Abstract
Aging is characterized by changes in the structure and quality of sleep. When the alterations in sleep become substantial, they can generate or accelerate cognitive decline, even in the absence of overt pathology. In fact, impaired sleep represents one of the earliest symptoms of Alzheimer’s disease (AD). This systematic review aimed to analyze the studies on sleep quality in aging, also considering mild cognitive impairment (MCI) and AD. The review process was conducted according to the PRISMA statement. A total of 71 studies were included, and the whole sample had a mean age that ranged from 58.3 to 93.7 years (62.8–93.7 healthy participants and 61.8–86.7 pathological populations). Of these selected studies, 33 adopt subjective measurements, 31 adopt objective measures, and 10 studies used both. Pathological aging showed a worse impoverishment of sleep than older adults, in both subjective and objective measurements. The most common aspect compromised in AD and MCI were REM sleep, sleep efficiency, sleep latency, and sleep duration. These results underline that sleep alterations are associated with cognitive impairment. In conclusion, the frequency and severity of sleep disturbance appear to follow the evolution of cognitive impairment. The overall results of objective measures seem more consistent than those highlighted by subjective measurements.
1. Introduction
Aging is associated with a physiological decline that also involves cognitive domains. In clinical conditions, such as mild cognitive impairment (MCI) or dementia, cognitive decline is altered compared to physiological ones [1,2]. Dementia currently affects more than 55 million people, and this figure is estimated to rise to 78 million in 2030 [3]. Alzheimer’s disease (AD) accounts for the majority of dementia cases [4]. For these reasons, the early detection of possible precursors of dementia and the diagnosis and treatment of modifiable risk factors are increasing in importance.
In recent years, evaluation of prodromal risk-state for dementia, such as MCI [5], has become relevant for its impact on older people’s quality of life. MCI is classified into two subtypes: amnestic (i.e., clinically significant memory impairment) and non-amnestic (i.e., decline in cognitive functions unrelated to memory). Moreover, other cognitive domains are considered and often compromised, allowing to classify MCI into single-domain amnestic/non-amnestic and multiple-domain amnestic/non-amnestic types [5,6]. To date, no reliable treatments are available for dementia. For these reasons, maintaining the well-being of people also in a prodromal state is an increasing priority, as well as identifying risk factors for cognitive impairment. In this sense, considering sleep quality and duration and sleep disorders can be relevant.
Sleep disturbances are characterized by a decreased sleep duration and quality, reduced sleep efficiency, increased sleep fragmentation, and diurnal sleepiness. Sleep disturbances are common in aging and there is an increase in sleep disorders in pathological aging [7,8,9,10,11,12]. Different studies showed that aging-related poor sleep quality is associated with a worsening in cognitive functions, e.g., [10,13,14], specifically associated with excessive diurnal sleepiness and attentional and executive impairments [15,16,17] as well as general cognitive impairment.
Sleep quality directly affects daily activities and is involved in individual psychological, cognitive, and physical well-being, e.g., [18,19]. Moreover, a cumulative index of sleep problems, rather than specific symptoms of poor sleep, represents the biggest risk factor for cognitive impairment [10]. However, some studies have not highlighted an association between sleep disturbance and impaired cognitive functioning [20]; on the contrary, other authors have reported higher cognitive functioning in a large sample of participants with insomnia [21].
Sleep disorders are common in AD and are involved in memory consolidation impairment [22] and metabolite removal from synapses (i.e., including β-amyloid), which are implicated in the neurogenesis of AD [22,23]. Sleep may also play an important role in cognitive reserve [24] and restoring neurobehavioral functions and psychological aspects, e.g., [25].
One out of four individuals with AD exhibits severe sleep dysfunctions, such as repetitive awakenings or other sleep disorders, i.e., insomnia, hypersomnia, or circadian rhythm disturbances [26,27,28]. Some authors identified sleep disorders in the preclinical phases of AD as a predictor of dementia incidence, such as in MCI [11,29,30,31,32,33,34,35,36]. A third of MCI people exhibit sleep disorders [29]. Most commonly reported sleep disorders are insomnia, sleep breathing-related disorders, restless legs syndrome, and REM sleep behavior disorders [7,32]. However, these results are inconsistent, with some studies reporting no association between sleep disturbances and cognitive decline [20].
This systematic review aims to clarify the relationship between sleep quality and aging, providing the main characteristics of sleep and sleep disturbances in the continuum from healthy aging to MCI and AD. Moreover, results are analyzed according to the type of measure—subjective or objective—used to assess sleep characteristics.
Specifically, this systematic review aims to: (1) evaluate the sleep characteristics of people with MCI compared to healthy older adults; (2) assess the sleep characteristics in people diagnosed with AD compared to healthy older adults; (3) evaluate the sleep characteristics in people diagnosed with MCI compared to AD; (4) compare the presence and severity of sleep disturbances among AD, MCI, and healthy subjects; (5) compare the differences between healthy, MCI and AD subjects in sleepiness, that is considered one of the more predominant features of poor sleep and sleep disorders; (6) assess whether poor sleep quality can be considered a reliable marker of cognitive impairment in a continuum from healthy older adult to mild cognitive impairment to Alzheimer disease.
Given that sleep disorders in the preclinical phases of AD are predictors of dementia incidence [7,29,30,31,32,33,34,35,36], we should find poorer sleep in people diagnosed with MCI or AD than in healthy older people. Furthermore, sleep should be worse, and sleep disturbances should be higher in AD than in MCI people.
2. Materials and Methods
The review process was conducted according to the PRISMA statement [37,38].
2.1. Research Strategies
Two independent researchers (IC, GF) consulted four electronic bibliographic database searches (PsychINFO, PubMed, Medline, PsycArticles). A list of keywords and MeSH terms was generated to identify studies (MCI OR Mild Cognitive Impairment OR dementia OR Alzheimer OR AD); and (sleep); and (elderly OR aged OR older OR elder OR geriatric OR elderly people OR old people OR senior). The last search was conducted on 18 December 2021. Restrictions were made, limiting the research to academic publications with English full texts and studies on human populations without restrictions regarding gender and ethnicity. Additionally, the bibliographical references of retrieved papers, reviews, and meta-analyses were screened manually to assess whether they contained relevant studies to include in the review.
Due to the wide variety of instruments used, diagnostic criteria, and data, a meta-analysis could not be performed.
The search strategies are presented in Table 1.

Table 1.
Research Strategies.
2.2. Eligibility Criteria
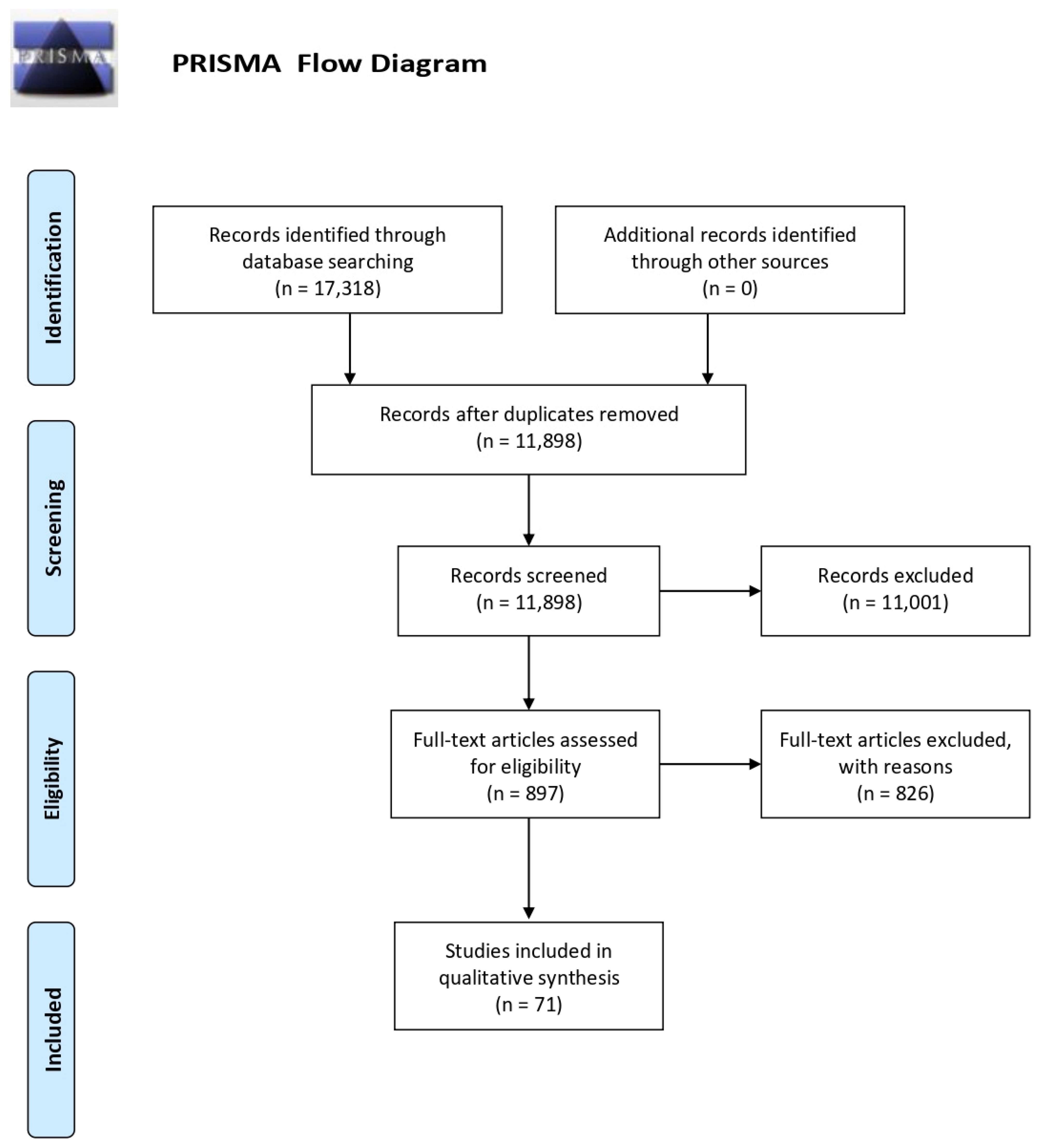
A total of 17,318 articles were obtained from the search procedure. The first step allows 5420 duplicates that were eliminated using the Mendeley software. Then, the list of potential articles produced by systematic research was revised. The reading of the title and abstract allowed the first exclusion of 11,001 studies. A further selection was made by reading the full text (see Figure 1). Two researchers independently performed the eligibility assessment. A supervisor (MC) resolved disagreements. Review and randomized control trials or intervention studies were excluded.
Figure 1.
PRISMA flow diagram.
We selected studies that included the adult population (age equal to or higher than 50 years), diagnosis of mild cognitive impairment; diagnosis of Alzheimer’s disease; healthy subjects; subjective sleep measurements; objective sleep measurements (polysomnography and actigraphy).
We enclosed both studies that included participants with a previous formal diagnosis and studies that diagnosed and classified participants based on their performance in a pool of neuropsychological tests.
We excluded studies including participants with (a) medical conditions that could potentially influence the investigated relationship (e.g., metabolic disorders; cardiovascular diseases; chronic conditions; cancer); (b) dementia (Parkinson’s disease; vascular dementia; frontotemporal dementia; dementia with Lewy bodies; Huntington’s disease); (c) psychiatric or neurological disorders; (d) strokes; (e) head traumas; (f) sleep disorders; (g) use of drugs that affect the nervous system or sleep; (h) studies that presented methodological criticisms; (i) studies that do not report essential data or have an assessment made by caregivers; (l) MCI participants included in healthy older people or AD groups.
2.3. Data Collection
According to the PICOS approach [38], information was extracted from each included study on (1) author(s) and year of publication; (2) characteristics of participants (including age, gender, Mini-Mental State Examination—MMSE score); (3) diagnostic criteria; (4) experimental paradigm; (5) results of the studies. Data were extracted by two researchers (IC and GF), and other researchers (FF and MC) were involved in case of controversy.
The methodological quality of studies was assessed.
2.4. Quality Assessment
The quality of the studies was assessed using the Cochrane Handbook for Systematic Reviews criteria [39], adapted ad hoc according to the objective of this review in order to reduce the risk bias. The analysis used five criteria to screen each study selected for systematic review: sampling bias, sleep measurements, diagnostic criteria, selective reporting bias, and methodological bias. Each criterion score ranges from 1 (low risk) to 3 (high risk). The overall quality shall be calculated by adding all the scores, ranging from 5 to 15. The study was considered at low risk of bias if the score was 5, while a 6–10 range score was considered an indicator of a moderate risk of bias, and an 11–15 range score meant a high risk of bias. The quality assessment was separately made for subjective and objective measurements.
Two independent researchers, IC and GF, rated all articles included in the study, and two other researchers (FF and MC) were involved in case of disputes.
3. Results
3.1. Studies Selection
The flow chart shows the number of studies identified from the databases, and the number of studies examined, assessed for eligibility, and included in the review with the reasons for possible exclusions (see Figure 1). A total of 71 studies were included in the final analysis. The most common exclusion criteria are: diagnosis of non-Alzheimer dementia, diagnosed sleep disorders (i.e., sleep apnea, insomnia, REM sleep behavior disorder), neurological disorders (i.e., stroke, head injury), and psychiatric disorders (i.e., depression, schizophrenia).
Of the 71 selected studies, 33 adopt subjective measurements (questionnaires, sleep diaries, and interviews), and 31 adopt objective measures (23 polysomnography and 8 actigraphy). Ten studies used both measures (6 a combination of questionnaires and polysomnography; 4 questionnaires and actigraphy).
Seventy of the seventy-one studies (98.6%) used a cross-sectional design.
Results will be presented in two subsections: (1) sleep in older age and (2) sleep in pathological older age, also considering the type of measurements adopted.
3.2. Quality Assessment of Subjective Measurements
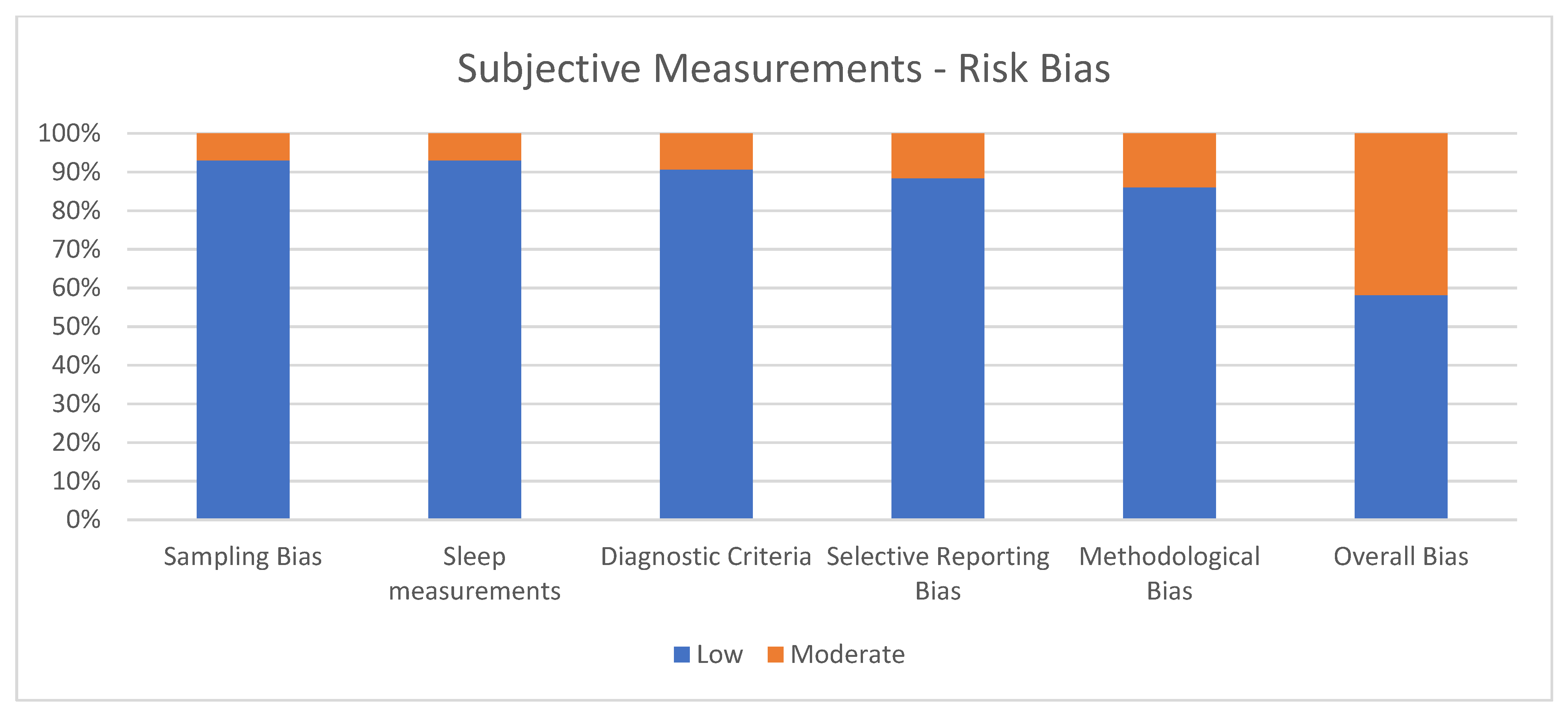
Figure 2 shows the percentage of articles adopting subjective measurements fulfilling each quality criterion assessed by the risk of bias assessment. On average, the quality of the studies was good since 25 out of 43 (58.1%) exhibited a low risk of bias. The high percentage of studies with low or no risk of bias increases the validity of this systematic review. Despite 18 studies (41.9%) showing moderate scores, no study reports a moderate risk of bias in more than two items. A large percentage of the studies adopted valid and reliable tools to measure sleep quality and included an appropriate sample size. Moreover, most studies were adequately controlled for confounding variables. The higher risk of bias was in the “methodological bias” and the lower in “sleep measurements”. The risk of bias ranged from 5 to 7 for every article included (see Figure 2).
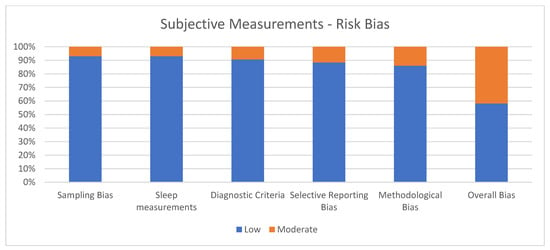
Figure 2.
Subjective measurements.
3.3. Quality Assessment of Objective Measurements
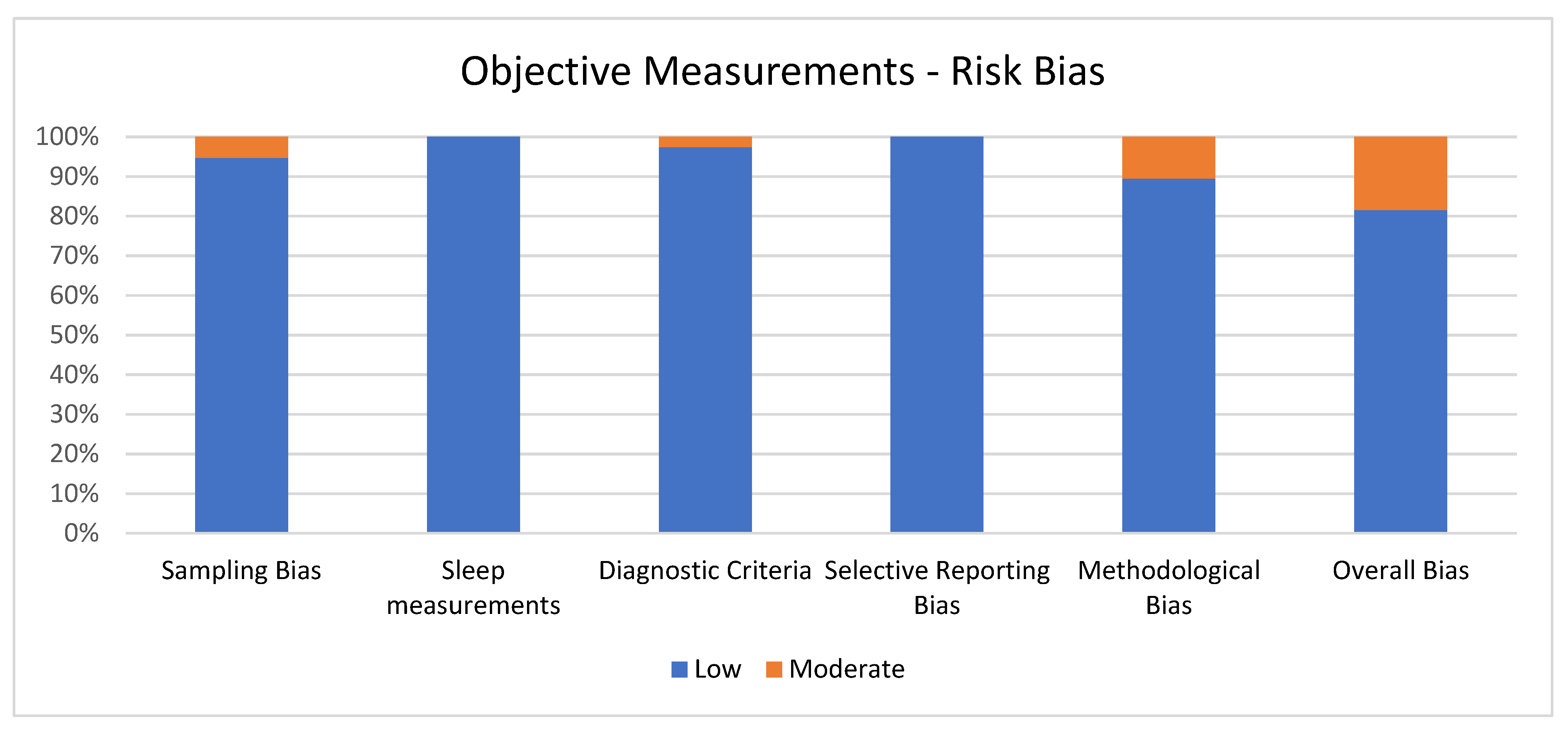
Figure 3 shows the percentage of articles adopting objective measurement fulfilling each quality criterion of risk of bias assessment. On average, the quality of the studies was good since 31 out of 38 (81.6%) exhibited low scores on the risk of bias. The high percentage of studies with low or no risk of bias increases the validity of this systematic review. Despite 7 studies (18.4%) showing moderate scores, no study reports a moderate risk of bias in more than one item. A large percentage of the studies used valid and reliable tools to measure sleep quality and included an appropriate sample size. Moreover, most studies were adequately controlled for confounding variables. The higher risk bias was in the “methodological bias” and the lower in “sleep measurements” and “selective reporting bias”. The score ranged from 5 to 6 for every article included for the overall bias (see Figure 3).
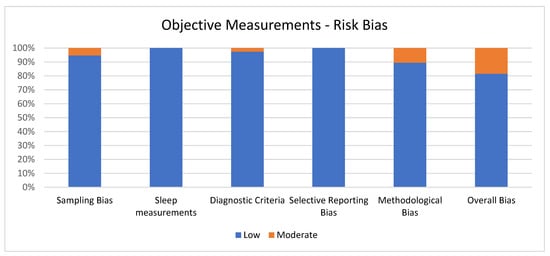
Figure 3.
Objective measurements.
3.4. Sleep in Healthy Older People
3.4.1. Subjective Measurements (N = 14)
Fourteen studies adopted subjective measures to assess sleep quality in healthy older people, with an overall sample size of 1680 individuals and a mean age ranging from 67.2 [40] to 93.7 years [41]. The most adopted tools to assess sleep quality and disturbances were the Pittsburgh Sleep Quality Index—PSQI [42] and the Neuropsychiatric Inventory—NPI. The PSQI assesses seven components of sleep (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunctions) and a global score that measures and discriminates “good sleepers” and “poor sleepers”. The NPI is a questionnaire evaluating twelve behavioral disturbances occurring in dementia, including sleep disturbances (i.e., hallucination, dysphoria, delusions, anxiety, agitation/aggression, euphoria, disinhibition, irritability, apathy, aberrant motor activity, eating abnormalities, and sleep disturbance) and measuring frequency and severity of the symptoms.
Pittsburgh Sleep Quality Index—PSQI Results (N = 11)
Eleven studies met the inclusion criteria and used the PSQI [42] to evaluate sleep quality in healthy older people [40,41,43,44,45,46,47,48,49,50,51]. Six studies considered the percentage of the sample with poor sleep quality [41,43,44,48,51], showing a poor sleep quality that varies from 11.2% [43] to 64% [48]. Five studies reported the average PSQI global score [40,45,46,47,50] with a range from 3.9 [45] to 6.3 [47]. One study [49] reported sleep duration (443.4 min) and sleep latency (12.5 min) of the participants.
Neuropsychiatric Inventory—NPI Results (N = 2)
Two studies adopted the NPI to evaluate sleep quality in healthy older people [52,53], underlining a prevalence of sleep disturbances of 3.8% [53] and 6.5% [52] in the considered samples.
Other Subjective Sleep Measurement Results (N = 4)
Two studies collected data about sleep routines through a sleep diary [48,54]. Bliwise et al. [54] analyzed gender differences in sleep habits but did not find significant differences in sleep quality between males and females. Landry et al. [48] reported a sleep latency of 22.6 min, an average number of awakenings of 2.1, and a sleep duration of 398 min in a sample of 78 participants with a mean age of 71.6. One study met the inclusion criteria and used the Insomnia Severity Index (ISI) to evaluate insomnia severity [43], underlining a prevalence of moderate or severe insomnia in 7.8% of participants with a mean age of 69.9. One study met the inclusion criteria and used the “St. Mary’s Hospital Sleep Questionnaire” [49] to evaluate the sleep-related routines, reporting a sleep latency of 12.4 min and sleep duration of 426 min in a sample of 14 participants with a mean age of 69.1.
Table 2 shows the main characteristics of the studies.

Table 2.
Sleep in older age. Summary of all the studies that use subjective measures.
3.4.2. Objective Measurements (N = 7)
Seven studies assessed the sleep characteristics in healthy older people, with an overall sample of 216 participants and ages ranging from 62.8 [55] to 82.2 [56].
Polysomnographic Results (N = 3)
Three studies met the inclusion criteria and adopted polysomnography to evaluate sleep quality in healthy older people [45,57,58]. The overall sample size was 57 participants, with a mean age ranging from 68.6 [45] to 69 years [57].
The most investigated index was the REM latency, ranging from 57.6 [58] to 91 min [45]. Curcio et al. [45] have compared various polysomnographic variables (total sleep time, total bedtime, sleep efficiency, number of awakenings, percentage, and latency of NREM1, NREM2, REM, and slow-wave sleep) in five different groups: young, healthy older people, subjects with depression, subjects with dementia, and subjects with obstructive sleep apnea syndrome. The healthy older people showed a higher percentage of NREM2 sleep than the other groups.
Prinz et al. [57] compared healthy older people with three other groups (subjects with mild, moderate, and severe dementia), underlining that healthy older people have a better sleep quality than people with dementia, considering time in bed (TIB), NREM 3–4 (%TIB), REM (%TIB), wakefulness (%TIB), number of awakenings, and REM latency (min)].
Reynolds III et al. [58] compared healthy older people with demented and depressed people, considering sleep Latency (min), wakefulness (min), time spent asleep (min), arousal (number), % sleep efficiency, % sleep maintenance, %NREM1, %NREM2, %NREM 3–4, %NREM, %REM, LREM (min), and REM time (min), finding a lower number of sleep-related disorders in healthy older people (see Table 3).

Table 3.
Sleep in older age. Summary of all the studies that use polysomnography.
Table 3 shows the main characteristics of the studies.
Actigraphy Results (N = 4)
Four studies met the inclusion criteria and used actigraphy to evaluate sleep quality in healthy older people [48,56,59,60]. The overall sample size was 159 participants, with a mean age ranging from 62.8 [55] to 82.2 years [56].
Both Wilckens et al. [59] and Kume et al. [56] reported a total sleep time ranging from 362.4 to 380.2 min. Paavilainen et al. [60] showed that time in bed in healthy older people (540 min) was lower than in people with dementia. Landry et al. [48] reported a 19.5% prevalence of poor sleep quality, evidenced by a composite score based on sleep fragmentation equal to or higher than 40, sleep efficiency equal to or less than 75, or sleep duration equal to or less than 360 min.
Table 4 shows the main characteristics of the studies.

Table 4.
Sleep in older age. Summary of all the studies that use actigraphy.
3.5. Sleep in Pathological Older People
The systematic review identified 49 studies assessing sleep quality or sleep disturbances in older people with pathological cognitive impairment. The age of the samples ranged from 62.1 [61] to 86.7 years [59]. Twenty-six studies included a sample with a diagnosis of AD [46,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86], and 28 included a sample with a diagnosis of MCI [63,65,66,68,69,84,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110].
3.5.1. Diagnostic Criteria
Different diagnostic criteria were adopted in the selected studies for the AD or MCI diagnosis.
Twenty studies adopted the diagnostic criteria of the “National Institute of Neurological and Communicative Diseases and Stroke -Alzheimer’s Disease and Related Disorders Association—NINCDS-ADRDA” [46,61,62,64,65,66,67,70,71,72,73,74,77,79,81,83,86,94,101,105,111]. Four studies followed the diagnostic criteria of the “Alzheimer’s Association and the National Institute on Aging—NIA-AA” [63,69,84,85,112]. Three studies used the diagnostic criteria of “Diagnostic and Statistical Manual of Mental Disorders—DSM” ed. III [71,76,80,113]. Three studies adopted the diagnostic criteria of “Diagnostic and Statistical Manual of Mental Disorders—DSM” ed. IV [46,63,93,114]. Two studies followed the diagnostic criteria of “Diagnostic and Statistical Manual of Mental Disorders—DSM” ed. 5 [68,88,115]. Eighteen studies adopted the diagnostic criteria of Petersen [5,63,66,69,87,88,90,92,96,97,99,100,101,102,104,106,110,116,117,118,119]. Five studies used the diagnostic criteria of Albert et al. [59,66,85,92,108,120]. Four studies used the “Mini-Mental State Examination—MMSE” [121] as a diagnostic criterion, with different cut-offs: MMSE lower than 24 [91], MMSE lower than 20 [78], MMSE lower than 21 [80], MMSE lower than 17 [109]. Seven studies adopted other criteria [122,123,124,125], for example, Portet et al. criteria [63,65,75,88,95,98,107,109,122].
3.5.2. Subjective Measurements (N = 27)
Twenty-seven studies assessed sleep quality in pathological older people with a subjective measure. The overall sample size was 4587 participants (540 AD, 1960 MCI, and 2086 healthy older people), with a mean age ranging from 63.7 [90] to 79 years [64].
Nine [62,63,64,65,66,67,68,69,70] studies reported data from AD people and twenty-four [63,65,66,68,69,87,88,89,90,91,92,93,94,95,96,97,98,100,101,102,103,106,107,109] studies on people with MCI diagnosis.
Pittsburgh Sleep Quality Index—PSQI Results (N = 11)
Eleven studies used the PSQI [42] to evaluate sleep quality in pathological older people [63,67,68,70,97,98,99,101,102,103,106]. Tuna et al. [98] included subjects with MCI, underlining a 53.6% prevalence of poor sleep quality in the sample. Shin et al. [67] included subjects with AD, highlighting a slightly poor sleep quality (mean score 5.4) in the sample. Two studies compared sleep quality in healthy, MCI, and AD older people [63,68]. Gorgoni et al. [63] did not report significant differences between the groups, while Tadokoro et al. [68] found a poorer sleep quality in AD subjects than in MCI and healthy subjects.
Six studies [97,99,101,102,103,106] compared the PSQI scores between healthy and MCI older people. Only Sun et al. [97] and Yu et al. [99] reported poorer sleep quality in MCI, while the other studies did not highlight any significant differences [101,102,103]. Zhou et al. [70] compared healthy and AD participants, finding higher PSQI scores and a higher prevalence of poor sleep quality (55.9%) in AD people than in healthy participants (15.2%).
Neuropsychiatric Inventory—NPI Results (N = 13)
Thirteen studies met the inclusion criteria and used the NPI [42] to evaluate the sleep quality of pathological older people [62,64,65,66,69,70,89,90,91,93,94,95].
Peters et al. [93], Reijs et al. [94], and Rozzini et al. [95] assessed sleep quality in older people with MCI diagnosis. The first two studies reported a prevalence of sleep disturbances of 39.9% and 22%, respectively, while Rozzini et al. [95] did not find evidence of sleep disturbances in older people with MCI (mean score: 0.5). Fernández-Martínez et al. [62] and Matsuoka et al. [64] evaluated the NPI in people with AD diagnosis and reported a prevalence of sleep disturbances of respectively 35.1% and 30.2%. Two studies [87,90] compared the sleep disturbances prevalence in healthy and MCI older people. Fernández-Martínez et al. [87] found significant differences between the two groups in sleep disturbances prevalence (MCI = 23,1% vs. healthy older people = 14%). Similarly, Muangpaisan et al. [90] reported a prevalence of sleep disturbances of 45.5% in MCI and 23.3% in the healthy control group. Ng et al. [91], comparing healthy older people and subjects affected by preclinical AD, showed that older people with preclinical AD reported poorer sleep quality than the control group. Lee et al. [89] investigated the difference in sleep disturbances between aMCI and naMCI, but did not find significant differences (18.8% aMCI, 18% naMCI). Two studies [66,69] compared older people with MCI and AD, underlining a poorer sleep quality in AD. In particular, Yatawara et al. [69] observed a prevalence of sleep disturbances of 31% in MCI and 40% in AD. Zhou et al. [70] compared a healthy group with an AD group, reporting a higher number of sleep disturbances in AD. Pocnet et al. [65], on the other hand, assessed the progression of sleep disturbances in a range of two years in healthy, MCI, and AD subjects. The results showed that sleep disturbances remained stable in healthy and AD older people while increased in MCI subjects.
Other Subjective Measurements Results (N = 9)
Sun et al. [97] used the “Insomnia Severity Index—ISI” to evaluate the insomnia severity in healthy and MCI subjects, underlining a greater severity in the MCI group.
One study adopted the “Karolinska Sleep Diary” [102] to evaluate the sleep routine but did not report differences between aMCI and healthy subjects.
One study collected data about sleep routines through an interview [88]. The MCI group showed higher sleep latency, awakenings, sleep after awakening, and lower sleep duration and quality than the healthy group. One study used the “Athens Insomnia Scale—AIS” [107] and reported higher sleep latency, number of sleep disturbances, total sleep time, night awakening, and earlier awakenings in MCI than in healthy participants. One study adopting the “Jenkins Sleep Questionnaire—JSS” [101] did not report any significant difference between aMCI and the healthy control group. One study collected data about sleep routines through an interview [109] and reported a higher sleep duration in older people with MCI than in healthy subjects. Finally, using the “Sleep Continuity Scale in Alzheimer’s Disease—SCADS” [92], no significant difference in sleep routine was observed in healthy and MCI people. However, older people with MCI showed a poor sleep quality in 21.7% of cases, while the prevalence observed in healthy participants was 15.3%. Table 5 shows the main characteristics of the studies.

Table 5.
Sleep in pathological older age. Summary of all the studies that use subjective measures.
3.5.3. Objective Measurements (N = 31)
Thirty-one studies assessed sleep quality in pathological older people using objective measures. The overall sample size was 1415 participants (478 AD, 306 MCI, and 631 healthy older people), with a mean age ranging from 58.3 [77] to 86.7 years [59].
Polysomnography Results (N = 23)
Participants assessed with polysomnography have a mean age ranging from 58.3 [77] to 76.9 years [79].
Twenty-three studies met the inclusion criteria and used the polysomnography to evaluate sleep quality in pathological older people [46,61,63,71,72,73,74,75,76,77,78,79,80,81,84,86,96,100,103,104,105,106,110].
Three studies [73,75,81] considered only AD. Tsuno et al. [81] assessed sleep latency in AD and subjects with multi-infarction dementia. No significant differences were reported between groups. Kundermann et al. [73] assessed sleep latency in AD, showing a mean time of 50 min. Total sleep time (ranges from 292.8 min [73] to 368.7 min [75]), sleep efficiency % (ranging from 59.5 [73] to 74.7 [75]); REM latency (that varies from 124.1 min [73] to 204.8 min [75]), and intra-sleep wakefulness (ranging from 130.8 min [75] to 136.2 min [73]) were investigated
Three studies [63,84,105] adopt polysomnography in healthy, MCI, and AD older people, and all reported some differences: Maestri et al. [105] reported a higher NREM stage 1 in AD than in MCI and healthy participants, while Gorgoni et al. [63] reported a lower slow-wave sleep percentage in AD than in the other two groups. Liguori et al. [85] analyzed the differences among healthy subjects, older people with MCI, mild AD (mAD), and mild-severe AD (msAD): msAD showed lower total sleep time, sleep efficiency %, REM %, and higher time in bed than the other groups; in addition, msAD had higher NREM1% than MCI and healthy subjects. Mild Alzheimer (mAD) presented higher total sleep time, NREM3%, REM% than msAD but lower than healthy and MCI groups; higher sleep efficiency % than msAD but lower than healthy subjects; higher time in bed than msAD, and higher intra-sleep wakefulness, REM latency and NREM1% than the healthy group. The older people group showed higher total sleep time, NREM3% than AD groups, higher sleep efficiency % than msAD but lower than the healthy older people group, lower time in bed than msAD, higher REM latency and intra-sleep wakefulness than the healthy group, higher REM % than AD group but lower than healthy older people, and higher NREM1% than healthy subjects but lower than AD groups.
Eleven studies [46,61,71,72,74,76,77,78,79,80,86] have compared AD and healthy subjects.
The participants with AD have shown: higher sleep latency [80,86], NREM1 and NREM2 latency [46], NREM sleep percentage [71], NREM1 sleep [74,86,105], REM latency [74,86], higher intra-sleep wakefulness [74,78,86]; other authors found lower NREM3 [74,86,105], slow wave [46,63,78], and REM sleep percentages [61,77,78,86,105], total sleep time [61,74,80,105], delta waves [76], K-complex [77], sleep spindles [77,79], and sleep efficiency [71,72,74,86].
Six studies [96,100,103,104,106,110] have compared MCI and healthy older people.
The MCI subjects have shown: higher REM Latency [106,110], intra-sleep wakefulness [106]; higher arousal index during the slow-wave sleep period [96,104]; REM % [96,100,104,105], slow-wave sleep % [103], total sleep time [110], and sleep efficiency % [110].
Furthermore, Carnicelli et al. [100] have observed that, after 2 years, 61.1% of MCI participants met AD diagnostic criteria. MCI converters showed a lower REM sleep % than MCI and healthy older people.
Table 6 shows the main characteristics of the studies.

Table 6.
Sleep in pathological older age. Summary of all the studies that use polysomnography.
Actigraphy Results (N = 8)
Participants assessed with actigraphy have a mean age ranging from 61.8 [85] to 86.7 years [59].
Eight studies met the inclusion criteria and used actigraphy to evaluate sleep quality in pathological older people [59,68,82,83,85,101,102,108].
Three studies compared AD and healthy subjects; Lee et al. [83] did not show a significant difference, while Khou et al. [82] reported a higher total sleep time and a higher time in bed in AD. Liguori et al. [84] observed a higher sleep latency and a lower sleep efficiency in AD.
Four studies compared MCI and healthy subjects; two did not show significant differences. One [101] observed some differences in sleep onset and higher variability in MCI, while the other [108] highlighted lower SE% in MCI subjects.
Tadokoro et al. [68] is the only study that compared MCI, AD, and healthy older people. It reported lower REM sleep and higher NREM1 and NREM2 sleep in AD than in MCI and healthy, and lower REM sleep in MCI than in healthy older people.
Table 7 shows the main characteristics of the studies.

Table 7.
Sleep in pathological older age. Summary of all the studies that use actigraphy.
3.6. Diurnal Sleepiness (N = 10)
Diurnal sleepiness is not a sleep disorder but a strong indirect index of poor sleep. The most widely used test to assess daytime sleepiness is the Epworth Sleepiness Scale—ESS, which evaluates sleepiness in eight different situations and the chances of feeling drowsiness and falling asleep.
3.6.1. Healthy Older People (N = 2)
Two studies used the Epworth Sleepiness Scale (ESS) to evaluate diurnal sleepiness in healthy older people [43,126]. The sample size includes 507 participants, with a mean age ranging from 69.9 [43] to 73.2 years [126].
Bernstein et al. [43] underlined a prevalence of 18% of sleepiness, while Ward et al. [127] reported a mean score of 5.9 in diurnal sleepiness in the respondents.
Table 8 shows the main characteristics of the studies.

Table 8.
Diurnal sleepiness in healthy older age. Summary of all the studies.
3.6.2. Pathological Older People (N = 8)
Eight studies met the inclusion criteria and used the ESS to evaluate the diurnal sleepiness in pathological older people [68,70,88,96,97,101,102,106]. The sample size includes 515 participants (105 AD, 160 MCI, and 250 healthy older people), with a mean age ranging from 66.5 [70] to 75.6 years [102].
Six of these studies [70,88,96,97,101,102,106] compared daytime sleepiness in healthy and MCI subjects, and only two studies [97,106] reported higher diurnal sleepiness in MCI than healthy older people. Zhou et al. [70] observed higher diurnal sleepiness in Alzheimer’s compared to the healthy group. Finally, Tadokoro et al. [68] compared healthy MCI and AD and did not find significant differences.
Table 9 shows the main characteristics of the studies.

Table 9.
Diurnal sleepiness in pathological older age. Summary of all the studies.
4. Discussion
Defining possible risks or exacerbating factors for pathological cognitive decline represents an important goal of the current research on aging and dementia. Accordingly, this systematic review aimed to clarify the possible role of sleep as a predictor of cognitive impairment in aging, analyzing sleep quality and sleep disturbances in healthy and pathological aging. The analyzed studies show that, in older people, the prevalence of sleep disturbance ranges from 3.8% [53] to 64% [48], in the MCI it ranges between 18% [89] and 53.6% [98], and finally in individuals with Alzheimer’s disease, the prevalence ranges between 30.2% [64] and 40% [69].
Twenty-six out of twenty-eight (92.8%) studies that used objective measures and compared healthy and pathological old people found worse sleep quality in the latter, while only nine out of twenty-two (40.9%) studies that used subjective measures showed these differences.
The results pointed out that thirty-four out of forty-five studies (75.5%) show a higher prevalence of sleep disturbances in pathological aging (AD and MCI) than in healthy older people. In the data provided by these studies, we can observe an overall poorer sleep quality in pathological older people, particularly a higher compromission in AD people than in MCI and healthy older people. These results underline that sleep alterations are associated with cognitive impairment [128,129,130,131,132,133,134].
The first goal of our systematic review was to evaluate the sleep characteristics of people with mild cognitive impairment compared to healthy older people, and 17 studies out of 23 (73.9%) observed a lower sleep quality in MCI than in healthy subjects. The main differences reported were lower REM%, slow-wave sleep and sleep efficiency and higher sleep latency, NREM1%, and sleep duration; these findings are confirmed by different studies [13,135,136]. Moreover, these results highlighted that sleep is more compromised in MCI participants despite the high prevalence of poor sleep and sleep disturbances in healthy older people (3.8–64%).
The second goal was to investigate the sleep characteristics in people diagnosed with Alzheimer’s disease compared to healthy older people. The more compromised sleep aspects in AD are slow-wave sleep and REM sleep; consequently, people show high sleep fragmentation and poor sleep efficiency. However, conflicting results emerged regarding stage 2 of NREM sleep [71,105]. In particular, Maestri et al. [105] observed that AD had a higher percentage of stage 2 NREM sleep than healthy older people, while Hoch et al. [71] reported that AD had a lower percentage of stage 2 NREM sleep than healthy older people. Additionally, polysomnographic results show a worsening of sleep, involving both sleep structure, e.g., [46,73,77,78,79,105] and sleep quality [66,68,70,84], in AD than in healthy older people.
The third goal was to assess the differences in the presence and severity of sleep disturbances between MCI and AD. People with AD were compared to older people with MCI in seven studies, and four studies out of seven (57.1%) found poorer sleep quality in AD. In particular, AD exhibited higher NREM1% and time in bed and lower total sleep time, sleep efficiency, NREM3%, and REM% compared to MCI.
The fourth goal was to compare the presence and severity of sleep disturbances among AD, MCI, and healthy subjects. However, only four studies compared these three groups concurrently [63,68,84,105]. Maestri et al. [105] observed that older people with MCI presented intermediate sleep disorders between healthy subjects and people with AD. In fact, MCI had poorer sleep quality than healthy subjects but higher than older people with AD; in addition, Tadokoro et al. [68] observed lower REM sleep percentage and higher percentages of stage 1 and stage 2 NREM sleep in AD than in MCI and healthy subjects, and lower REM sleep in MCI than in healthy older people. While Liguori et al. [85] observed that older people with MCI had lower sleep efficiency, REM% than healthy subjects but higher than subjects with AD. Furthermore, older people with MCI had higher NREM1% than control groups but lower than participants with AD. These results are in line with previous studies analyzing sleep in normal and pathological older age, e.g., [7,8,9,12,28].
The fifth and final goal was to evaluate the differences between healthy, MCI and AD subjects in daytime sleepiness, considered one of the more predominant features of poor sleep and sleep disorders. Only two out of eight studies (25%) found higher sleepiness in subjects with AD and MCI than in healthy older people. The fact that 75% of the studies found no significant difference between the groups can be explained by the increased diurnal sleepiness in the healthy older people control group compared to younger subjects [137,138,139].
Finally, most of the studies were cross-sectional (70 out of 71; 98.6%); therefore, it is impossible to indicate a causal relationship. However, the only longitudinal study (1 out of 71; 1.4%) observed that MCI participants showed a greater increase in sleep disturbances after two years compared to healthy older people and people with AD [65]. Furthermore, other studies [90,140] observed that poor sleep quality was associated with deposits of beta-amyloid plaques and lower volumes in the amygdala, hippocampi, and bilateral parietal lobules compared. These findings agree with results showing that beta-amyloid plaques and hippocampi atrophies seem to be predictors of dementia [140]. According to these data, we can infer that sleep tends to worsen with age until it develops into overt sleep disturbances as MCI and AD progress [7,29,30,32,33,34]. Additionally, the present systematic review results strengthen the hypothesis that a decrease in sleep time and quality, including sleep disturbances, constitutes a strong biological marker of cognitive impairment in aging and for the transition from healthy aging to MCI and from the latter to AD. These results are consistent with well-known data suggesting that sleep loss can lead to cognitive decline [15,16,141,142]. The future direction of this study involves implementing a meta-analysis to also have a quantitative estimate of sleep problems. we also plan to study other dementia conditions, as studying only these two pathological conditions is a limiting factor.
Limitation
Although the encouraging results, this review holds some limitations.
Despite the considerable number of studies that assessed sleep subjects with AD, just a few studies evaluated sleep in mild cognitive impairment or the influence of poor sleep quality on the cognitive functions of subjects with MCI. The small number of studies on older people with MCI, the multiplicity of instruments used to assess sleep, and the different criteria for diagnosing MCI and AD limited this review, particularly the subjective measures, making comparisons difficult. Another limiting factor was the lack of a meta-analysis, which was difficult to conduct due to the large variety of instruments, diagnostic criteria, and reported results from the examined studies. Another limiting aspect is the lack of longitudinal studies. An additional limiting factor is the presence of multiple types of dementia (mixed dementia) that overlap making it difficult to identify a specific sleep pattern for each dementia phenotype. Lastly, this review exclusively analyzed two types of pathological aging (mild cognitive impairment and Alzheimer’s disease), omitting the other types of dementia (e.g., vascular dementia, frontotemporal dementia).
5. Conclusions
This systematic review has shown a higher prevalence of sleep disturbances in older people with mild cognitive impairment and Alzheimer’s disease than in healthy older people. AD and MCI showed modifications over every aspect of sleep: sleep quality, sleep structure, sleep disturbance, and duration of wakefulness intra-sleep. This study could be useful because it tried to investigate the differences between three groups of participants (subjects with AD, MCI, and healthy older people) and highlights the different sleep disturbances found in healthy and pathological aging.
An important goal for the next studies will be to figure out whether sleep disturbances and poor sleep quality are early symptoms of dementia or whether dementia leads to poorer sleep due to the more significant alterations of the nervous system occurring in pathologically older age than in healthy older people.
Author Contributions
Conceptualization, I.C. and M.C.; methodology, M.C., G.F., F.F. and I.C.; investigation, I.C., G.F. and F.F.; writing—original draft preparation, M.C., G.F., F.F. and I.C.; writing—review and editing, M.C., G.F., F.F. and I.C.; supervision, M.C. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Casagrande, M. Executive functions in Alzheimer disease: A systematic review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive Functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 1 September 2021).
- Ashraf, G.M.; Chibber, S.; Mohammad; Zaidi, S.K.; Tabrez, S.; Ahmad, A.; Shakil, S.; Mushtaq, G.; Baeesa, S.S.; Kamal, M.A. Recent Updates on the Association Between Alzheimer’s Disease and Vascular Dementia. Med. Chem. 2016, 12, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild Cognitive Impairment. Minneap. Minn. 2016, 22, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.R.; Ancoli-Israel, S. Normal and abnormal sleep in the elderly. Handb. Clin. Neurol. 2011, 98, 653–665. [Google Scholar]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 2000, 17, 285–311. [Google Scholar] [CrossRef]
- Espiritu, J.R.D. Aging-Related Sleep Changes. Clin. Geriatr. Med. 2008, 24, 1–14. [Google Scholar] [CrossRef]
- Gadie, A.; Shafto, M.; Leng, Y.; Kievit, R.A. How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 2017, 7, e014920. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and human aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Monk, T.H. Aging human circadian rhythms: Conventional wisdom may not always be right. J. Biol. Rhythm. 2005, 20, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R. Executive functions in Alzheimer disease: A systematic review. Sleep Sci. 2015, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fortier-Brochu, É.; Beaulieu-Bonneau, S.; Ivers, H.; Morin, C.M. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Med. Rev. 2012, 16, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Marotta, A.; Fuentes, L.J.; Casagrande, M. Inhibition of Return, but not Facilitation, Disappears under Vigilance Decrease due to Sleep Deprivation. Exp. Psychol. 2014, 61, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Casagrande, M.; Lupianez, J. Alerting, Orienting and Executive Control: The effects of sleep deprivation on the attentional networks. Exp. Brain Res. 2011, 210, 81–89. [Google Scholar] [CrossRef]
- Maccari, L.; Martella, D.; Marotta, A.; Sebastiani, M.; Banaj, N.; Fuentes, L.J.; Casagrande, M. Effects of sleep loss on emotion recognition: A dissociation between face and word stimuli. Exp. Brain Res. 2014, 232, 3147–3157. [Google Scholar] [CrossRef]
- Casagrande, M.; Forte, G.; Tambelli, R.; Favieri, F. The Coronavirus Pandemic: A possible Model of the Direct and Indirect Impact of the Pandemic on Sleep Quality in Italians. Nat. Sci. Sleep 2021, 13, 191–199. [Google Scholar] [CrossRef]
- Gothe, N.P.; Ehlers, D.K.; Salerno, E.A.; Fanning, J.; Kramer, A.F.; McAuley, E. Physical activity, sleep and quality of life in older adults: Influence of physical, mental and social well-being. Behav. Sleep Med. 2020, 18, 797–808. [Google Scholar] [CrossRef]
- Mecca, A.P.; Michalak, H.R.; McDonald, J.W.; Kemp, E.C.; Pugh, E.A.; Becker, M.L.; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Sleep disturbance and the risk of cognitive decline or clinical conversion in the ADNI cohort. Dement. Geriatr. Cogn. Disord. 2018, 45, 232–242. [Google Scholar] [CrossRef]
- Kyle, S.D.; Sexton, C.E.; Feige, B.; Luik, A.I.; Lane, J.; Saxena, K.; Spiegelhalder, K. Sleep and cognitive performance: Cross-sectional associations in the UK Biobank. Sleep 2017, 38, 85–91. [Google Scholar] [CrossRef]
- Prince, T.M.; Abel, T. The impact of sleep loss on hippocampal function. Learn. Mem. 2013, 20, 558–569. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.E.; Bigal, M.E.; Katz, M.J.; Brickman, A.M.; Lipton, R.B. Sleep onset/maintenance difficulties and cognitive function in nondemented older adults: The role of cognitive reserve. J. Int. Neuropsychol. Soc. 2012, 18, 461. [Google Scholar] [CrossRef]
- Casagrande, M.; Favieri, F.; Tambelli, R.; Forte, G. The enemy who sealed the world: Effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep 2020, 75, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.; Lynch, C.A.; Walsh, C.; Coen, R.; Coakley, D.; Lawlor, B.A. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep 2005, 6, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Davis, J.E. A Qualitative Examination of the Phenomenon of Sundowning. J. Nurs. Scholarsh 2007, 39, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Lee, C.U.; Lim, H.K. Role of Sleep Disturbance in the Trajectory of Alzheimer’s Disease. Clin. Psychopharmacol. Neurosci. 2017, 15, 89–99. [Google Scholar] [CrossRef]
- Beaulieu-Bonneau, S.; Hudon, C. Sleep disturbances in older adults with mild cognitive impairment. Int. Psychogeriatr. 2009, 21, 654–666. [Google Scholar] [CrossRef]
- Diem, S.J.; Blackwell, T.L.; Stone, K.L.; Yaffe, K.; Tranah, G.; Cauley, J.A.; Ensrud, K.E. Measures of sleep–wake patterns and risk of mild cognitive impairment or dementia in older women. Am J. Geriatr. 2016, 24, 248–258. [Google Scholar] [CrossRef]
- Dlugaj, M.; Weinreich, G.; Weimar, C.; Stang, A.; Dragano, N.; Wessendorf, T.E. Heinz Nixdorf Recall Study Investigative Group; Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J. Alzheimer’s Dis. 2014, 41, 479–497. [Google Scholar] [CrossRef]
- Guarnieri, B.; Adorni, F.; Musicco, M.; Appollonio, I.; Bonanni, E.; Caffarra, P.; Caltagirone, C.; Cerroni, G.; Concari, L.; Cosentino, F.I.I.; et al. Prevalence of Sleep Disturbances in Mild Cognitive Impairment and Dementing Disorders: A Multicenter Italian Clinical Cross-Sectional Study on 431 Patients. Dement. Geriatr. Cogn. Disord. 2012, 33, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.E.S.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Holtzman, D.M. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013, 70, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.E.S.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat. Rev. Neurol. 2014, 10, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.S.; Kowgier, M.; Buchman, A.S.; Bennett, D.A. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 2013, 36, 1027–1032. [Google Scholar] [CrossRef]
- Sterniczuk, R.; Theou, O.; Rusak, B.; Rockwood, K. Sleep disturbance is associated with incident dementia and mortality. Curr. Alzheimer Res. 2013, 10, 767–775. [Google Scholar] [CrossRef]
- Liberati, A.; Douglas, G.A.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, 1–34. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Schmidt, C.; Peigneux, P.; Cajochen, C.; Collette, F. Adapting test timing to the sleep-wake schedule: Effects on diurnal neurobehavioral performance changes in young evening and older morning chronotypes. Chronobiol. Int. 2012, 29, 482–490. [Google Scholar] [CrossRef]
- Jirong, Y.; Changquan, H.; Hongmei, W.; Hongmei, D.; Bi-Rong, D. Association of sleep quality and dementia among long-lived Chinese older adults. Age 2013, 35, 1423–1432. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new Instrument for Psychiatric Practice and Research. J. Psychiatr. Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Bernstein, J.P.K.; De Vito, A.; Weitzner, D.S.; MacAulay, R.; Calamia, R.; Keller, J.N. Examining Relationships between Multiple Self-Reported Sleep Measures and Gait Domains in Cognitively Healthy Older Adults. J. Gerontol. 2019, 66, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Rainey-Smith, S.R.; Villemagne, V.L.; Weinborn, M.; Bucks, R.S.; Sohrabi, H.R.; Martins, R.N. The relationship between sleep quality and brain amyloid burden. J. Sleep Res. 2016, 39, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef] [PubMed]
- De Gennaro, L.; Gorgoni, M.; Reda, F.; Lauri, G.; Truglia, I.; Cordone, S.; Rossini, P.M. The Fall of Sleep K-Complex in Alzheimer Disease. Sci. Rep. 2017, 7, 39688. [Google Scholar] [CrossRef]
- Fjell, A.; Idland, A.V.; Sala-Llonch, R.; Watne, L.O.; Borza, T.; Brækhus, A.; Walhovd, K.B. Neuroinflammation and tau interact with amyloid in predicting sleep problems in aging independently of atrophy. Cereb. Cortex 2018, 28, 2775–2785. [Google Scholar] [CrossRef]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Mary, A.; Bourguignon, M.; Wens, V.L.; Op de Beeck, M.; Leproult, R.; De Tiège, X.; Peigneux, P. Aging reduces experience-induced sensorimotor plasticity A magnetoencephalographic study. Neuroimage Clin. 2015, 104, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rainey-Smith, S.R.; Mazzucchelli, G.N.; Villemagne, V.L.; Brown, B.M.; Porter, T.; Weinborn, M.; Laws, S.M. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Trans. Psychiatry 2018, 8, 47. [Google Scholar] [CrossRef]
- Sani, T.P.; Bond, R.L.; Marshall, C.R.; Hardy, C.J.D.; Russell, L.L.; Moore, K.M.; Warren, J.D. Sleep symptoms in syndromes of frontotemporal dementia and Alzheimer’s disease: A proof-of-principle behavioural study. eNeurologicalSci 2019, 17, 100212. [Google Scholar] [CrossRef]
- Squelard, G.P.; Missotten, P.A.; Paquay, L.; De Lepeleire, J.; Buntinx, F.J.V.M.; Fontaine, O.; Ylieff, M.J.D. Neuropsychiatric Inventory data in a Belgian sample of elderly persons with and without dementia. Clin. Interv. Aging 2012, 7, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Tatsch, M.F.; Bottino, C.M.D.; Azevedo, D.J.; Hototian, S.R.; Moscoso, M.A.; Folquitto, J.C.; Louza, M.R. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: Prevalence and relationship with dementia severity. Am. J. Geriatr. Psychiatry 2006, 14, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Bliwise, D.L.; Tinklenberg, J.R.; Yesavage, J.A. Timing of sleep and wakefulness in Alzheimer’s disease patients residing at home. Biol. Psychiatry 1992, 31, 1163–1165. [Google Scholar] [CrossRef]
- Wilckens, K.A.; Woo, S.G.; Erickson, K.I.; Wheeler, M.E. Sleep continuity and total sleep time are associated with task-switching and preparation in young and older adults. J. Sleep Res. 2014, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Kume, Y.; Kodama, A.; Sato, K.; Kurosawa, S.; Ishikawa, T.; Ishikawa, S. Sleep/awake status throughout the night and circadian motor activity patterns in older nursing-home residents with or without dementia, and older community-dwelling people without dementia. Int. Psychogeriatr. 2016, 28, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.N.; Vitaliano, P.P.; Vitiello, M.V.; Bokan, J.; Raskind, M.; Peskind, E.; Gerber, C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging 1982, 3, 361–370. [Google Scholar] [CrossRef]
- Reynolds, C.F.; Kupfer, D.J.; Taska, L.S.; Hoch, C.C. Slow wave sleep in elderly depressed, demented, and healthy subjects. J. Sleep Res. 1985, 8, 155–159. [Google Scholar] [CrossRef][Green Version]
- Wilckens, K.A.; Tudorascu, D.L.; Snitz, B.E.; Prince, J.C.; Aizenstein, H.J.; Lopez, O.L.; Cohen, A.D. Sleep moderates the relationship between amyloid beta and memory recall. Neurobiol. Aging 2018, 71, 142–148. [Google Scholar] [CrossRef]
- Paavilainen, P.; Korhonen, I.B.; Lötjönen, J.; Cluitmans, L.O.; Jylhä, M.; Särelä, A.; Partinen, M. Circadian activity rhythm in demented and non-demented nursing-home residents measured by telemetric actigraphy. J. Sleep Res. 2005, 14, 61–68. [Google Scholar] [CrossRef]
- Dykierek, P.; Stadtmüller, G.; Schramm, P.; Bahro, M.; van Calker, D.; Braus, D.F.; Riemann, D. The value of REM sleep parameters in differentiating Alzheimer’s disease from old-age depression and normal aging. J. Psychiatr. Res. 1998, 32, 1–9. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Castro, J.; Molano, A.; Zarranz, J.J.; Rodrigo, R.M.; Ortega, R. Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr. Alzheimer Res. 2008, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, M.; Lauri, L.; Truglia, I.; Cordone, S.; Sarasso, S.; Scarpelli, S.; De Gennaro, L. Parietal Fast Sleep Spindle Density Decrease in Alzheimer’s Disease and Amnesic Mild Cognitive Impairment. J. Neural Transpl. Plast. 2016, 2016, 8376108. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Imai, A.; Fujimoto, H.; Kato, Y.; Shibata, K.; Nakamura, K.; Narumoto, J. Neural Correlates of Sleep Disturbance in Alzheimer’s Disease: Role of the Precuneus in Sleep Disturbance. J. Alzheimer’s Dis. 2018, 63, 957–964. [Google Scholar] [CrossRef]
- Pocnet, C.; Antonietti, J.P.; Donati, A.; Popp, J.; von Gunten, A. Behavioral and psychological symptoms and cognitive decline in patients with amnestic MCI and mild AD: A two-year follow-up study. Int. Psychogeriatr. 2015, 27, 79–1389. [Google Scholar] [CrossRef]
- Scaricamazza, E.; Colonna, I.; Sancesario, G.M.; Assogna, F.; Orfei, M.D.; Franchini, F.; Liguori, C. Neuropsychiatric symptoms differently affect mild cognitive impairment and Alzheimer’s disease patients: A retrospective observational study. Neurol Sci 2019, 40, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Han, H.J.; Shin, D.J.; Park, H.M.; Lee, Y.B.; Park, K.H. Sleep Problems Associated with Behavioral and Psychological Symptoms as Well as Cognitive Functions in Alzheimer’s Disease. Clin. Neurol. Neurosurg. 2014, 10, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, K.; Ohta, Y.; Hishikawa, N.; Nomura, E.; Wakutani, Y.B.; Takao, Y.; Abe, K. Discrepancy of subjective and objective sleep problems in Alzheimer’s disease and mild cognitive impairment detected by a home-based sleep analysis. J. Clin. Neurosci. 2020, 74, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yatawara, C.; Hiu, S.; Tan, L.; Kandiah, N. Neuropsychiatric symptoms in South-East Asian patients with mild cognitive impairment and dementia: Prevalence, subtypes, and risk factors. Int. J. Geriatr. Psychiatry 2017, 33, 122–130. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Yu, X.; Zhao, X.; Ma, L.; Shan, P. High prevalence of sleep disorders and behavioral and psychological symptoms of dementia in late-onset Alzheimer disease: A study in Eastern China. Am. J. Med. 2019, 98, e18405. [Google Scholar] [CrossRef]
- Hoch, C.C.; Reynolds, C.F., III; Houck, P.R. Sleep patterns in Alzheimer, depressed, and healthy elderly. West. J. Nurs. Res. 1998, 10, 239–256. [Google Scholar] [CrossRef]
- Hot, P.; Rauchs, G.; Bertran, F.; Denise, P.; Desgranges, B.; Clochon, P.; Eustache, F. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer’s disease. Biol. Psychol. 2011, 87, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kundermann, B.; Thum, A.; Rocamora, R.; Haag, A.; Krieg, J.C.; Hemmeter, U. Comparison of polysomnographic variables and their relationship to cognitive impairment in patients with Alzheimer’s disease and frontotemporal dementia. J. Psychiatr. Res. 2011, 45, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Chiaravalloti, A.; Nuccetelli, M.; Izzi, F. Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer’s disease. J. Neurol. 2017, 264, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Mercuri, M.B.; Nuccetelli, M.; Izzi, F.; Bernardini, S.; Placidi, F. Cerebrospinal Fluid Orexin Levels and Nocturnal Sleep Disruption in Alzheimer’s Disease Patients Showing Neuropsychiatric Symptoms. J. Neurol. 2018, 66, 993–999. [Google Scholar] [CrossRef]
- Loewenstein, R.J.; Weingartner, H.; Gillin, J.C.; Kaye, W.; Ebert, W.; Mendelson, W.B. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol. Aging 1982, 3, 371–377. [Google Scholar] [CrossRef]
- Montplaisir, J.; Petit, D.; Lorrain, D.; Gauthier, S. Sleep in Alzheimer’s disease: Further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. J. Sleep Res. 1995, 18, 145–148. [Google Scholar] [CrossRef]
- Prinz, P.N.; Peskind, E.R.; Vitaliano, P.P.; Raskind, M.A.; Eisdorfer, C.; Zemcuznikov, N.; Gerber, C.J. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J. Am. Geriatr. Soc. 1982, 30, 86–93. [Google Scholar] [CrossRef]
- Rauchs, G.; Schabus, M.; Parapatics, S.; Bertran, F.; Clochon, P.; Anderer, P. Is there a link between sleep changes and memory in Alzheimer’s disease? NeuroReport: For Rapid. Neurosci. Res. Commun. 2008, 19, 1159–1162. [Google Scholar]
- Reynolds, C.F.; Kupfer, D.J.; Taska, L.S.; Hoch, C.C.; Spiker, D.G.; Sewitch, D.E.; Morycz, R. EEG sleep in elderly depressed, demented, and healthy subjects. Biol. Psychiatry 1985, 20, 431–442. [Google Scholar] [CrossRef]
- Tsuno, N.; Shigeta, M.; Hyoki, K.; Faber, P.L.; Lehman, D. Fluctuations of Source of EEG Activity during Transation. Neuropsychobiology 2004, 50, 267–272. [Google Scholar] [CrossRef]
- Khou, C.S.; Rendzia, B.; Watts, A. Evaluation of waist-worn actigraphy monitors for the assessment of sleep in older adults with and without Alzheimer’s disease. J. Rehabil. Assist. Technol. Eng. 2018, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Friedland, R.; Whitehouse, P.I.; Woo, J.I. Twenty-Four-Hour Rhythms of Sleep-Wake Cycle and Temperature in Alzheimer’s Disease. J. Neuropsychiatry Clin. 2004, 16, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Spanetta, M.; Izzi, F.; Franchini, F.; Nuccetelli, M.; Sancesario, G.M.; Di Santo, S.; Bernardini, S.; Mercuri, N.B.; Placidi, F. Sleep-wake cycle in Alzheimer’s disease is associated with tau pathology and orexin dysregulation. J. Alzheimer’s Dis. 2020, 74, 501–508. [Google Scholar] [CrossRef]
- Liguori, C.; Placidi, F.; Izzi, F.; Spanetta, M.; Mercuri, N.B.; Di Pucchio, A. Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer’s disease course. Alzheimer’s Res. Ther. 2020, 12, 1–13. [Google Scholar]
- Liguori, C.; Romigi, A.; Nuccetelli, M.; Zannino, S.; Sancesario, G.; Martorana, A.; Placidi, F. Orexinergic System Dysregulation, Sleep Impairment, and Cognitive Decline in Alzheimer Disease. JAMA Neurol. 2014, 71, 1498–1505. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Molano, A.; Castro, J.; Zarranz, J.J. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease, and its relationship with cognitive impairment. Curr. Alzheimer Res. 2010, 7, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Hita-Yañez, E.; Atienza, M.; Cantero, J.L.; Hita-Yanez, E.; Atienza, M.; Cantero, J.L. Polysomnographic and subjective sleep markers of mild cognitive impairment. J. Sleep Res. 2013, 36, 1327–1334. [Google Scholar] [CrossRef]
- Lee, K.S.; Cho, H.S.; Hong, C.H.; Kim, D.G.; Oh, B.H. Differences in neuropsychiatric symptoms according to mild cognitive impairment subtypes in the community. Dement. Geriatr. Cogn. Disord. 2008, 26, 212–217. [Google Scholar] [CrossRef]
- Muangpaisan, W.; Intalapaporn, S.; Assantachai, P. Neuropsychiatric symptoms in the community-based patients with mild cognitive impairment and the influence of demographic factors. Int. J. Geriatr. Psychiatry 2008, 23, 699–703. [Google Scholar] [CrossRef]
- Ng, K.P.; Pascoal, T.A.; Mathotaarachchi, S.; Chung, C.O.; Benedet, A.L.; Shin, M.; Initi, A.D.N. Neuropsychiatric symptoms predict hypometabolism in preclinical Alzheimer disease. Neurology 2017, 88, 1814–1821. [Google Scholar] [CrossRef]
- Palmer, K.; Mitolo, M.; Burgio, F.; Meneghello, F.; Venneri, A. Sleep Disturbance in Mild Cognitive Impairment and Association with Cognitive Functioning. A Case-Control Study. Front. Aging Neurosci. 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.R.; Rockwood, K.; Black, S.E.; Hogan, D.B.; Gauthier, S.G.; Loy-English, I.; Feldman, H.H. Neuropsychiatry symptom clusters and functional disability in cognitively-impaired-not-demented individuals. Am J. Geriatr. 2008, 16, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Reijs, B.L.R.; Vos, S.J.B.; Soininen, H.; Lotjonen, J.; Koikkalainen, J.; Pikkarainen, M.; Visser, P.J. Association between Later Life Lifestyle Factors and Alzheimer’s Disease Biomarkers in Non-Demented Individuals: A Longitudinal Descriptive Cohort Study. J. Alzheimer’s Dis. 2017, 60, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Rozzini, L.; Chilovi, B.V.; Peli, M.; Conti, M.; Rozzini, R.; Trabucchi, M.; Padovani, A. Anxiety symptoms in mild cognitive impairment. Int. J. Geriatr. Psychiatry 2009, 24, 300–305. [Google Scholar] [CrossRef]
- Sanchez-Espinosa, M.P.; Atienza, M.; Cantero, J.L. Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-beta and cortical thinning. Neuroimage Clin. 2014, 98, 395–404. [Google Scholar] [CrossRef]
- Sun, Q.; Lou, L.; Ren, H.; Wei, C.; Xing, M.; Cheng, Y.; Zhang, N. Semantic clustering and sleep in patients with amnestic mild cognitive impairment or with vascular cognitive impairment-no dementia. Int. Psychogeriatr. 2016, 28, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Tuna, F.; Üstündağ, A.; Başak, C.H.; Tuna, H. Rapid Geriatric Assessment, Physical Activity, and Sleep Quality in Adults Aged more than 65 Years: A Preliminary Study. J. Nutr. Health Aging 2019, 23, 617–622. [Google Scholar] [CrossRef]
- Yu, J.; Mahendran, R.; Rawtaer, I.; Kua, E.H.; Feng, L. Poor sleep quality is observed in mild cognitive impairment and is largely unrelated to depression and anxiety. Aging Ment. Health 2017, 21, 823–828. [Google Scholar] [CrossRef]
- Carnicelli, L.; Maestri, M.; Di Coscio, E.; Tognoni, G.; Fabbrini, M.; Schirru, A.; Bonanni, E. A longitudinal study of polysomnographic variables in patients with mild cognitive impairment converting to Alzheimer’s disease. J. Sleep Res. 2019, 28, e12821. [Google Scholar] [CrossRef]
- Wams, E.J.; Wilcock, G.K.; Foster, R.G.; Wulff, K. Sleep-Wake Patterns and Cognition of Older Adults with Amnestic Mild Cognitive Impairment (aMCI): A Comparison with Cognitively Healthy Adults and Moderate Alzheimer’s Disease Patients. Curr. Alzheimer Res. 2017, 14, 1030–1041. [Google Scholar] [CrossRef]
- Westerberg, C.E.; Lundgren, E.M.; Florczak, S.M.; Mesulam, M.M.; Weintraub, S.; Zee, P.C.; Paller, K.A. Sleep Influences the Severity of Memory Disruption in Amnestic Mild Cognitive Impairment Results from Sleep Self-assessment and Continuous Activity Monitoring. Alzheimer Dis. Assoc. Disord. 2010, 24, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, C.E.; Mander, B.A.; Florczak, S.M.; Weintraub, S.; Mesulam, M.M.; Zee, P.C.; Paller, K.A. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. J. Int. Neuropsychol. Soc. 2012, 18, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hita-Yañez, E.; Atienza, M.; Cantero, J.L.; Hita-Yanez, E.; Atienza, M.; Cantero, J.L. Sleep patterns in elders with mild cognitive impairment: The role of memory decline and ApoE ε4 genotype. Curr. Alzheimer Res. 2012, 9, 290–297. [Google Scholar] [CrossRef]
- Maestri, M.; Carnicelli, L.; Tognoni, G.; Di Coscio, E.; Giorgi, F.S.; Volpi, L.; Bonanni, E. Non-rapid eye movement sleep instability in mild cognitive impairment: A pilot study. Sleep 2015, 16, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Naismith, S.L.; Hickie, I.B.; Terpening, Z.; Rajaratnam, S.M.W.; Hodges, J.R.; Bolitho, S.; Lewis, S.J.G. Circadian Misalignment and Sleep Disruption in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2014, 38, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.Q.; Yang, B.X.; Liao, Y.H.; Gao, G.X.; Jiang, N.; Zhou, J.; Richmond, C.J. Sleep Disturbance in Older Adults with or without Mild Cognitive Impairment and Its Associated Factors Residing in Rural Area, China. Int. J. Geriatr. Psychiatry 2020, 33, 122–130. [Google Scholar] [CrossRef]
- Alfini, A.; Albert, M.; Faria, A.V.; Soldan, A.; Pettigrew, C.; Wanigatunga, S.; Zipunnikov, V.; Spira, A.P. Associations of actigraphic sleep and circadian rest/activity rhythms with cognition in the early phase of Alzheimer’s disease. Sleep Adv. 2021, 2, zpab007. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Z.; Du, Y.; Sun, T.; Na, L.; Niu, Y. Social capital and cognitive decline: Does sleep duration mediate the association? PLoS ONE 2021, 16, e0252208. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Lei, Q.; He, L.; Zhong, B.; Meng, Y.; Li, Z. Spontaneous K-Complexes may be biomarkers of the progression of amnestic mild cognitive impairment. Sleep Med. 2020, 67, 99–109. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; American Psychiatric Publishing Inc.: Washington, DC, USA, 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Publishing Inc.: Washington, DC, USA, 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Kokmen, E.; Tangelos, E.G. Aging, memory, and Mild Cognitive Impairment. Int. Psychogeriatr. 1997, 9, 65–69. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. N. Engl. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef]
- Petersen, R.C.; Aisen, P.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Knopman, D.S.; Mielke, M.; Pankratz, V.S.; Roberts, R.; Rocca, W.A.; et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann. Neurol. 2013, 74, 199–208. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Cockrell, J.R.; Folstein, M.F.; Copeland, J.R.M.; Abou-Saleh, M.T.; Blazer, D.G. Principles and Practice of Geriatric Psychiatry; John Wiley & Sons: New York, NY, USA, 2002; pp. 147–158. [Google Scholar]
- Portet, F.; Ousset, P.J.; Visser, P.J.; Frisoni, G.B.; Nobili, F.; Scheltens, P.; Vellas, B.; Touchon, J.; MCI Working Group of the European Consortium on Alzheimer’s Disease (EADC). Mild cognitive impairment (MCI) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Artero, S.; Petersen, R.; Touchon, J.; Ritchie, K. Revised criteria for mild cognitive impairment: Validation within a longitudinal population study. Dement. Geriatr. Cogn. Disord. 2006, 22, 465–470. [Google Scholar] [CrossRef]
- Johar, I.; Mollenhauer, B.; Aarsland, D. Cerebrospinal fluid biomarkers of cognitive decline in Parkinson’s disease. Int. Rev. Neurobiol. 2017, 132, 275–294. [Google Scholar]
- Morris, J.C. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 1997, 9, 173–176. [Google Scholar] [CrossRef]
- Vitiello, V.M. Sleep Disorders and aging: Understanding the causes. J. Gerontol. A Biol. Sci. Med. 1997, 52, M189–M191. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.M.; McLaren, D.G.; Schultz, A.P.; Chhatwal, J.; Boot, B.P.; Hedden, T.; Sperling, R.A. Daytime sleepiness is associated with decreased default mode network connectivity in both young and cognitively intact elderly subjects. J. Sleep Res. 2013, 36, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Bombois, S.; Derambure, P.; Pasquier, F.; Monaca, C. Sleep Disorders in Aging and Dementia. J. Nutr. Health Aging 2010, 14, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Frohnhofen, H.Y.; Schlitzer, J.; Netzer, N. Sleep in older adults and in subject with dementia. Z Gerontol. Geriat. 2017, 50, 603–608. [Google Scholar] [CrossRef]
- Lim, A.S.; Yu, L.; Kowgier, M.; Schneider, J.A.; Buchman, A.S.; Bennett, D.A. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2017, 70, 1544–1551. [Google Scholar] [CrossRef]
- Lucey, B.P.; McCullough, A.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; Zaza, A.M.; Holtzman, D.M. Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Trans. Med. 2019, 11, 474. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Wang, G.J.; Wiers, C.E.; Demiral, S.B.; Gou, M.; Kim, S.W.; Volkow, N.D. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Sci. 2018, 115, 4483–4488. [Google Scholar] [CrossRef]
- Vaou, O.E.; Lin, S.H.; Branson, C.; Auerbach, S. Sleep and dementia. Curr. Sleep Med. Rep. 2008, 4, 134–142. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, P.; Li, C.; Tan, Y.; Li, G.; Xu, D.; Chen, L. Sleep disturbance in mild cognitive impairment: A systematic review of objective measures. Neurol. Sci. 2017, 38, 1363–1371. [Google Scholar] [CrossRef]
- D’Rozario, A.L.; Chapman, J.L.; Phillips, C.L.; Palmer, J.R.; Hoyos, C.M.; Mowszowski, L.; Duffy, S.L.; Marshall, N.S.; Benca, R.; Naismith, S.L. Objective measurement of sleep in mild cognitive impairment: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 52, 101308. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Van den Hoed, J.; Dement, W.C. Sleep and daytime sleepiness in the elderly. J. Ger. Psychiat. 1980, 13, 135–151. [Google Scholar]
- Ohayon, M.M.; Vecchierini, M.F. Daytime sleepiness and cognitive impairment in the elderly population. Arch. Intern. Medic. 2002, 162, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sforza, E.; Pichot, V.; Saint Martin, M.; Barthélémy, J.C.; Roche, F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep Med. 2015, 16, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.; Wiltshire, J.; Lee, S.H.; Ramos, A.R.; Hernandez-Cardenache, R.; Rundek, T.; Curiel Cid, R.; Loewenstein, D. Effect of sleep quality on amnestic mild cognitive impairment vulnerable brain regions in cognitively normal elderly individuals. Sleep 2019, 42, zsy254. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Wiste, H.J.; Vemuri, P.; Weigand, S.D.; Senjem, M.L.; Zeng, G.; Bernstein, M.A.; Gunter, J.L.; Pankratz, V.S.; Aisen, P.S.; et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 2010, 133, 3336–3348. [Google Scholar] [CrossRef]
- Marotta, A.; Martella, D.; Maccari, L.; Sebastiani, M.; Casagrande, M. Poor vigilance affects attentional orienting triggered by central uninformative gaze and arrow cues. Cogn. Proc. 2014, 15, 503–513. [Google Scholar] [CrossRef]
- Casagrande, M.; Martella, D.; Di Pace, E.; Pirri, F.; Guadalupi, F. Orienting and alerting: Effects of 24 h of prolonged wakefulness. Exp. Brain Res. 2006, 171, 184–193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).