 | 0.87 | 0.27 | 0.22 | 0.32 |
| 2 | 85 | 0.28 | 0.13 | 0.27 | 0.12 | 0.99 | 0.27 | 0.21 | 0.32 |
| 3 | 51 | 0.28 | 0.11 | 0.27 | 0.16 | 0.67 | 0.28 | 0.21 | 0.32 |
| 4 | 68 | 0.33 | 0.14 | 0.30 | 0.09 | 0.78 | 0.28 | 0.24 | 0.38 |
| On Clinical Defined Groups: | |||||||||
| Fe: | |||||||||
| Externalising Using CBCL Criteria | Children (n) | Mean | Std Dev | Geo-mean | Mini | Maxi | Median | 25th Percentile | 75th Percentile |
| Normal | 190 | 103.6 | 153.4 | 72.5 | 13.7 | 1868.7 | 65.4 | 42.7 | 121.3 |
| Borderline | 47 | 102.8 | 76.8 | 83.2 | 23.0 | 369.3 | 83.5 | 54.1 | 117.7 |
| Clinical | 40 | 114.7 | 138.8 | 79.9 | 17.5 | 823.8 | 74.7 | 48.7 | 121.9 |
| Cu: | |||||||||
| Externalising CBCL Criteria Range | Children (n) | Mean | Std Dev | Geo-mean | Mini | Maxi | Median | 25th Percentile | 75th Percentile |
| Normal | 190 | 18.2 | 8.9 | 16.8 | 5.4 | 78.0 | 16.3 | 13.1 | 21.1 |
| Border line | 47 | 21.3 | 9.3 | 19.7 | 11.9 | 54.4 | 18.9 | 14.5 | 26.2 |
| Clinical | 40 | 22.0 | 17.2 | 18.7 | 2.5 | 116.8 | 18.6 | 13.9 | 24.7 |
| B: | |||||||||
| Externalising CBCL Criteria | Children (n) | Mean | Std Dev | Geo-mean | Mini | Maxi | Median | 25th Percentile | 75th Percentile |
| Normal | 190 | 0.29 | 0.12 | 0.27 | 0.12 | 0.99 | 0.27 | 0.21 | 0.32 |
| Border line | 47 | 0.32 | 0.14 | 0.30 | 0.17 | 0.76 | 0.28 | 0.22 | 0.38 |
| Clinical | 40 | 0.32 | 0.14 | 0.30 | 0.09 | 0.78 | 0.29 | 0.24 | 0.38 |
| Cd | Mg | Zn | Ca | |
|---|---|---|---|---|
| Cd | 1 | |||
| Mg | −0.71 | 1 | ||
| Zn | −0.76 | 0.86 | 1 | |
| Ca | −0.74 | 0.89 | 0.97 | 1 |
| Se | Mo | Hg | I | |
|---|---|---|---|---|
| Se | 1 | |||
| Mo | 0.91 | 1 | ||
| Hg | −0.94 | −0.89 | 1 | |
| I | −0.76 | −0.73 | 0.78 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatela, S.; Ward, N.I.; Paterson, J.; Zeng, I.S. Environmental Influences on the Behavioural and Emotional Outcomes of Children: A Network Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8479. https://doi.org/10.3390/ijerph19148479
Karatela S, Ward NI, Paterson J, Zeng IS. Environmental Influences on the Behavioural and Emotional Outcomes of Children: A Network Analysis. International Journal of Environmental Research and Public Health. 2022; 19(14):8479. https://doi.org/10.3390/ijerph19148479
Chicago/Turabian StyleKaratela, Shamshad, Neil I. Ward, Janis Paterson, and Irene Suilan Zeng. 2022. "Environmental Influences on the Behavioural and Emotional Outcomes of Children: A Network Analysis" International Journal of Environmental Research and Public Health 19, no. 14: 8479. https://doi.org/10.3390/ijerph19148479
APA StyleKaratela, S., Ward, N. I., Paterson, J., & Zeng, I. S. (2022). Environmental Influences on the Behavioural and Emotional Outcomes of Children: A Network Analysis. International Journal of Environmental Research and Public Health, 19(14), 8479. https://doi.org/10.3390/ijerph19148479







