Abstract
Jellyfish are ubiquitous animals registering a high and increasing number of contacts with humans in coastal areas. These encounters result in a multitude of symptoms, ranging from mild erythema to death. This work aims to review the state-of-the-art regarding pathophysiology, diagnosis, treatment, and relevant clinical and forensic aspects of jellyfish stings. There are three major classes of jellyfish, causing various clinical scenarios. Most envenomations result in an erythematous lesion with morphological characteristics that may help identify the class of jellyfish responsible. In rare cases, the sting may result in delayed, persistent, or systemic symptoms. Lethal encounters have been described, but most of those cases happened in the Indo-Pacific region, where cubozoans, the deadliest jellyfish class, can be found. The diagnosis is mostly clinical but can be aided by dermoscopy, skin scrapings/sticky tape, confocal reflectance microscopy, immunological essays, among others. Treatment is currently based on preventing further envenomation, inactivating the venom, and alleviating local and systemic symptoms. However, the strategy used to achieve these effects remains under debate. Only one antivenom is currently used and covers merely one species (Chironex fleckeri). Other antivenoms have been produced experimentally but were not tested on human envenomation settings. The increased number of cases, especially due to climate changes, justifies further research in the study of clinical aspects of jellyfish envenoming.
1. Introduction
Jellyfish is a designation given to the free-swimming pelagic state of some members of the phylum Cnidaria [1]. Although most species live in tropical and temperate waters, they can also be found in cold marine environments. It is known that jellyfish populations go through major oscillations every 20 years [2], and their numbers have been increasing globally in recent years, with more geographical areas affected and an increased number of outbreaks [3]. The causes of the current population surge are yet to be clarified, however, global warming-induced warmer marine water temperatures, overfishing of natural predators and industrialization seem to play a role [4]. Of the roughly 10,000 jellyfish species, approximately 100 are accountable for the majority of human envenomations. Around 2006, 150 million cnidarian envenomations are estimated to occur [5]. Cnidarian venom is responsible for local as well as systemic symptoms, though the severity varies greatly, depending on the animal, area of the sting, and individual susceptibility [6]. The encounters with jellyfish can range from a mere nuisance to lethal events. As jellyfish populations are rising due to climate changes and traveling becomes increasingly more accessible, it is expected that more clinicians worldwide will encounter patients stung by jellyfish. As such, it is increasingly important that physicians and forensic experts all over the world are familiar with the clinical picture of jellyfish envenomation, its treatment, and possible complications. Previous studies of the research group have been highlighting the relevance of image in the clinical and forensic suspicion and different aspects of toxicology have been reviewed in the last few years [7,8,9,10,11]. This work follows this major objective by fully reviewing the state-of-the-art concerning pathophysiology, diagnosis, treatment, and relevant clinical and forensic features of jellyfish stings.
2. Materials and Methods
A thorough search in PubMed (U.S. National Library of Medicine) was undertaken without a date or language constraint, focusing on pathophysiology, signs and symptoms, history and physical examination, diagnostic, treatment, and forensic aspects of jellyfish stings. Furthermore, the keyword “jellyfish” was crossed with syndrome, poisoning, envenomation, scar, and cnidaria. A total of 232 scientific documents were considered for this review offering a complete overview of major clinical and forensic aspects of jellyfish stings.
3. Jellyfish Biology
The phylum Cnidaria encompasses six classes: Scyphozoa, Hydrozoa, Cubozoa, Anthozoa, Myxozoa, and Staurozoa [12]. Of these, only Scyphozoa, Hydrozoa and Cubozoa contain animals referred to as jellyfish. Scyphozoans are considered “true jellyfish” and encompass most of the jellyfish species. They can be found worldwide, and their stings are usually mild. Some of the Scyphozoan with medical relevance include the Pelagia noctiluca, Aurelia aurita, Chrysaora quinquecirrha, Linuche unguiculata, Nemopilema nomurai, Rhizostoma pulmo, and Cyanea capillata. Only a few members of the class Hydrozoa are regarded as jellyfish. Physalia physalis (also known as Portuguese man o’war or the man-of-war) is probably the most relevant member of the class, as it is responsible for most hydrozoan envenomations and elicits a distinctive clinical picture. It is not a single organism, but a colony that operates as a single individual. Olindias sambaquiensis is also a Hydrozoan jellyfish but it is only found in Brazil. Cubozoa includes the deadliest jellyfish species. They have cube-shaped bodies and usually inhabit tropical and subtropical oceans. The most dangerous species, namely Carukia barnesi, and Chironex fleckeri are, however, restricted to the Indo-Pacific region. Jellyfish bell sizes range from about 1 mm to several centimeters (excluding the tentacles and oral arms). Cyanea capillata (scyphozoan) is the biggest known jellyfish, with tentacles capable of reaching 36.5 m in length [13]. These animals are equipped with a special type of stinging cell, the nematocyte or cnidocyte, used for defense, capturing prey, and spatial competition. It contains many extrusive organelles derived from the Golgi apparatus, called nematocysts. These consist of a casing with a hinged operculum, inside which an inverted coiled tubule is immersed in the jellyfish venom. Mechanical or chemical stimulation prompts the tubule’s quick eversion, therefore inoculating the venomous substances [14]. The nematocysts are present in several regions of the cnidarian’s body; they are most often seen on tentacles and oral arms, but they can also be found on the bell [6]. These organelles are responsible for human envenomation.
4. Toxicokinetics of Jellyfish Venom
Not much is known about the toxicokinetics of this venom. Jellyfish venom enters the human body through the epidermis when it is pierced by a nematocyst tubule. It should be noted that these organelles may remain viable even in dead organisms and in small fragments of tentacles that broke loose from the animal [15]. The venom exerts its effects locally, but it can also enter the bloodstream, causing systemic symptoms.
In Irukandji syndrome (caused by envenomation from Cubozoan jellyfish), the reason for the delay between the sting and the manifestation of the systemic symptoms is suspected to be related to the path the toxins take to reach their target. Carukia barnesi venom has large elements (50–100 kDa) and may thus move through the lymphatic system, in a route similar to that of snake venoms, giving the distinctive 30 min delay in the onset of symptoms [16].
5. Pathophysiology
Cnidarian venom consists of a complex combination of bioactive elements, including components such as serotonin and histamine, along with high molecular weight proteins. Of these, there have been described lipases, proteases, serine protease inhibitors, hyaluronidases, deoxyribonucleases, l-amino acid oxidases, c-type lectins, neurotoxins, ion channel blockers, pore-forming toxins, and cysteine-rich secretory protein [14].
The effect of the jellyfish venom seems to be predominantly toxic in nature. However, it has been described that some components of the venom can act as antigens, eliciting an innate immune response, antibody formation, and activating the immunological memory [17,18,19,20].
Despite extensive research, the pathophysiological processes and mechanisms of this venom remain unknown. In general, cardiotoxicity is thought to be the leading cause of mortality [21], whereas hemolytic activity seems to be a preliminary damaging factor, providing a method for disentangling the complex venom. Cardiovascular toxicity is, however, independent from hemolysis [22]. Hemolysis is a common effect of several jellyfish venoms. Some venom components attain a hemolytic effect by altering cell permeability, causing ion currents, cell swelling, and osmotic lysis, while others degrade the phospholipids bilayer or form pores in the membrane [23,24,25].
The best-described jellyfish toxic activity is cell lysis by pore-forming toxins [26]. There have also been isolated neurotoxins, targeting ionic channels and neurotransmitter receptors [27]. Oxidative stress has also been reported as a pathophysiologic mechanism [28].
5.1. Cardiotoxicity, Pore Formation, and Intracellular Ca2+ Overload
Jellyfish venom cardiac effect shows great variation, ranging from no apparent cardiotoxicity to raised troponin I levels [29], Tako-Tsubo cardiomyopathy [30], and acute myocardial infarction [31]. Some species seem to be particularly cardiotoxic, such as Carukia barnesi, responsible for the Irukandji syndrome. Patients with Irukandji syndrome, despite having no or minor skin markings, have substantial and continuous pain, tachycardia, hypertension followed by hypotension, and pulmonary edema, implying serious cardiac dysfunction [32,33].
Although the precise mechanism of the acute cardiac malfunction is yet to be fully understood, intracellular Ca2+ excess caused by extracellular Ca2+ entry through pore-forming toxins, as well as Ca2+ intracellular release led by β adrenergic signaling have been identified as key factors driving the cardiotoxicity of jellyfish venom [34].
The Ca2+ antagonists verapamil, diltiazem, and nifedipine have all been shown to reduce the cardiotoxicity of venom from Physalia physalis (hydrozoan) [35], Carybdea rastonii (cubozoan) [36,37], Chironex fleckeri (cubozoan) [38], and Cyanea capillata [39]. Another study, however, found that verapamil did not affect Ca2+ influx, but it was inhibited by La3+, a non-specific channel and pore blocker. As such, if the Ca2+ channels were blocked and the Ca2+ still entered the cell, it can be inferred that the venom creates a bypass to this system, such as a non-selective pore, which cannot be blocked by verapamil. This supports the presence of a pore-forming toxin in the venom of Chironex fleckeri [40], and lends credence to the notion that the mechanism of extracellular Ca2+ entrance is dominated by non-specific translocation through pores in the cell membrane. More than ten hemolytic proteins have been isolated from jellyfish venom thus far. These new proteins have been shown to operate as non-selective cation pore-forming proteins, hence contributing to extracellular Ca2+ entry [24,41,42,43,44].
Chironex fleckeri venom was also shown to cause extracellular Na+ entry and intracellular Ca2+ overload in cardiomyocytes. This effect was not hindered by Ca2+ or Na+ channel blockers or inhibitors of Na+/H+ or Na+/K+ ATPase exchange but was blocked by prior exposure to a solution containing no Na+ and by Ni2+. This supports a possible role of the Na+/Ca2+ exchange on the Ca2+ overload [34]. Another study found that Chiropsalmus quadrigatus (cubozoan) venom exhibits both vasoconstrictor and cardiodepressive effects in rabbits and associated that effect with the activation of voltage-dependent Ca2+ channels and consequent Ca2+ overload [45].
5.2. Induction of Na+ and K+ Currents
The venom of P. noctiluca (scyphozoan) nematocysts can induce an ionic current, mainly Na+, through the plasma membrane, most likely due to a pore-forming process. The hemolytic and cytolytic action of P. noctiluca venom is presumably due to this NaCl inflow followed by water and subsequent cell enlargement. Amiloride, an Na+ channel blocker, can inhibit this effect. It has been demonstrated that this venom is thermolabile, having its hemolytic activity significantly reduced above 40 °C and abolished after boiling. It is known that heat exposure changes the three-dimensional structure of proteins, inducing their loss of activity. As such, this result supports the hypothesis of a protein component of the venom being involved in pore formation [46]. A zinc metalloproteinase has been identified on P. noctiluca venom, which contains a ShK toxin domain. This neurotoxin inhibits K+ channels and is probably involved in sting toxicity [47]. Similar metalloproteinases have also been described in Rhopilema esculenta (scyphozoan), Cyanea nozaki (scyphozoan), Nemopilema nomurai (scyphozoan), and Aurelia aurita (scyphozoan) [48]. The metalloproteinases identified exhibited caseinolytic, gelatinolytic and fibrinolytic activity, and seem to give a significant contribution to the toxic effects of jellyfish venom.
5.3. Targeting the Adrenergic System
The adrenergic system also seems to play a role in the cardiovascular toxicity of jellyfish venom. This was first demonstrated when the aorta contraction induced by Carybdea rastonii (cubozoan) venom was inhibited by trifluoperazine and phentolamine [37]. Further research revealed that prazosin and propranolol reduced the tachycardia and venom-induced pressor response in anesthetized rats [49,50]. These results, however, were not consistent between jellyfish species.
Propranolol, but not prazosin, significantly reduced the concentration-dependent inotropic response in the left atria elicited by Malo maxima (cubozoan) venom [51]. However, previous treatment of prazosin did not significantly reduce hypertension or prevent cardiovascular collapse caused by the sting of the jellyfish Chiropsalmus sp. (cubozoan) [52]. Prazosin had no discernible impact on the cardiovascular effects, but greatly reduced the pressor response to tentacle extract from Chironex fleckeri [53]. Another investigation on Chironex fleckeri venom found that propranolol and prazosin have no effect on the cardiovascular toxicity of this venom [54].
In another investigation, Cyanea capillata venom caused intracellular Ca2+ overload via intracellular Ca2+ release on mouse cardiomyocytes. Esmolol, atenolol and propranolol were able to inhibit this effect. Both cyclic adenosine monophosphate concentration (cAMP) and protein kinase A (PKA) activity increased proportionally, showing that beta-adrenergic signaling is involved in the cardiotoxicity of jellyfish venom. The significant extracellular Ca2+ influx through pore-forming components, bypassing the adrenergic mechanism of Ca2+ overload, may explain previous unfavorable results with adrenergic blockers. This hypothesis is supported by the fact that, while in the absence of extracellular Ca2+, propranolol completely inhibits the Ca2+ overload, this inhibition is only partial when the cardiomyocytes were placed in a solution containing Ca2+ [55]. O. sambaquiensis (hydrozoan) and Chiropsalmus quadrumanus extracts were found to strongly interfere with noradrenergic neurotransmission of the rat vas deferens without affecting purinergic response or smooth muscle structure [56].
5.4. Endothelial Nitric Oxide Synthase (eNOS) Induction
Endothelial nitric oxide synthase (eNOS) induction by Cyanea capillata tentacle extract leads to nitric oxide (NO) production in a dose and time-dependent manner. Studies revealed that the extract caused the phosphorylation and activation of eNOS mostly via phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt)-dependent, protein kinase C/inositol trisphosphate receptor (PKC/IP3R)-sensitive, and Ca2+-dependent pathways. These results confirm that the jellyfish venom-induced vasodilation is mediated by NO release via the stimulation of eNOS in endothelial cells [57].
5.5. Tubule Length of the Nematocyst and Severity of the Pain
Recent data suggest that the variation in pain severity could be attributed to the tubule length of the nematocyst, this way causing variable epithelial lesions. Kitatani et al. [58] found that nematocyst tubules from the dangerous jellyfish species Carybdea brevipedalia (cubozoan), Chrysaora pacifica (scyphozoan), and Chironex yamaguchii (cubozoan) are long enough (>200 m) to pierce the human epidermis and stimulate pain receptor fibers’ free nerve ends. Less harmful species like Aurelia aurita, on the other hand, have shorter tubules [58]. While the primary noxious stimulus may be triggered by the tubule penetration, which stimulates pain receptor neurons, the persistent pain may be due to venom injected into the skin. In fact, the jellyfish venom itself activates the transient receptor potential vanilloid-1 (TRPV1) non-selective cation channels present in nociceptive neurons, therefore causing pain [59,60].
5.6. Hemostasis Disturbance
Rhizostoma pulmo (scyphozoan) venom was found to be able to influence the hemostatic system on three separate levels, exhibiting fibrinolysis, fibrinogenolysis, and suppression of ADP-induced platelet aggregation. It also demonstrated considerable hemolytic activity against human red blood cells, as well as high proteolytic activity against substrates such as (azo) casein and gelatin. However, EDTA (metalloproteinase inhibitor) reduced this proteolytic activity but not PMSF (serine proteinase inhibitor). Rhizoprotease, a new metalloproteinase of 95 kDa, was isolated in the venom [61]. Nemopilema nomurai (scyphozoan) venom contains a chymotrypsin-like serine protease with fibrinolytic activity [62]. Aurelia aurita shows strong fibrinogenolytic activity [63].
5.7. Other Reported Mechanisms of Toxicity
The venom of Nemopilema nomurai (scyphozoan) can induce significant edema. This venom does not seem to operate as an acute proinflammatory agent, but rather it plays a role in the persistence of inflammation. Its effect on edema is mostly ascribed to its influence on vascular permeability via a mechanism of direct degrading basement membrane components. These findings support the use of antihistaminic drugs in scyphozoan stings. Phospholipase is not involved in the vasopermeability shift generated by Nemopilema nomurai venom [64]. Pelagia noctiluca and Cyanea capillata have an oxidative effect, inducing inflammation and apoptosis [28,65,66,67]. Phospholipase A2 plays an important role in inflammatory pathways and is an active component of some animal venoms. Nevalainen et al. [68] found phospholipase A2 in cnidarian venom and suggested it may play a role in its toxicity. Histamine release is induced by Physalia physalis venom by fast, short-duration exocytosis of granules and lengthier lysis of mast cells [69]. Cyanea capillata venom was also found to induce histamine release [70].
5.8. Delayed Reactions
A type IV hypersensitivity reaction triggered either by a sequestered antigen persisting in the skin or by a cross-reacting antigen in the venom is postulated to be in the genesis of the delayed, persistent reactions after jellyfish stings [71].
5.9. Irukandji Syndrome
Carukia barnesi venom can activate neural Na+ channels, inducing catecholamine release and vasoconstriction. This mechanism seems to be accountable for the sympathomimetic-like symptoms of Irukandji syndrome, namely hypertension, and tachycardia [50]. Like Carukia barnesi, Malo maxima venom activates a neural sodium channel, releasing endogenous noradrenaline and calcitonin gene-related peptide antagonist (CGRP) [51]. The following mechanisms have been implicated in acute heart failure: (i) stress-related cardiomyopathy caused by hypertension or direct cardiomyocyte toxicity of catecholamines [72], and (ii) pore formation on cardiomyocytes, which disturbs cellular function and permits poisons to enter and cardiac enzymes to exit [73].
6. Envenomation Syndromes
As mentioned above, the toxic effect of jellyfish venom varies greatly. The different classes of jellyfish tend to cause different syndromes, although most signs and symptoms are nonspecific and shared between classes.
6.1. Class Scyphozoa
Scyphozoans (true jellyfish) are abundant and ubiquitous. Although responsible for most jellyfish envenomations worldwide, only a very small fraction of these are severe, rendering them less dangerous than the other jellyfish classes [14]. Symptoms related to this envenomation are compiled in Table 1. Upon contact of the skin with the jellyfish, pain of variable intensity is the most frequent and often the only clinical manifestation of the envenomation [13]. A vesiculourticarial eruption may appear, possibly resembling the shape of the tentacles or the bell of the offending animal. Edema, pruritus, hemorrhage, or necrosis may be present as well. Even though erythema and pain typically subside in hours to days, sequelae of the sting may persist, namely scaring and hyperpigmentation [27]. In some cases, the eruption may become generalized, persistent, or be delayed [74]. Systemic symptoms, even though rare, may occur. Lethal cases have been reported [75].

Table 1.
Jellyfish envenomation symptoms.
6.2. Class Hydrozoa
Physalia physalis, the most prominent member of this class, is a colonial organism that has a gas bladder and numerous tentacles made up of cell populations with diverse roles. Morphologically, it has a vivid color that ranges from green to violet [150]. Hemolytic, cardiotoxic, and neurotoxic activities of Physalia venom have been demonstrated. Typical lesions develop immediately after contact with the organism, including linear urticariform plaques, as well as severe pain. Systemic symptoms may arise, such as nausea and vomiting, cold sweats, syncope, heart arrhythmia, and even death. Furthermore, allergic reactions to the venom might cause anaphylactic shock, which can result in death within minutes [150,151]. Rhabdomyolysis and acute renal failure have also been described [122].
6.3. Class Cubozoa
The members of this class, also known as box jellyfish, are one of the most hazardous marine organisms in the world [50]. Even though they cause a smaller number of envenomations, compared to the other classes, most encounters result in severe symptoms. This class encompasses two families, Chirodropidae (such as Chironex fleckeri) and Carybdeidae (to which belong the species most associated with Irukandji syndrome). Their venom is cardiotoxic, hemolytic, dermonecrotic, and neurotoxic [152]. Envenomations from these organisms can result in cardiorespiratory depression and death within minutes [34,73,153,154]. Skin necrosis has also been described [82]. A delayed soft tissue necrosis might occur, which is thought to be caused by an immune response to the tubules lingering in the lesion or by the triggering of undischarged nematocysts [19].
Irukandji Syndrome
Irukandji syndrome is a severe illness produced by the envenomation of some species of small jellyfish from the Cubozoa class [155]. It consists of a clinical picture dominated by systemic symptoms similar to a catecholamine surge, including hypertension, tachycardia, intense pain, and muscle cramping, eventually leading to pulmonary edema, shock and cerebral hemorrhage [156]. So far, the species implicated in the syndrome are Carukia barnesi [102], Alatina mordens, Carybdea alata, Malo maxima, Carybdea xaymacana [157], Morbakka fenneri, Malo kingi, Carukia shinju, Gerongia rifkinae [33], Alatina reinensis, Gonionemus oshoro [16] and Alatina alata [158]. However, species identification is not necessary to diagnose Irukandji syndrome.
The first cases described happened in the northern Australian territories [159]. However, similar disorders have been observed all over the tropical waters, including Thailand, the Caribbean, Florida, and Hawaii, although not all instances have been linked to a specific species [103,104,105,156,160,161]. Stings seem to occur in short, pandemic outbreaks, so patients often present themselves to the hospital in clusters [162].
The offending jellyfish often goes unnoticed, but sufferers frequently experience severe pain at the afflicted region after the triggering incident. Erythema, edema, and tentacle marks may be present [163]. The initial local discomfort is generally mild and subsides in less than half an hour. During this time, an erythemato-papular skin lesion of around 2 cm, the dimension of the offending animal, might appear, popularly referred to as “goose pimples”, which normally dissipates quickly but can last for days [16]. The onset of severe systemic symptoms, the cornerstone of the typical Irukandji syndrome, can range from 5 to 120 min, but most often takes 30 min [164]. Muscle cramps, serious back, thoracic, and abdominal pain, nausea, vomiting, diaphoresis, anxiety, restlessness, headache, localized sweating, and piloerection are the main symptoms [73,159,165,166]. The condition is often linked to a sense of “impending doom” [157]. Because the toxin is hyperadrenergic, hypertension and tachycardia are common. There have been reports of blood pressures as high as 300/180 mmHg [167]. Pallor, peripheral cyanosis, oliguria, tremor, and cerebral edema have also been described [168]. Ventricular tachycardia, myocardial injury [72,106], cardiomyopathy with abrupt pulmonary edema [147,169] and cardiogenic shock [32,156] may occur. In extreme cases, ventilatory failure may ensue, necessitating admission to an intensive care unit [32]. Pancreatitis, priapism, and acute renal failure were also associated with this syndrome, notwithstanding their rarity [156].
Although exceedingly painful and frequently necessitating narcotic analgesia and inpatient hospitalization, Irukandji syndrome is typically not deadly, especially if supportive care is given early [156]. Most patients who seek emergent care following a sting can be returned home in the same day. Besides, not all encounters with species capable of producing Irukandji syndrome result in this clinical state [170,171]. Even though the pain only lasts for some hours, there are reports of pain recurrence, necessitating additional hospital visits [156]. In one reported case, the pain recurred up to a year later [104]. The first described fatal Irukandji disease cases were caused by intracranial hemorrhage [148,149]. These are thought to have occurred because of extreme hypertension.
6.4. Seabather’s Eruption
Seabather’s eruption is a severely pruriginous papule-erythematous dermatitis that arises following exposure to marine water in areas of the body covered by bathing clothes. It is caused by the larval form of Linuche unguiculata, which becomes trapped in the bathing suit and releases its toxin [172,173]. Lesions usually occur in the gluteal region, the most afflicted areas among surfers. However, the chest, belly, arms, and thighs, which were in direct contact with the surfboard, activated the larvae nematocysts [174]. Lesions could also be found in flexural regions. Chills, fever, nausea, vomiting, diarrhea, headache, and abdominal pain are found on rare occasions and usually occur in children or cases of severe envenomation [76,175].
6.5. Delayed Reactions
Jellyfish stings commonly result in immediate local skin reactions, typically characterized by erythema, edema, vesicles, and severe localized pain. However, in rare cases, the patients may present with delayed allergic reactions with similar symptoms days or months after the sting, even without having experienced an immediate reaction. While a toxic mechanism is held accountable for the immediate reaction, the delayed eruptions are immune-mediated [83,90]. Delayed cutaneous reactions can take multiple presentations, such as keloid-like plaques [71], nodular and papular eruptions [84,85,139,176], linear pigmentation [86], vesicles [177], and granulomatous infiltration [87].
6.6. Eye Lesions
Jellyfish venom has toxic effects on ocular tissue. A sting to the eye may result in pain, photophobia, conjunctival injection, punctate epithelial keratitis, iritis, persistent mydriasis, peripheral anterior synechiae, corneal stromal edema, foreign body sensation, and increased intraocular pressure, and usually resolves without sequelae [100,178,179,180,181]. However, severe fundus lesions have also been described, namely retinal vascular occlusion, thinning of the retina, optic atrophy, and scar formation in the macular area [182]. Table 1 compiles the various symptoms related to jellyfish envenomation reported in the literature.
7. Diagnosis
The diagnosis of a jellyfish sting is predominantly clinical. Some clinical characteristics of the lesion may raise suspicion as to which cnidaria class caused the envenomation. If a cnidarian sting is suspected but the patient cannot recall having contact with a jellyfish, a skin scrape/sticky tape test may reveal nematocysts in the lesion. Dermoscopy, histology, and reflectance confocal microscopy can be useful as well.
7.1. Signs and Symptoms
Scyphozoan stings can take a multitude of appearances (Figure 1). Unlike other classes, these species may leave the “imprint” of their bodies on the skin, giving rise to a jellyfish-like erythematous lesion. The tentacle marks can be flat, edematous, papular, and vesicular. Since these organisms are ubiquitous, a scyphozoan sting should be suspected when a patient has a history of sea bath and comes in with a variably painful, erythematous, linear, or jellyfish-shaped lesion.
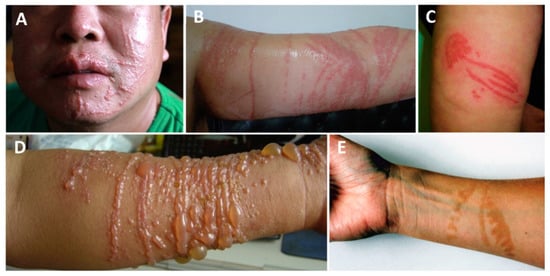
Figure 1.
Scyphozoan jellyfish stings: (A,B)—multiple linear erythematous beaded marks of a Cyanea nozakii sting; (C)—confluent erythematous papules in the shape of a jellyfish; (D)—linear, vesicular lesions after a Stomolophus meleagris sting; (E)—hyperpigmentation two months after the contact with a Pelagia noctiluca jellyfish. (A,B) reproduced from [183], (C) from [184], (D) from [132], (E) from [78].
Regarding the class Hydrozoa, upon contact with Physalia physalis tentacles, an immediate, painful skin rash will appear (Figure 2). The severity of the lesion ranges from erythematous urticarial linear beaded plaques to vesiculobullous eruptions. On some occasions, the lesion may blister or even become necrotic. Lesions may take a “frosted” appearance due to superficial skin necrosis. A sting from this species should be suspected when a sea bather reports intense pain and a linear rash with a “string of beads” appearance.
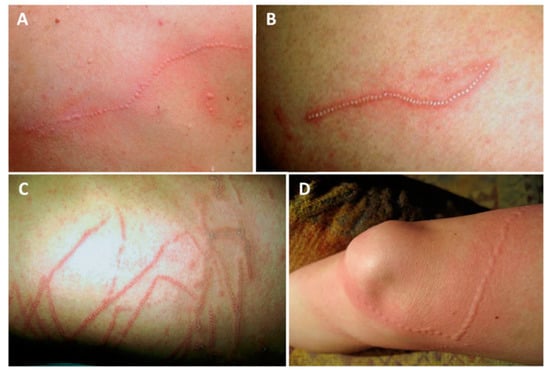
Figure 2.
Physalia physalis jellyfish sting marks: (A)—typical linear, beaded tentacle marks, 2 h after a sting on the dorsum; (B)—12 h after a sting on the dorsum, showing the “frosted” appearance; (C)—12 h after a sting to the chest; (D)—beaded, urticariform eruption following a sting. (A–C) reproduced from [133], with permission.
Cubozoan stings leave relatively wide, ladder-like, cross-hatched marks, resembling those of a whip (Figure 3). They often have a “frosted” appearance due to superficial skin necrosis [185].
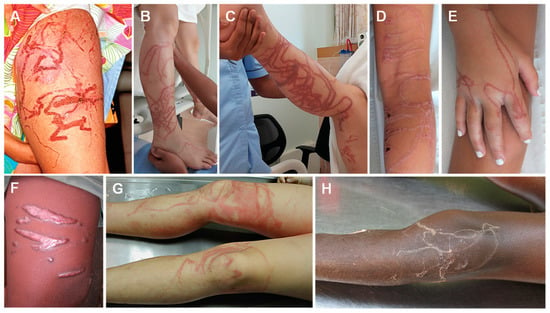
Figure 3.
Cubozoan jellyfish stings: (A)—Chironex fleckeri sting; (B,C)—near-fatal cubozoan sting on a 31-year-old man; (D,E)—tentacle-shaped skin necrosis on two children stung by Chironex sp.; (F)—scar on the thigh of a 28-year-old Thai man, 6 months after a sting; (G)—erythematous urticarial lesions following a fatal Chironex fleckeri sting; (H)—pale, serpiginous tentacles adherent to a 6-year-old boy after a fatal Chironex fleckeri sting. (A) reproduced from [186], (B,C) from [187], (D,E) from [142], (F) from [141], (G) from [188] and (H) from [143], with permission.
The lesions may complicate with necrosis and take several weeks to fully heal (Figure 4). A cubozoan sting should be suspected in a patient bathing in Indo-Pacific shallow waters that presents with severe pain, skin marks as described above, and possibly with distressing systemic symptoms.
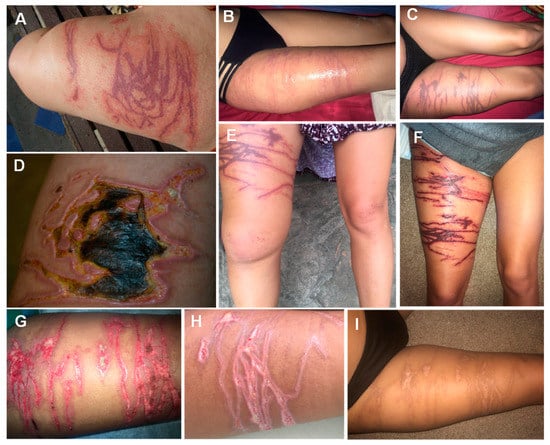
Figure 4.
Evolution of Cubozoan jellyfish stings: (A)—brownish, erythematous tentacle marks a few hours after a cubozoan sting; (B)—some weeks after the envenomation, the wound showed signs of infection and gangrene; (C)—erythematous, linear marks on the day of the envenomation; (D)—day 2, dark purple marks corresponding to the tentacle contact sites—right leg edema; (E)—day 4, worsening edema of the right leg; (F)—day 12, scab formation with exposure of textured granulation tissue underneath and serous exudate; (G)—day 19, increased granulation tissue, the wound edges have started to re-epithelize; (H)—day 24, the wound is healing by secondary intention; I)—wound appearance 120 days after the sting. (A,B) reproduced from [77], (C–I) from [141], with permission.
Irukandji syndrome occurs mostly in the Indo-Pacific region. Morbakka spp., a possible agent of Irukandji syndrome, can leave a caterpillar track mark on the site of the sting [108]. However, most Irukandji stings leave only “goose pimples” or no mark at all. Localized sweating can often be seen (Figure 5). When Irukandji syndrome is suspected, the clinician should bear in mind the following differential diagnosis: other cnidaria stings, hyperthyroidism, sympathomimetic toxicity, pancreatitis, pheochromocytoma, rhabdomyolysis, anaphylaxis, acute decompensated heart failure, acute coronary syndrome, and decompression illness [155].
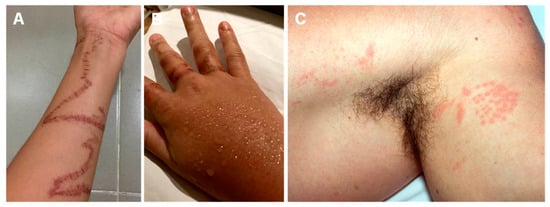
Figure 5.
Irukandji jellyfish stings: (A,B)—Morbakka spp., a possible agent of Irukandji syndrome, can leave a caterpillar track mark on the site of the sting; (B)—localized sweating can be found on sting sites; (C)—”Goose pimples” appearance on the contact site. (A,B) reproduced from [108], (C) from [162], with permission.
Seabather’s eruption should be suspected in a patient bathing in the Atlantic coast of Central and South America, and the Atlantic African coast, from Mauritania to Gabon, presenting with a highly pruritic papular rash in the areas covered by the bathing suit (Figure 6). Seabather’s eruption can be confused with swimmer’s itch, which occurs after bathing in freshwater and is found all over the world. Swimmer’s itch affects only exposed regions of the body, and the agents responsible are Schistosoma spp. cercariae [174]. Insect bites and scabies are other prominent differential diagnoses for swimmer’s itch [189].
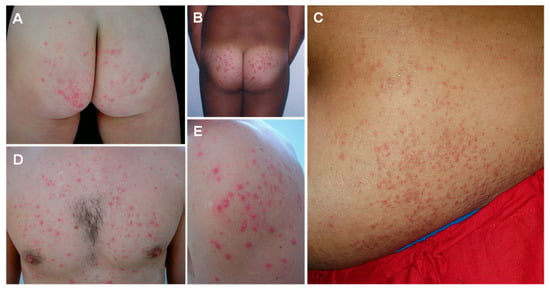
Figure 6.
Seabather’s eruption caused by jellyfish stings: highly pruritic papules and pustules on the gluteal region (A,B), abdomen (C), thorax (D) and shoulder (E), corresponding to friction areas covered by the bathing suit. (A) reproduced from [175], (B) from [190], (C) from [191], (D) from [175], (E) from [191], with permission.
After a sting to the eye, conjunctival edema, epithelial corneal defects, and foreign bodies may be seen [178] (Figure 7).
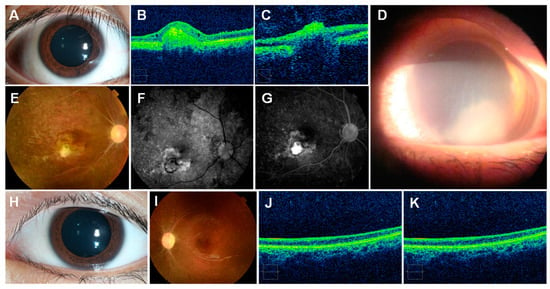
Figure 7.
Jellyfish stings to the human eye: (A)—injected conjunctiva following an ocular sting; (B)—optical coherence tomography shows elevated lesion and thinning of the retina; (C)—severe thinning of the retinal nerve fiber layer; (D)—three days after an ocular sting, showing marked corneal edema with an area of diffuse severe keratitis occupying the lower half of the cornea; (E)—fundoscopy, revealing optic disc pallor, retinal vascular occlusion, retinal pigmentation, scar; (F,G)—fundus fluorescein angiography; (H)—normal examination; (I)—pale optic disc, retinal vascular occlusion, pigmentation of the retina; (J,K)—thinning of the retinal layer. (A–C,E–K) reproduced from [182], (D) from [192], with permission.
7.2. Medical Exams
Dermoscopic findings may be species-specific and represent a diagnostic tool of jellyfish sting. A study on the dermoscopy of Pelagia noctiluca stings identified four dermoscopic features: brown dots, brown ‘Chinese characters’ pattern, pinpoint brown and whitish-yellow crusts. When a clear history of interaction with the cnidarian is unavailable, observation of these dermoscopic characteristics in typical cases of Pelagia noctiluca stings may aid the diagnosis [136] (Figure 8).
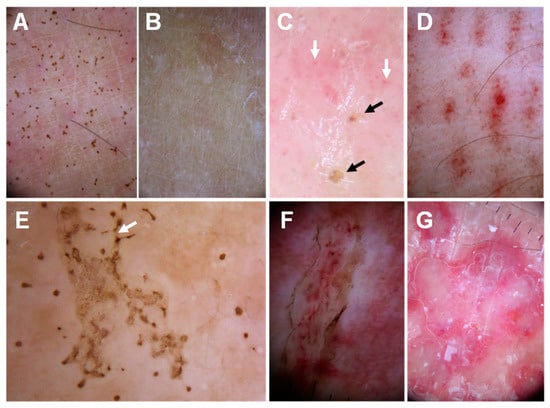
Figure 8.
Dermoscopic findings in Pelagia noctiluca jellyfish stings: (A)—brown dots on a 1-week-old sting; (B)—21 days after the sting, the brown dots have completely disappeared; (C)—pinpoint brown crusts (black arrows) and red dots (white arrows) in a pink background; (D)—‘Linear purpura’: linear bands composed of red dots regularly spaced in a tabby pattern; (E)—‘Chinese characters’ pattern: brown dots connected by light brown granular lines (white arrow), matching a linear blistered lesion; (F)—‘Serpentine ulceration’. Scales and brown dots delimit the edges of the ulcer, with linear purpura inside; (G)—‘Circular milky-red areas’ corresponding to a recurrent, persistent, inflammatory reaction to a sting. Reproduced from [136], with permission.
Histology of the lesions may demonstrate the presence of nematocysts, as well as inflammation signs (Figure 9).
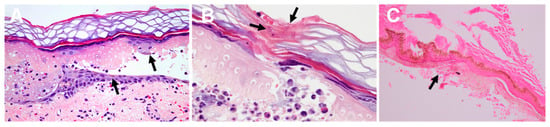
Figure 9.
Histology of jellyfish stings: (A)—epidermal necrosis and vesicle formation following a Pelagia noctiluca sting. Pigmented keratinocytes (black arrows), vasodilatation, edema, and erythrocyte extravasation are displayed; (B)—fragments of nematocyst tubules are shown in the stratum corneum (black arrow); (C)—remains of nematocysts (arrow) following a Chironex fleckeri sting. (A,B) reproduced from [136], and (C) from [143], with permission.
Nematocyst identification on skin scrapings/sticky tape confirms the occurrence of a jellyfish sting [193]. Furthermore, as nematocyst morphology is species-specific, a skin scraping may help identify the species responsible for the envenomation or, at least, the class of the jellyfish [170] (Figure 10).
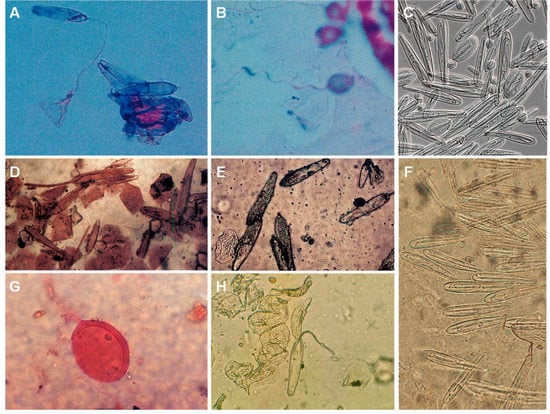
Figure 10.
Jellyfish nematocysts retrieved from human skin: (A)—ruptured nematocyst in a skin scraping from a Chiropsalmus quadrumanus sting lesion; (B)—nematocyst from Physalia physalis jellyfish; (C)—nematocysts isolated from Chironex fleckeri tentacles; (D)—Chironex fleckeri nematocysts (skin scraping); (E)—Chironex fleckeri nematocysts (sticky tape); (F)—nematocysts harvested from Chironex spp. tentacles with sticky tape; (G)—skin scraping from a patient with Irukandji syndrome (species is unknown); (H)—sticky tape from a Chironex fleckeri sting. (A,B) reproduced from [194], (C) from [44], (D,E) from [195], (F) from [196], (G) from [156] and (H) from [143], with permission.
The radioallergosorbent test (RAST), enzyme-linked immunoabsorbent assay (ELISA), and the Ouchterlony immunodiffusion test are useful for detecting allergic responses to unnoticed cnidarian encounters. By detecting antibodies against jellyfish, these may also identify people who have become sensitized during a previous encounter with these animals and, therefore, are at risk of developing a more severe reaction following a future interaction [15,197].
Color Doppler Ultrasonography (CDUS) has been used for evaluating the therapeutic response in delayed allergic reactions to cnidarian stings. In all described cases, the initial evaluation portrayed dermal thickening and decreased echogenicity, when compared to that of healthy individuals. It is worth noting that CDUS enabled clinicians to determine that the cutaneous inflammation had subsided and, as a result, discontinue the therapy, even when the clinical examination of the lesions remained nearly unaltered. The Doppler mode revealed hypovascularization, which could be attributed to vasoconstriction caused by jellyfish stings. Color or power Doppler imaging is essential for ruling out vascular contraction diseases that could lead to ischemia, namely severe vasospasm caused by jellyfish envenomation [83].
Seabather´s eruption: although the histological aspect of the biopsy is not specific, it may aid the differential diagnosis by displaying superficial and deep interstitial and perivascular infiltrates composed of eosinophils, neutrophils, and lymphocytes. The ELISA (enzyme-linked immunosorbent assay) technique allows for the detection of IgG antibodies to Linuche unguiculata in afflicted people’s serum [175].
8. Treatment
The most significant intervention is primary prevention. Many places have created information, warnings, and beach closures as a result of local observations and climate analyses [198]. A full-body lycra “stinger suit” can prevent some envenomations, particularly those caused by the smaller Irukandji jellyfish [162]. Secondary prevention, such as the use of sunscreen lotion containing jellyfish sting inhibitors, has been shown to lessen symptoms following jellyfish exposure [199,200]. The initial approach to a jellyfish envenomation should be guided by the following steps:
- (a)
- Patient stabilization—victims should be removed from the water to prevent further stinging and drowning. If needed, life support maneuvers should be performed. The victim should be brought to emergency care if needed;
- (b)
- Preventing nematocyst discharge—care should be taken to minimize the venom load by preventing further discharge of the nematocysts. As such, vinegar is usually employed to prevent the discharge of the remaining nematocysts, but this use is based largely on empirical knowledge. Research regarding this use is conflicting. It is generally advised to use vinegar in Hydrozoan and Cubozoan stings and to avoid its use on Scyphozoan. Ballesteros et al. showed that vinegar leads to Pelagia noctiluca nematocysts discharge in vitro [201]. However, the most recent review on this topic suggested vinegar to be beneficial in Cyanea capillata and Pelagia noctiluca envenomation, so further research is needed [14,202]. After inactivating the nematocysts, adherent tentacles must be removed gently, using tweezers or a similar tool. Tentacle removal can also be attempted by rinsing with seawater [203], however, some studies recommend against this practice, as it can induce further venom delivery [202]. Freshwater must not be used for rinsing, as the osmotic challenge would induce nematocyst discharge [119]. An in vitro study found that lidocaine might block nematocyst discharge from Pelagia noctiluca [25]. Moreover, topical lidocaine was demonstrated to both reduce pain and prevent nematocyst release [25].
- (c)
- Alleviating local venom effects—heat has shown significant pain relief for many jellyfish stings, particularly those caused by Physalia physalis [160,204,205].
- (d)
- Controlling systemic effects—analgesics, antihistamines, corticosteroids, anti-hypertensive drugs are some of the treatments that may be required, depending on the symptoms;
- (e)
- Preventing complications—if a sting is at risk of becoming infected, antibiotics should cover Streptococcus spp., Staphylococcus spp., and marine organisms, such as Vibrio spp. According to Center for Disease Control recommendations, tetanus prophylaxis is indicated whenever there is a minor wound, and the patient is not up to date with the tetanus vaccination scheme. As such, tetanus prophylaxis must also be considered [6,76,206].
8.1. Particularities of Each Class
Regarding scyphozoan stings, most cases are mild, and management usually includes oral or topical antihistamines and topical corticosteroids. Systemic corticosteroids may be required in severe cases. Analgesics (acetaminophen, non-steroidal anti-inflammatory, opiates) and topical antibiotics can be useful to control pain and prevent or treat infections [13].
In hydrozoan stings, only a small fraction of nematocysts discharge on initial contact. As such, tentacles should be removed carefully, ideally after being doused with an inactivating product. A recent study [207] showed that, while different kinds of vinegar prevented discharge in Physalia spp., even a minor dilution of vinegar significantly diminished inhibitory effects or resulted in partial nematocyst discharge. In addition, a 30 s vinegar irrigation is enough to decrease stinging. The suppression of discharge was not solely a function of pH, as this effect was not obtained with different acidic solutions. Alcohols promoted discharge, resulting in enhanced hemolysis, and should therefore be avoided. Finally, the findings confirm prior research [204,208,209] that supports the use of heat in the treatment of Physalia spp. stings, since heat greatly decreased hemolysis. The use of cold not only failed to diminish hemolysis but also exacerbated stinging. The only systematic review concerning pain relief following Physalia spp. stings revealed that hot water, compared to ice packs, was capable of a ≥50% pain reduction after 20 min of treatment, with a number need to benefit of 1.8 (1.4 to 2.7, CI 95%) [208].
In the case of cubozoan stings, the venom load released into the sting site is linked to tentacle contact length and sequelae severity. Because only about 1% of nematocysts discharge upon initial contact [210], successful and careful removal of clinging tentacles is critical in the treatment of potentially fatal cubozoan stings, as ineffective removal of adherent tentacles has the potential to significantly aggravate sting results by increasing the venom load [211]. Vinegar has been proven in vitro to be a powerful, irreversible inhibitor of cubozoan nematocyst discharge [210,211,212]. Some case series show improved outcomes, including a better chance of survival, when vinegar is used in first aid [80,187]. A recent in vitro study illustrates that rinsing the sting with sea water, scraping tentacles away, and using cold packs can exacerbate venom-induced hemolysis, a measure of sting sequelae, in the cubozoans studied. Furthermore, its findings support the use of vinegar or the commercial over-the-counter spray Sting No MoreTM (composed of vinegar, urea, magnesium sulfate, and copper gluconate) before tentacle removal as they were effective in deactivating the nematocysts. Besides, the authors suggest that the sting site should be immersed in 45 °C water or a 45 °C hot pack for 45 min after the removal of the tentacles, as it inactivates the hemolytic activity of the venom [211]. Other small studies support heat application [213]. However, in a small randomized controlled trial [214], hot water immersion only offered mild relief from the acute pain of Chironex fleckeri stings, and its performance was similar to ice pack application. This is surprising given the proven benefit of hot water on Physalia spp. stings. The lack of effect of the heat might be attributed to the fact that, in this study, treatment was postponed until the patient arrived at the emergency department. As a result, the effect of heat was probably symptomatic rather than venom inactivation. A Chironex fleckeri antivenom is available and should be administered as soon as possible to a suspected Chironex fleckeri victim in the following circumstances: cardiorespiratory instability, unconsciousness, airway or ventilation compromise, severe pain, and the possibility of significant skin scarring [215].
8.2. Irukandji Syndrome
As with most jellyfish stings, first aid includes removing the victim from the water and liberally dousing the sting site with vinegar. It has been demonstrated that vinegar irrigation can improve the outcomes of Irukandji victims [19,108,119,203,210]. The commercial copper gluconate-based product Sting No More™ has also revealed its merit in the prevention and first aid treatment of Irukandji syndrome [210].
This syndrome is, most of the time, extremely painful, often requiring opioids [155,216]. All patients with Irukandji syndrome must have active pain management, which includes regular pain assessments using a validated tool [106].
Because of the possible cardio-respiratory consequences of envenomation, patients’ cardiac and respiratory states must be centrally monitored in order to recognize and actively treat them [106].
Nitroglycerin is the first-line therapy for Irukandji syndrome-related hypertension. Its impact on venous and arterial dilatation provides advantages in victims with life-threatening pulmonary edema. In persistent hypertension, an infusion of nitroglycerin can be initiated and adjusted to the targeted blood pressure. Its usage is contraindicated in people using phosphodiesterase inhibitors, as it is in other uses of nitrates [33].
Phentolamine: Because of its alpha-adrenergic antagonist properties, phentolamine is also suggested as a therapy for Irukandji syndrome-related hypertension [119,164]. Because of the risk of hypotension, delayed cardiac failure, and pulmonary edema in extreme instances, phentolamine is preferred over phenoxybenzamine, due to it being reversible and having a shorter half-life. Nonetheless, unless contraindicated, a titratable vasodilator such as nitroglycerin should be administered initially, particularly in victims with concomitant heart failure, and phentolamine should be reserved for individuals resistant to nitrates [155].
Benzodiazepines: In Irukandji syndrome, benzodiazepines are advised as adjunctive therapy for pain and hypertension. Usually, a combination of suitable analgesics and benzodiazepines will alleviate the hypertension caused by Irukandji syndrome [217].
Magnesium sulfate (MgSO4) administration is a current practice [218]. However, there are no strong data to support or advise against this use [167]. The only randomized controlled trial on this topic showed no difference between MgSO4 administration and placebo on Irukandji patients, though minimal doses of MgSO4 and large exclusion criteria were applied [219]. The current practice is based on the effectiveness documented in case series. The reported symptom recurrence after dosage reduction suggests a dose-response association. More randomized controlled trials are necessary to establish the function of MgSO4 as part of the treatment of this envenomation. In the meantime, it can be considered in severe cases.
8.3. Seabather´s Eruption
This syndrome is usually mild and benign. Management often includes topical and systemic antihistamines, as well as strong topical steroids. In some cases, fever and extensive lesions may demand a short course of oral glucocorticoids. Swimmers affected by seabather´s eruption should remove their swimsuit prior to showering, as the fresh water may cause further discharge. The swimwear must be thoroughly washed with soap before reuse to eliminate any remaining larvae [76,220].
8.4. Corneal Lesions
Jellyfish venom is harmful to the eyes as well. Stings cause damage to the eyelid, conjunctiva, cornea, and anterior chamber [221]. Following a sting, 3% NaCl and 0.3% Norfloxacin eyedrops have been successful in the treatment of epithelial keratitis and corneal edema. The lesion healed within 2 weeks, with minimal scarring [222]. Treatment with nematocyst removal, topical antihistamines, topical steroids, cycloplegics, and topical antibiotics has also been described, with good results [178]. In case of a Physalia ocular sting, it is recommended to inactivate the toxin with heat, apply analgesia, and debride the cornea [181].
8.5. Other Treatments
Systemic antihistamines, topical/oral corticosteroids, or tacrolimus ointment may be beneficial in severe delayed reactions [85,90,177]. Extracorporeal shock wave therapy was successful in the treatment of a chronic recurrent dermatitis following a Physalia physalis sting that was resistant to local cortisone treatment [93]. Early radical debridement and vacuum-assisted wound care appear to be useful in preventing skin necrosis and consequently progressive tissue loss caused by stings [82].
9. Forensic and Toxicological Aspects
Forensic identification of humans can be based on primary and secondary methods. The latter include anthropology, evidence, and medical and personal characteristics, such as scars and tattoos. Although categorized as secondary, these methods may play a fundamental role in the identification of a victim [223]. In some cases, jellyfish stings leave a permanent scar on the victim, becoming a distinctive characteristic of that person. As such, these stings can be used as a secondary identification method, improving identification accuracy.
Reports of postmortem examinations after a jellyfish sting are scarce, and mostly reveal cerebral hemorrhage, as well as visceral and cerebral congestion.
A 4-year-11-months-old boy was stung by a jellyfish and died 40 min later. His autopsy, 19 h hours after the death, revealed whitish foam in the oral endotracheal tube, a reticular pattern of eruptions on the left arm and left lateral thorax. The skin scraping of the lesions showed nematocyst similar to those of Chiropsalmus quadrumanus. A total of 75 mL of serous fluid was removed from each pleural space. The larynx, trachea, and bronchial tree were filled with frothy white foam. The lungs weighed 530 g and had a purple, congested appearance. The cut surface was edematous and congested, with a prominence of the septa. There was subendocardial hemorrhage on the septal wall of the left ventricle. Acute passive congestion was evident in the liver, spleen, and kidneys. Histological exam revealed widespread interstitial and perivascular lymphoid infiltration of the myocardium. There were a few areas of early changes of hypoxia, but no necrosis. There was focal subendocardial hemorrhage in the septum of the left ventricle. Many alveolar capillaries were congested with monocytes. Interstitial and perivascular lymphocytic infiltrates were also seen in the salivary glands, thyroid, trachea, esophagus, hepatic portal areas, and the dermis. Lymphoid nodules were found in the bone marrow [194].
A 67-year-old obese woman stung by a Physalia physalis became comatose shortly after and died 5 days later. Linear, erythematous, vesicular lesions were seen on her right arm, with a total length of about 75 to 350 cm. A microscopic inspection of the affected skin revealed numerous nematocysts. Subepidermal separations were apparent at the dermo-epidermal junction. Patchy necrosis with neutrophils and lymphocytes was detected in the epidermis, while erythrocyte extravasation was found in the dermis. There were focal regions of arteriosclerotic cardiovascular disease and signs of coagulopathy, but no myocardial death [224].
Postmortem examination of a previously healthy 44-year-old male diagnosed with Irukandji syndrome revealed a substantial intracranial hemorrhage with no arteriovenous malformations. The nematocyst retrieved from the skin of the patient matched those of Carukia barnesi [149].
10. Climate Changes and the Increased Risk of Jellyfish Stings
The rising water temperatures are expected to prompt jellyfish blooms, both by directly increasing asexual reproduction and by promoting eutrophization, therefore boosting jellyfish food availability. Specifically, the complex life cycle of scyphozoan jellyfish alternates between benthic (polyp) and pelagic (medusa) phases. Medusae reproduce sexually by producing planula larvae, which land on surfaces and evolve into polyps that reproduce asexually. Under specific environmental circumstances, a mechanism known as strobilation allows polyps to discharge microscopic jellyfish (ephyrae) into the water. Polyp asexual reproduction rate has been shown to increase with water temperature, up to a certain optimal point [225,226,227,228]. However, overwintering temperatures are required to enable strobilation, with some species tolerating higher strobilation temperatures, while others were unable to thrive in warmer conditions. As such, although it has been hypothesized that jellyfish will flourish in rising water temperatures, future climate change might prohibit species that can only strobilate under certain circumstances, limiting the biodivesrsity of jellyfish [229]. Eutrophication has also been implicated in jellyfish blooms. In fact, food availability greatly increases jellyfish populations, as it improves fertility and triggers a shift from asexual to sexual reproduction, resulting in more individuals in the medusa stage [230].
11. Conclusions
Major characteristics of jellyfish stings are summarized in Figure 11. Major characteristics of jellyfish stings. Most patients and doctors alike are unaware of the clinical picture and treatment of a jellyfish envenomation. Some stings, particularly those caused by scyphozoans (i.e., true jellyfish) are usually mild and limited to an erythematous rash, while others (mostly cubozoan stings) can be lethal. Seabather’s eruption is a peculiar syndrome caused by the larval form of some jellyfish (mostly Linuche unguiculata), consisting of a highly pruritic papular rash in the regions covered by bathing clothes. Irukandji syndrome also deserves particular attention, as it is severe and often lethal. It resembles a catecholamine surge, and almost all cases described occurred in the Indo-Pacific region. The diagnosis is mainly clinical, based on the history of sea bath, the characteristics of the skin lesions, and, possibly, systemic symptoms. Skin scraping/sticky tape, dermoscopy, and reflectance confocal microscopy may help determine the occurrence of a jellyfish sting and identify the responsible species. Immunological tests may aid in determining if a patient has been previously exposed to a jellyfish and the risk of developing an allergic reaction.
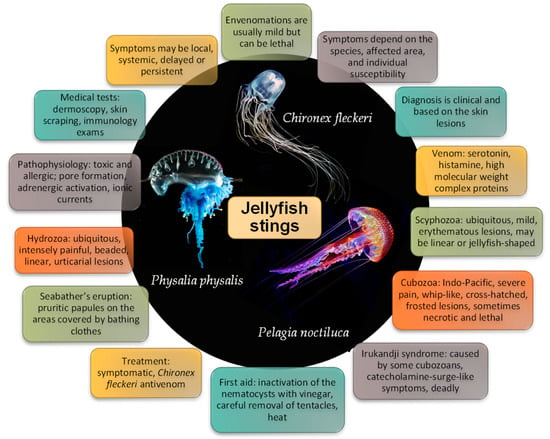
Figure 11.
Major characteristics of jellyfish stings.
The treatment of jellyfish stings remains controversial. It is widely accepted that inactivating the nematocysts and preventing further venom discharge is of utmost importance. However, research is conflicting regarding the safest method to achieve that goal. Vinegar irrigation is the most consensual treatment for inactivating nematocysts and heat application has been reported to inactivate the venom and offer pain relief. Careful removal of the tentacles with tweezers is also advocated. Further research is needed in this regard, particularly randomized controlled trials in different places of the world, as different jellyfish species seem to react differently to the treatments. The only specific treatment for a cnidarian envenomation in use thus far is a Chironex fleckeri antivenom. Other antivenoms are still being developed but were not tested in humans [231,232]. Finally, the way in which the venom exerts its toxic effects on humans is yet to be fully clarified and that may help to develop targeted treatments for jellyfish stings.
Author Contributions
Conceptualization and supervision, R.J.D.-O. Data curation, formal analysis, and writing of original draft preparation, S.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, and royalties.
Institutional Review Board Statement
All procedures were performed according to the ethical and legal standards of the institution.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest. No writing assistance was used in the production of this manuscript.
References
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Needleman, R.K.; Neylan, I.P.; Erickson, T.B. Environmental and Ecological Effects of Climate Change on Venomous Marine and Amphibious Species in the Wilderness. Wilderness Environ. Med. 2018, 29, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; De Haro, L. Clinical marine toxicology: A European perspective for clinical toxicologists and poison centers. Toxins 2013, 5, 1343–1352. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Kim, J.S.; Yoon, W.D.; Lim, D.; Hart, A.J.; Hodgson, W.C. Cardiovascular effects of Nemopilema nomurai (Scyphozoa: Rhizostomeae) jellyfish venom in rats. Toxicol. Lett. 2006, 167, 205–211. [Google Scholar] [CrossRef]
- Boulware, D.R. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J. Travel Med. 2006, 13, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Killi, N.; Mariottini, G.L. Cnidarian Jellyfish: Ecological Aspects, Nematocyst Isolation, and Treatment Methods of Sting. Mar. Org. Model Syst. Biol. Med. 2018, 65, 477–513. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. One image is worth more than a thousand words: Producing an atlas of medical signs for teaching clinical and forensic toxicology. Forensic Sci. Res. 2022, in press. [CrossRef]
- Dinis-Oliveira, R.J.; Carvalho, F.; Duarte, J.A.; Proença, J.B.; Santos, A.; Magalhães, T. Clinical and forensic signs related to cocaine abuse. Curr. Drug Abus. Rev. 2012, 5, 64–83. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Carvalho, F.; Moreira, R.; Duarte, J.A.; Proenca, J.B.; Santos, A.; Magalhaes, T. Clinical and forensic signs related to opioids abuse. Curr. Drug Abus. Rev. 2012, 5, 273–290. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolic Profiles of Propofol and Fospropofol: Clinical and Forensic Interpretative Aspects. Biomed. Res. Int. 2018, 2018, 6852857. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Magalhães, T.; Moreira, R.; Proença, J.B.; Pontes, H.; Santos, A.; Duarte, J.A.; Carvalho, F. Clinical and forensic signs related to ethanol abuse: A mechanistic approach. Toxicol. Mech. Methods 2014, 24, 81–110. [Google Scholar] [CrossRef]
- Horton, T.; Kroh, A.; Ahyong, S.; Bailly, N.; Bieler, R.; Boyko, C.B.; Brandão, S.N.; Gofas, S.; Hooper, J.N.A.; Hernandez, F.; et al. World Register of Marine Species (WoRMS); WoRMS Editorial Board: Ostend, Belgium, 2022. [Google Scholar]
- Sujanitha, V.; Sivansuthan, S.; Luckshman, W.V.; Gnaneswaran, R.; Jeyakanth, T.; Gunarathna, U. The clinical manifestations, outcome and identification of jellyfish stings in Jaffna, Sri Lanka. Trop. Dr. 2017, 47, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Costa, R.; Morabito, R.; La Spada, G.; Marino, A.; Dossena, S. Impact of Scyphozoan Venoms on Human Health and Current First Aid Options for Stings. Toxins 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Calton, G.J.; Burnett, H.W.; Mandojana, R.M. Local and systemic reactions from jellyfish stings. Clin. Dermatol. 1987, 5, 14–28. [Google Scholar] [CrossRef]
- Carrette, T.J.; Underwood, A.H.; Seymour, J.E. Irukandji syndrome: A widely misunderstood and poorly researched tropical marine envenoming. Diving Hyperb. Med. 2012, 42, 214–223. [Google Scholar]
- Horiike, T.; Nagai, H.; Kitani, S. Identification of allergens in the box jellyfish Chironex yamaguchii that cause sting dermatitis. Int. Arch. Allergy Immunol. 2015, 167, 73–82. [Google Scholar] [CrossRef]
- Haddad, V., Jr. Environmental dermatology: Skin manifestations of injuries caused by invertebrate aquatic animals. An. Bras. Dermatol. 2013, 88, 496–506. [Google Scholar] [CrossRef]
- Tibballs, J.; Yanagihara, A.A.; Turner, H.C.; Winkel, K. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets 2011, 10, 438–446. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Pane, L. Mediterranean jellyfish venoms: A review on scyphomedusae. Mar. Drugs 2010, 8, 1122–1152. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.S.; Wang, Q.Q.; He, Q.; Liu, S.H.; Li, Y.; Zhang, J.; Zhang, L.M. The lethality of tentacle-only extract from jellyfish Cyanea capillata is primarily attributed to cardiotoxicity in anaesthetized SD rats. Toxicon 2010, 55, 838–845. [Google Scholar] [CrossRef]
- Liang, X.; Beilei, W.; Ying, L.; Qianqian, W.; Sihua, L.; Yang, W.; Guoyan, L.; Jia, L.; Xuting, Y.; Liming, Z. Cardiovascular effect is independent of hemolytic toxicity of tentacle-only extract from the jellyfish Cyanea capillata. PLoS ONE 2012, 7, e43096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.; Xiao, L.; Zhang, L. Intervention effects of five cations and their correction on hemolytic activity of tentacle extract from the jellyfish Cyanea capillata. PeerJ 2017, 5, e3338. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, G.L. Hemolytic venoms from marine cnidarian jellyfish—An overview. J. Venom. Res. 2014, 5, 22–32. [Google Scholar] [PubMed]
- Morabito, R.; Marino, A.; Dossena, S.; La Spada, G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon 2014, 83, 52–58. [Google Scholar] [CrossRef]
- Ponce, D.; Brinkman, D.L.; Potriquet, J.; Mulvenna, J. Tentacle Transcriptome and Venom Proteome of the Pacific Sea Nettle, Chrysaora fuscescens (Cnidaria: Scyphozoa). Toxins 2016, 8, 102. [Google Scholar] [CrossRef]
- Badré, S. Bioactive toxins from stinging jellyfish. Toxicon 2014, 91, 114–125. [Google Scholar] [CrossRef]
- Morabito, R.; Condello, S.; Currò, M.; Marino, A.; Ientile, R.; La Spada, G. Oxidative stress induced by crude venom from the jellyfish Pelagia noctiluca in neuronal-like differentiated SH-SY5Y cells. Toxicol. In Vitro 2012, 26, 694–699. [Google Scholar] [CrossRef]
- Mc, D.T.D.; Pereira, P.; Seymour, J.; Winkel, K.D. A sting from an unknown jellyfish species associated with persistent symptoms and raised troponin I levels. Emerg. Med. 2002, 14, 175–180. [Google Scholar] [CrossRef]
- Bianchi, R.; Torella, D.; Spaccarotella, C.; Mongiardo, A.; Indolfi, C. Mediterranean jellyfish sting-induced Tako-Tsubo cardiomyopathy. Eur. Heart J. 2011, 32, 18. [Google Scholar] [CrossRef][Green Version]
- Salam, A.M.; Albinali, H.A.; Gehani, A.A.; Al Suwaidi, J. Acute myocardial infarction in a professional diver after jellyfish sting. Mayo Clin. Proc. 2003, 78, 1557–1560. [Google Scholar] [CrossRef]
- Little, M.; Pereira, P.; Mulcahy, R.; Cullen, P.; Carrette, T.; Seymour, J. Severe cardiac failure associated with presumed jellyfish sting. Irukandji syndrome? Anaesth. Intensive Care 2003, 31, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J.; Li, R.; Tibballs, H.A.; Gershwin, L.A.; Winkel, K.D. Australian carybdeid jellyfish causing “Irukandji syndrome”. Toxicon 2012, 59, 617–625. [Google Scholar] [CrossRef]
- Mustafa, M.R.; White, E.; Hongo, K.; Othman, I.; Orchard, C.H. The mechanism underlying the cardiotoxic effect of the toxin from the jellyfish Chironex fleckeri. Toxicol. Appl. Pharm. 1995, 133, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Gean, C.J.; Calton, G.J.; Warnick, J.E. The effect of verapamil on the cardiotoxic activity of Portuguese man-o’war (Physalia physalis) and sea nettle (Chrysaora quinquecirrha) venoms. Toxicon 1985, 23, 681–689. [Google Scholar] [CrossRef]
- Azuma, H.; Ishikawa, M.; Nakajima, T.; Satoh, A.; Sekizaki, S. Calcium-dependent contractile response of arterial smooth muscle to a jellyfish toxin (pCrTX: Carybdea rastonii). Br. J. Pharmacol. 1986, 88, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Karaki, H.; Nagase, H.; Urakawa, N.; Azuma, H.; Nakajima, T. Contractile effects of jellyfish toxin extracted from Carybdea rastonii on isolated rabbit aorta. Jpn. J. Pharmacol. 1986, 42, 425–430. [Google Scholar] [CrossRef]
- Burnett, J.W.; Calton, G.J. Response of the box-jellyfish (Chironex fleckeri) cardiotoxin to intravenous administration of verapamil. Med. J. Aust. 1983, 2, 192–194. [Google Scholar] [CrossRef]
- Beilei, W.; Lin, Z.; Qian, H.; Qianqian, W.; Tao, W.; Jia, L.; Xiaojuan, W.; Xuting, Y.; Liang, X.; Liming, Z. Direct cardiac toxicity of the tentacle-only extract from the jellyfish Cyanea capillata demonstrated in isolated rat heart. J. Cardiovasc. Pharm. 2012, 59, 331–338. [Google Scholar] [CrossRef]
- Bailey, P.M.; Bakker, A.J.; Seymour, J.E.; Wilce, J.A. A functional comparison of the venom of three Australian jellyfish—Chironex fleckeri, Chiropsalmus sp., and Carybdea xaymacana—On cytosolic Ca2+, haemolysis and Artemia sp. lethality. Toxicon 2005, 45, 233–242. [Google Scholar] [CrossRef]
- Chung, J.J.; Ratnapala, L.A.; Cooke, I.M.; Yanagihara, A.A. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish (Carybdea alata) venom. Toxicon 2001, 39, 981–990. [Google Scholar] [CrossRef]
- Nagai, H.; Takuwa, K.; Nakao, M.; Ito, E.; Miyake, M.; Noda, M.; Nakajima, T. Novel proteinaceous toxins from the box jellyfish (Sea Wasp) Carybdea rastoni. Biochem. Biophys. Res. Commun. 2000, 275, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A novel protein toxin from the deadly box jellyfish (Sea Wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Aziz, A.; Loukas, A.; Potriquet, J.; Seymour, J.; Mulvenna, J. Venom proteome of the box jellyfish Chironex fleckeri. PLoS ONE 2012, 7, e47866. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Noguchi, K.; Matsuzaki, T.; Sakanashi, M.; Nakasone, J.; Miyagi, K.; Sakanashi, M.; Sakanashi, M. Haemodynamic effects of the crude venom from nematocysts of the box-jellyfish Chiropsalmus quadrigatus (Habu-kurage) in anaesthetized rabbits. Toxicon 2003, 41, 621–631. [Google Scholar] [CrossRef]
- Morabito, R.; Costa, R.; Rizzo, V.; Remigante, A.; Nofziger, C.; La Spada, G.; Marino, A.; Paulmichl, M.; Dossena, S. Crude venom from nematocysts of Pelagia noctiluca (Cnidaria: Scyphozoa) elicits a sodium conductance in the plasma membrane of mammalian cells. Sci. Rep. 2017, 7, 41065. [Google Scholar] [CrossRef]
- Frazão, B.; Campos, A.; Osório, H.; Thomas, B.; Leandro, S.; Teixeira, A.; Vasconcelos, V.; Antunes, A. Analysis of Pelagia noctiluca proteome Reveals a Red Fluorescent Protein, a Zinc Metalloproteinase and a Peroxiredoxin. Protein J. 2017, 36, 77–97. [Google Scholar] [CrossRef]
- Lee, H.; Jung, E.S.; Kang, C.; Yoon, W.D.; Kim, J.S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vivo cardiovascular effects of the Irukandji jellyfish (Carukia barnesi) nematocyst venom and a tentacle extract in rats. Toxicol. Lett. 2005, 155, 135–141. [Google Scholar] [CrossRef]
- Winkel, K.D.; Tibballs, J.; Molenaar, P.; Lambert, G.; Coles, P.; Ross-Smith, M.; Wiltshire, C.; Fenner, P.J.; Gershwin, L.A.; Hawdon, G.M.; et al. Cardiovascular actions of the venom from the Irukandji (Carukia barnesi) jellyfish: Effects in human, rat and guinea-pig tissues in vitro and in pigs in vitro. Clin. Exp. Pharmacol. Physiol. 2005, 32, 777–788. [Google Scholar] [CrossRef]
- Li, R.; Wright, C.E.; Winkel, K.D.; Gershwin, L.A.; Angus, J.A. The pharmacology of Malo maxima jellyfish venom extract in isolated cardiovascular tissues: A probable cause of the Irukandji syndrome in Western Australia. Toxicol. Lett. 2011, 201, 221–229. [Google Scholar] [CrossRef]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vivo cardiovascular effects of an Australasian box jellyfish (Chiropsalmus sp.) venom in rats. Toxicon 2005, 45, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri) venom and a tentacle-only extract in rats. Toxicol. Lett. 2005, 155, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.J.; Angus, J.A.; Winkel, K.D.; Wright, C.E. A pharmacological investigation of the venom extract of the Australian box jellyfish, Chironex fleckeri, in cardiac and vascular tissues. Toxicol. Lett. 2012, 209, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Wang, B.; Wang, C.; Xiao, L.; Zhang, L. β adrenergic receptor/cAMP/PKA signaling contributes to the intracellular Ca(2+) release by tentacle extract from the jellyfish Cyanea capillata. BMC Pharmacol. Toxicol. 2017, 18, 60. [Google Scholar] [CrossRef]
- Bueno, T.C.; Collaço, R.C.; Cardoso, B.A.; Bredariol, R.F.; Escobar, M.L.; Cajado, I.B.; Gracia, M.; Antunes, E.; Zambelli, V.O.; Picolo, G.; et al. Neurotoxicity of Olindias sambaquiensis and Chiropsalmus quadrumanus extracts in sympathetic nervous system. Toxicon 2021, 199, 127–138. [Google Scholar] [CrossRef]
- Wang, B.; Liu, D.; Wang, C.; Wang, Q.; Zhang, H.; Liu, G.; Tao, X.; Zhang, L. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by tentacle extract from the jellyfish Cyanea capillata. PeerJ 2017, 5, e3172. [Google Scholar] [CrossRef]
- Kitatani, R.; Yamada, M.; Kamio, M.; Nagai, H. Length Is Associated with Pain: Jellyfish with Painful Sting Have Longer Nematocyst Tubules than Harmless Jellyfish. PLoS ONE 2015, 10, e0135015. [Google Scholar] [CrossRef]
- Cuypers, E.; Yanagihara, A.; Karlsson, E.; Tytgat, J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006, 580, 5728–5732. [Google Scholar] [CrossRef]
- Cromer, B.A.; McIntyre, P. Painful toxins acting at TRPV1. Toxicon 2008, 51, 163–173. [Google Scholar] [CrossRef]
- Rastogi, A.; Sarkar, A.; Chakrabarty, D. Partial purification and identification of a metalloproteinase with anticoagulant activity from Rhizostoma pulmo (Barrel Jellyfish). Toxicon 2017, 132, 29–39. [Google Scholar] [CrossRef]
- Bae, S.K.; Lee, H.; Heo, Y.; Pyo, M.J.; Choudhary, I.; Han, C.H.; Yoon, W.D.; Kang, C.; Kim, E. In vitro characterization of jellyfish venom fibrin (ogen) olytic enzymes from Nemopilema nomurai. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Biswas, S.; Sarkar, A.; Chakrabarty, D. Anticoagulant activity of Moon jellyfish (Aurelia aurita) tentacle extract. Toxicon 2012, 60, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yu, H.; Li, R.; Li, P. Topical Exposure to Nemopilema nomurai Venom Triggers Oedematogenic Effects: Enzymatic Contribution and Identification of Venom Metalloproteinase. Toxins 2021, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Impellizzeri, D.; Morabito, R.; Marino, A.; Ahmad, A.; Spanò, N.; Spada, G.L.; Cuzzocrea, S.; Esposito, E. Pelagia noctiluca (Scyphozoa) crude venom injection elicits oxidative stress and inflammatory response in rats. Mar. Drugs 2014, 12, 2182–2204. [Google Scholar] [CrossRef]
- Wang, T.; Wen, X.J.; Mei, X.B.; Wang, Q.Q.; He, Q.; Zheng, J.M.; Zhao, J.; Xiao, L.; Zhang, L.M. Lipid peroxidation is another potential mechanism besides pore-formation underlying hemolysis of tentacle extract from the jellyfish Cyanea capillata. Mar. Drugs 2013, 11, 67–80. [Google Scholar] [CrossRef]
- Ayed, Y.; Chayma, B.; Hayla, A.; Abid, S.; Bacha, H. Is cell death induced by nematocysts extract of medusa Pelagia noctiluca related to oxidative stress? Environ. Toxicol. 2013, 28, 498–506. [Google Scholar] [CrossRef]
- Nevalainen, T.J.; Peuravuori, H.J.; Quinn, R.J.; Llewellyn, L.E.; Benzie, J.A.; Fenner, P.J.; Winkel, K.D. Phospholipase A2 in cnidaria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 731–735. [Google Scholar] [CrossRef]
- Cormier, S.M. Exocytotic and cytolytic release of histamine from mast cells treated with Portuguese man-of-war (Physalia physalis) venom. J. Exp. Zool. 1984, 231, 1–10. [Google Scholar] [CrossRef]
- Uvnas, B. A toxic (histamine-releasing) principle from tentacles of Cyanea capillata (the stinging jelly-fish). J. Med. Pharm. Chem. 1961, 4, 511–515. [Google Scholar] [CrossRef]
- Guevara, B.E.; Dayrit, J.F.; Haddad, V., Jr. Delayed allergic dermatitis presenting as a keloid-like reaction caused by sting from an Indo-Pacific Portuguese man-o’-war (Physalia utriculus). Clin. Exp. Derm. 2017, 42, 182–184. [Google Scholar] [CrossRef]
- Tiong, K. Irukandji syndrome, catecholamines, and mid-ventricular stress cardiomyopathy. Eur. J. Echocardiogr. 2009, 10, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon 2006, 48, 830–859. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Calton, G.J. Jellyfish envenomation syndromes updated. Ann. Emerg. Med. 1987, 16, 1000–1005. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.B.; Durey, A. Fatal Pulmonary Edema in a Child After Jellyfish Stings in Korea. Wilderness Environ. Med. 2018, 29, 527–530. [Google Scholar] [CrossRef]
- Sridhar, S.C.; Deo, S.C. Marine and Other Aquatic Dermatoses. Indian J. Dermatol. 2017, 62, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, P.S.; Gupta, D.; Van Hoesen, K.; Zavala, A. Dermatological Progression of a Probable Box Jellyfish Sting. Wilderness Environ. Med. 2019, 30, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Tønseth, K.A. Health damage after jellyfish stings. Tidsskr. Den Nor. Legeforen. 2007, 127, 1777–1778. [Google Scholar]
- Shabani, M.; Saffaei, A.; Asghari, M.; Sahraei, Z. Jellyfish Stings Rarely Induced Infectious Cellulitis: First Aid Remedies as Double-Edged Sword. Adv. J. Emerg. Med. 2020, 4, e33. [Google Scholar] [CrossRef] [PubMed]
- Thaikruea, L.; Siriariyaporn, P. Severe Dermatonecrotic Toxin and Wound Complications Associated With Box Jellyfish Stings 2008–2013. J. Wound Ostomy Cont. Nurs. 2015, 42, 599–604. [Google Scholar] [CrossRef]
- Rossetto, A.L.; Cruz, C.C.B.; Pereira, I.C.C.; Nunes, J.A.; Martins, M.M.; Nicolacópulos, T.; Rossetto, A.L.; Haddad Junior, V. Diagnostic confusion between seabather’s eruption as well as dermatophytosis and parasitic infestations. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190462. [Google Scholar] [CrossRef]
- Desax-Willer, D.; Krebs, T.; Christen, S. Delayed deep dermal necrosis after jellyfish sting in a 4-year-old female infant. Case Rep. Plast Surg. Hand Surg. 2018, 5, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Giavedoni, P.; Velasco, V.; Morgado-Carrasco, D.; Nogué, S.; Mascaró Galy, J.M. Delayed Allergic Reactions to Jellyfish Stings: Usefulness of Ultrasonographic Evaluation. J. Ultrasound Med. 2018, 37, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Mabuchi, T.; Kawai, M.; Ota, T.; Ikoma, N.; Ozawa, A.; Horita, T. A Case of Delayed Flare-up Allergic Dermatitis Caused by Jellyfish Sting. Tokai J. Exp. Clin. Med. 2014, 39, 90–94. [Google Scholar] [PubMed]
- Veraldi, S.; Carrera, C. Delayed cutaneous reaction to jellyfish. Int. J. Dermatol. 2000, 39, 28–29. [Google Scholar] [CrossRef]
- Ohtaki, N.; Satoh, A.; Azuma, H.; Nakajima, T. Delayed flare-up reactions caused by jellyfish. Dermatologica 1986, 172, 98–103. [Google Scholar] [CrossRef]
- Ulrich, H.; Landthaler, M.; Vogt, T. Granulomatous jellyfish dermatitis. J. Dtsch Dermatol. Ges. 2007, 5, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Yaffee, H.S. A delayed cutaneous reaction following contact with jellyfish. Dermatol. Int. 1968, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, L.; Balato, N.; Zagaria, O.; Balato, A. Successful management of a delayed and persistent cutaneous reaction to jellyfish with pimecrolimus. J. Dermatol. Treat. 2009, 20, 179–180. [Google Scholar] [CrossRef]
- Rallis, E.; Limas, C. Recurrent dermatitis after solitary envenomation by jellyfish partially responded to tacrolimus ointment 0.1%. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1287–1288. [Google Scholar] [CrossRef]
- Burnett, J.W.; Calton, G.J. Recurrent eruption following a solitary envenomation by the cnidarian Stomolophous meleagris. Toxicon 1985, 23, 1010–1014. [Google Scholar] [CrossRef]
- Menahem, S.; Shvartzman, P. Recurrent dermatitis from jellyfish envenomation. Can. Fam. Phys. 1994, 40, 2116–2118. [Google Scholar]
- Dellanna, L.; Hirche, F.; Capra, V. Successful Treatment of Recurrent Dermatitis after Physalia physalis (Portuguese Man O’ War) Envenomation with Extracorporeal Shock Wave Therapy. Case Rep. Derm. 2021, 13, 202–208. [Google Scholar] [CrossRef]
- Chand, R.P.; Selliah, K. Reversible parasympathetic dysautonomia following stinging attributed to the box jelly fish (Chironex fleckeri). Aust. New Zealand J. Med. 1984, 14, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Ponampalam, R. An unusual case of paralytic ileus after jellyfish envenomation. Emerg. Med. J. 2002, 19, 357–358. [Google Scholar] [CrossRef]
- Burnett, J.W. Prolonged urinary incontinence and biliary dyskinesia following abdominal contact with jellyfish tentacles. Wilderness Environ. Med. 2006, 17, 180–186. [Google Scholar] [CrossRef]
- Adiga, K.M. Brachial artery spasm as a result of a sting. Med. J. Aust. 1984, 140, 181–182. [Google Scholar] [CrossRef]
- Williamson, J.A.; Burnett, J.W.; Fenner, P.J.; Hach-Wunderle, V.; Hoe, L.Y.; Adiga, K.M. Acute regional vascular insufficiency after jellyfish envenomation. Med. J. Aust. 1988, 149, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.; Chan, H.Z.; Faruk, N.A.; Bea, K.C.; Zulkiflee, O. Jellyfish Envenomation Resulting In Vascular Insufficiency And Neurogenic Injury of Upper Limb. Malays. Orthop. J. 2015, 9, 49–51. [Google Scholar] [CrossRef]
- Glasser, D.B.; Noell, M.J.; Burnett, J.W.; Kathuria, S.S.; Rodrigues, M.M. Ocular jellyfish stings. Ophthalmology 1992, 99, 1414–1418. [Google Scholar] [CrossRef]
- Burnett, H.W.; Burnett, J.W. Prolonged blurred vision following coelenterate envenomation. Toxicon 1990, 28, 731–733. [Google Scholar] [CrossRef]
- Little, M.; Seymour, J. Another cause of “Irukandji stingings”. Med. J. Aust. 2003, 179, 654. [Google Scholar] [CrossRef] [PubMed]
- de Pender, A.M.; Winkel, K.D.; Ligthelm, R.J. A probable case of Irukandji syndrome in Thailand. J. Travel Med. 2006, 13, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Lippmann, J. Severe Irukandji-like jellyfish stings in Thai waters. Diving Hyperb Med. 2009, 39, 175–177. [Google Scholar] [PubMed]
- Grady, J.D.; Burnett, J.W. Irukandji-like syndrome in South Florida divers. Ann. Emerg. Med. 2003, 42, 763–766. [Google Scholar] [CrossRef]
- Sando, J.J.; Usher, K. Case review: A 28-year-old Korean man with Irukandji syndrome. Int. Emerg. Nurs. 2009, 17, 72–76. [Google Scholar] [CrossRef] [PubMed]
- McIver, L.J.; Tjhung, I.G.; Parish, S.T.; Derkenne, R.C.; Kippin, A.N. Irukandji sydrome in the Torres Strait: A series of 8 cases. Wilderness Environ. Med. 2011, 22, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Thaikruea, L. Irukandji-like syndrome caused by single-tentacle box jellyfish found in Thailand, 2007–2019. Int. Marit. Health 2020, 71, 91–96. [Google Scholar] [CrossRef]
- Filling-Katz, M.R. Mononeuritis multiplex following jellyfish stings. Ann. Neurol. 1984, 15, 213. [Google Scholar] [CrossRef]
- Burnett, J.W.; Williamson, J.A.; Fenner, P.J. Mononeuritis multiplex after coelenterate sting. Med. J. Aust. 1994, 161, 320–322. [Google Scholar] [CrossRef]
- Peel, N.; Kandler, R. Localized neuropathy following jellyfish sting. Postgrad. Med. J. 1990, 66, 953–954. [Google Scholar] [CrossRef]
- Al-Ajmi, A.M.; Jayappa, S.; Rousseff, R.T. Isolated severe median mononeuropathy caused by a jellyfish sting. J. Clin. Neuromuscul. Dis. 2013, 14, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Hach-Wunderle, V.; Mebs, D.; Frederking, K.; Breddin, H.K. Jellyfish poisoning. Dtsch. Med. Wochenschr. 1987, 112, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Laing, J.H.; Harrison, D.H. Envenomation by the box-jellyfish--an unusual cause of ulnar nerve palsy. J. R. Soc. Med. 1991, 84, 115–116. [Google Scholar] [CrossRef]
- Pang, K.A.; Schwartz, M.S. Guillain-Barré syndrome following jellyfish stings (Pelagia noctiluca). J. Neurol. Neurosurg. Psychiatry 1993, 56, 1133. [Google Scholar] [CrossRef]
- Devere, R. Guillain-Barré syndrome after a jellyfish sting. J. Clin. Neuromuscul. Dis. 2011, 12, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Azari, P.; Lu, Y.; Clarke, C.F.; Collins, T.; Briones, D.; Huh, B. Pathophysiology of the spreading of complex regional pain syndrome revisited: A case report. Neuromodulation 2011, 14, 428–431; discussion 431. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W. Dysphonia: A new addition to jellyfish envenomation syndromes. Wilderness Environ. Med. 2005, 16, 117–118. [Google Scholar] [CrossRef]
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish stings and their management: A review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef]
- Spielman, F.J.; Bowe, E.A.; Watson, C.B.; Klein, E.F., Jr. Acute renal failure as a result of Physalia physalis sting. South Med. J. 1982, 75, 1425–1426. [Google Scholar] [CrossRef]
- Pereira, J.C.C.; Szpilman, D.; Haddad Junior, V. Anaphylactic reaction/angioedema associated with jellyfish sting. Rev. Soc. Bras. Med. Trop. 2018, 51, 115–117. [Google Scholar] [CrossRef]
- Maldonado, E.; Maillaud, C.; Barguil, Y.; Labadie, M. Rhabdomyolysis during envenomation by Physalia sp. envenomation in New Caldonia. Med. St. Trop. 2017, 27, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Rengel, A.C.; Davel, S.; O’Connor, S.; Medcraft, N. Rhabdomyolysis due to jellyfish envenomation in Western Australian waters. Med. J. Aust. 2022, 216, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, P.S.; Hays, J.T. Erythema nodosum following a jellyfish sting. J. Emerg. Med. 1987, 5, 487–491. [Google Scholar] [CrossRef]
- Al-Ebrahim, K.; Tahir, M.Z.; Rustom, M.; Shafei, H. Jellyfish-venom-induced deep venous thrombosis. A case report. Angiology 1995, 46, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, J.F.; O’Sullivan, E.A. A case report of an unusual mandibular swelling in a 4-year-old child possibly caused by a jellyfish sting. Int. J. Paediatr. Dent. 1998, 8, 51–54. [Google Scholar] [CrossRef]
- Tortell, A.; Baldacchino, R.V.; Grech, J.A. The Boy With the Jellyfish Tattoo and Facial Swelling. Wilderness Environ. Med. 2021, 32, 545–547. [Google Scholar] [CrossRef]
- Binnetoglu, F.K.; Kizildag, B.; Topaloglu, N.; Kasapcopur, O. Severe digital necrosis in a 4-year-old boy: Primary Raynaud’s or jellyfish sting. Case Rep. 2013, 2013, bcr2013201478. [Google Scholar] [CrossRef]
- Lam, S.C.; Hung, Y.W.; Chow, E.C.; Wong, C.W.; Tse, W.L.; Ho, P.C. Digital ischaemia: A rare but severe complication of jellyfish sting. Hong Kong Med. J. 2014, 20, 460–463. [Google Scholar] [CrossRef]
- Lo, A.H.; Chan, Y.C.; Law, Y.; Cheng, S.W. Successful treatment of jellyfish sting-induced severe digital ischemia with intravenous iloprost infusion. J. Vasc. Surg. Cases 2016, 2, 31–33. [Google Scholar] [CrossRef]
- Chiang, F.; Castillo, M. Seastrokes: A new threat for north Carolina swimmers? A case report. Neuroradiol. J. 2014, 27, 499–502. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Haddad, V., Jr.; Virga, R.; Bechara, A.; Silveira, F.L.; Morandini, A.C. An outbreak of Portuguese man-of-war (Physalia physalis—Linnaeus, 1758) envenoming in Southeastern Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Kapamajian, M.A.; Ahmad, A.; Burnett, J.W.; Burnett, H.W.; Frenkel, S. Unilateral orbital inflammation in a child after a jellyfish sting to the lower extremities. Ophthalmic Plast Reconstr. Surg. 2009, 25, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Bandyopadhyay, D.; Haldar, S. Lichen planus-like eruption resulting from a jellyfish sting: A case report. J. Med. Case Rep. 2009, 3, 7421. [Google Scholar] [CrossRef]
- Del Pozo, L.J.; Knöpfel, N.; Martín-Santiago, A.; Escudero-Góngora, M.M.; Saus, C.; Izquierdo-Herce, N.; Bauzà-Alonso, A. Dermoscopic Findings of Jellyfish Stings Caused by Pelagia noctiluca. Actas Dermosifiliogr. 2016, 107, 509–515. [Google Scholar] [CrossRef]
- Gunn, M.A. Localized fat atrophy after jellyfish sting. Br. Med. J. 1949, 2, 687. [Google Scholar] [CrossRef]
- Drury, J.K.; Noonan, J.D.; Pollock, J.G.; Reid, W.H. Jelly fish sting with serious hand complications. Injury 1980, 12, 66–68. [Google Scholar] [CrossRef]
- Tamanaha, R.H.; Izumi, A.K. Persistent cutaneous hypersensitivity reaction after a Hawaiian box jellyfish sting (Carybdea alata). J. Am. Acad. Dermatol. 1996, 35, 991–993. [Google Scholar] [CrossRef]
- Pérez-López, I.; Garrido-Colmenero, C.; Blasco-Morente, G.; Tercedor-Sánchez, J. Koebner phenomenon in a patient with lichen sclerosus following a jellyfish sting: An exceptional morphology. Actas Dermosifiliogr. 2015, 106, 238–239. [Google Scholar] [CrossRef]
- Thaikruea, L.; Siriariyaporn, P.; Wutthanarungsan, R.; Smithsuwan, P. Review of fatal and severe cases of box jellyfish envenomation in Thailand. Asia Pac. J. Public Health 2015, 27, NP1639–NP1651. [Google Scholar] [CrossRef]
- Anziani Vente, A.; Jaloux, C.; Morand, J.J.; Bel, S.L.; Bosdure, E.; Dubus, J.C.; Morand, A. Severe box jellyfish envenomation in 2 children. Travel Med. Infect. Dis. 2022, 45, 102217. [Google Scholar] [CrossRef] [PubMed]
- Tiemensma, M.; Currie, B.J.; Byard, R.W. Fatal jellyfish envenoming-Pediatric and geographic vulnerabilities. J. Forensic Sci. 2021, 66, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Togias, A.G.; Burnett, J.W.; Kagey-Sobotka, A.; Lichtenstein, L.M. Anaphylaxis after contact with a jellyfish. J. Allergy Clin. Immunol. 1985, 75, 672–675. [Google Scholar] [CrossRef]
- Friedel, N.; Scolnik, D.; Adir, D.; Glatstein, M. Severe anaphylactic reaction to mediterranean jellyfish (Ropilhema nomadica) envenomation: Case report. Toxicol. Rep. 2016, 3, 427–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armoni, M.; Ohali, M.; Hay, E. Severe dyspnea due to jellyfish envenomation. Pediatr. Emerg. Care 2003, 19, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Williamson, J.A.; Burnett, J.W.; Colquhoun, D.M.; Godfrey, S.; Gunawardane, K.; Murtha, W. The “Irukandji syndrome” and acute pulmonary oedema. Med. J. Aust. 1988, 149, 150–156. [Google Scholar] [CrossRef]
- Fenner, P.J.; Hadok, J.C. Fatal envenomation by jellyfish causing Irukandji syndrome. Med. J. Aust 2002, 177, 362–363. [Google Scholar] [CrossRef]
- Pereira, P.; Barry, J.; Corkeron, M.; Keir, P.; Little, M.; Seymour, J. Intracerebral hemorrhage and death after envenoming by the jellyfish Carukia barnesi. Clin. Toxicol. 2010, 48, 390–392. [Google Scholar] [CrossRef]
- Bastos, D.M.; Haddad, V.J.; Nunes, J.L. Human envenomations caused by Portuguese man-of-war (Physalia physalis) in urban beaches of São Luis City, Maranhão State, Northeas.st Coast of Brazil. Rev. Soc. Bras. Med. Trop. 2017, 50, 130–134. [Google Scholar] [CrossRef]
- Moleiro, S.; Pereira, A.; Paiva Lopes, M.J. Marine dermatitis following exposure to a Portuguese-man-of-war. Acta Med. Port. 2013, 26, 66–69. [Google Scholar]
- Endean, R.; Monks, S.A.; Cameron, A.M. Toxins from the box-jellyfish Chironex fleckeri. Toxicon 1993, 31, 397–410. [Google Scholar] [CrossRef]
- Flecker, H. Fatal stings to North Queensland bathers. Med. J. Aust. 1952, 1, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Lumley, J.; Williamson, J.A.; Fenner, P.J.; Burnett, J.W.; Colquhoun, D.M. Fatal envenomation by Chironex fleckeri, the north Australian box jellyfish: The continuing search for lethal mechanisms. Med. J. Aust. 1988, 148, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.L.; Nappe, T.M. Irukandji Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nickson, C.P.; Waugh, E.B.; Jacups, S.P.; Currie, B.J. Irukandji syndrome case series from Australia’s Tropical Northern Territory. Ann. Emerg. 2009, 54, 395–403. [Google Scholar] [CrossRef]
- Little, M.; Pereira, P.; Carrette, T.; Seymour, J. Jellyfish responsible for Irukandji syndrome. J. Assoc. Phys. 2006, 99, 425–427. [Google Scholar] [CrossRef]
- Bouyer-Monot, D.; Pelczar, S.; Ferracci, S.; Boucaud-Maitre, D. Retrospective study of jellyfish envenomation in emergency wards in Guadeloupe between 2010 and 2016: When to diagnose Irukandji syndrome? Toxicon 2017, 137, 73–77. [Google Scholar] [CrossRef]
- Flecker, H. Irukandji sting to North Queensland bathers without production of weals but with severe general symptoms. J. Aust. 1952, 2, 89–91. [Google Scholar] [CrossRef]
- Yoshimoto, C.M.; Yanagihara, A.A. Cnidarian (coelenterate) envenomations in Hawai’i improve following heat application. Trans. R Soc. Trop. Med. Hyg. 2002, 96, 300–303. [Google Scholar] [CrossRef]
- Cheng, A.C.; Winkel, K.D.; Hawdon, G.M.; McDonald, M. Irukandji-like syndrome in Victoria. Aust. N. Z. J. Med. 1999, 29, 835. [Google Scholar] [CrossRef]
- Gershwin, L.A.; Richardson, A.J.; Winkel, K.D.; Fenner, P.J.; Lippmann, J.; Hore, R.; Avila-Soria, G.; Brewer, D.; Kloser, R.J.; Steven, A.; et al. Biology and ecology of Irukandji jellyfish (Cnidaria: Cubozoa). Adv. Mar. Biol. 2013, 66, 1–85. [Google Scholar] [CrossRef]
- Greenland, P.; Hutchinson, D.; Park, T. Irukandji Syndrome: What nurses need to know. Nurs. Health Sci. 2006, 8, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Harrison, S.L. Irukandji and Chironex fleckeri jellyfish envenomation in tropical Australia. Wilderness Environ. Med. 2000, 11, 233–240. [Google Scholar] [CrossRef]
- Fenner, P.J.; Williamson, J.; Callanan, V.I.; Audley, I. Further understanding of, and a new treatment for, “Irukandji” (Carukia barnesi) stings. Med. J. Aust. 1986, 145, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Hadok, J.C. “Irukandji” syndrome: A risk for divers in tropical waters. Med. J. Aust. 1997, 167, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, J.; Franklin, R.; Gibbs, C.; Williams, D. Review article: Role of magnesium sulphate in the management of Irukandji syndrome: A systematic review. Emerg. Med. Australas. 2017, 29, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sando, J.J.; Usher, K.; Buettner, P. ‘To swim or not to swim’: The impact of jellyfish stings causing Irukandji Syndrome in Tropical Queensland. J. Clin. Nurs. 2010, 19, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Audley, I. Cardiac failure following Irukandji envenomation. Med. J. Aust. 1990, 153, 164–166. [Google Scholar] [CrossRef]
- Huynh, T.T.; Seymour, J.; Pereira, P.; Mulcahy, R.; Cullen, P.; Carrette, T.; Little, M. Severity of Irukandji syndrome and nematocyst identification from skin scrapings. Med. J. Aust. 2003, 178, 38–41. [Google Scholar] [CrossRef]
- Little, M.; Mulcahy, R.F. A year’s experience of Irukandji envenomation in far north Queensland. Med. J. Aust 1998, 169, 638–641. [Google Scholar] [CrossRef]
- Segura Puertas, L.; Burnett, J.W.; de la Cotera, E.H. The medusa stage of the coronate scyphomedusa Linuche unguiculata (‘thimble jellyfish’) can cause seabather’s eruption. Dermatology 1999, 198, 171–172. [Google Scholar] [CrossRef]
- Segura-Puertas, L.; Ramos, M.E.; Aramburo, C.; De La Cotera, E.P.H.; Burnett, J.W. One Linuche mystery solved: All 3 stages of the coronate scyphomedusa Linuche unguiculata cause seabather’s eruption. J. Am. Acad. Dermatol. 2001, 44, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.E.; Meinking, T.L.; Rosen, L.B.; Taplin, D.; Hogan, D.J.; Burnett, J.W. Seabather’s eruption. Clinical, histologic, and immunologic features. J. Am. Acad. Dermatol. 1994, 30, 399–406. [Google Scholar] [CrossRef]
- Rossetto, A.L.; Dellatorre, G.; Silveira, F.L.; Haddad Júnior, V. Seabather’s eruption: A clinical and epidemiological study of 38 cases in Santa Catarina State, Brazil. Rev. Do Inst. De Med. Trop. De São Paulo 2009, 51, 169–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Donnell, B.F.; Tan, C.Y. Persistent contact dermatitis from jellyfish sting. Contact Dermat. 1993, 28, 112–113. [Google Scholar] [CrossRef]
- Uri, S.; Marina, G.; Liubov, G. Severe delayed cutaneous reaction due to Mediterranean jellyfish (Rhopilema nomadica) envenomation. Contact Dermat. 2005, 52, 282–283. [Google Scholar] [CrossRef]
- Mao, C.; Hsu, C.C.; Chen, K.T. Ocular Jellyfish Stings: Report of 2 Cases and Literature Review. Wilderness Environ. Med. 2016, 27, 421–424. [Google Scholar] [CrossRef]
- Winkel, K.D.; Hawdon, G.M.; Ashby, K.; Ozanne-Smith, J. Eye injury after jellyfish sting in temperate Australia. Wilderness Environ. Med. 2002, 13, 203–205. [Google Scholar] [CrossRef]
- Mitchell, J.H. Eye injuries due to jellyfish (Cyanea annaskala). Med. J. Aust. 1962, 49, 303–305. [Google Scholar] [CrossRef]
- Chui, J.J.; Ooi, K.G.; Reeves, D.; Francis, I.C. Bluebottle envenomation-induced crystalline keratopathy. Cornea 2011, 30, 835–837. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Cheng, D.J.; Lian, L.H.; Zhang, R.T.; Yu, X.Y. Severe fundus lesions induced by ocular jellyfish stings: A case report. World J. Clin. Cases 2020, 8, 4544–4549. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Yue, Y.; Liu, S.; Xing, R.; Chen, X.; Li, P. Combined proteomics and transcriptomics identifies sting-related toxins of jellyfish Cyanea nozakii. J. Proteom. 2016, 148, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Abellaneda, C.; Navarra, R.; Martín-Urda, M.T.; Gómez, M. Jellyfish sting or tattoo? Actas Dermo-Sifili. 2012, 103, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Pearn, J. The sea, stingers, and surgeons: The surgeon’s role in prevention, first aid, and management of marine envenomations. J. Pediatr. Surg. 1995, 30, 105–110. [Google Scholar] [CrossRef]
- Law, Y.H. Stopping the sting. Science 2018, 362, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Thaikruea, L.; Siriariyaporn, P. The magnitude of severe box jellyfish cases on Koh Samui and Koh Pha-ngan in the Gulf of Thailand. BMC Res. Notes 2016, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D. Jellyfish sting injuries. Hautarzt 2014, 65, 873–878. [Google Scholar] [CrossRef]
- Haddad, V., Jr.; Cardoso, J.L.; Silveira, F.L. Seabather’s eruption: Report of five cases in southeast region of Brazil. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 171–172. [Google Scholar] [CrossRef]
- Haddad Junior, V.; Silveira, F.L.; Migotto, A.E. Skin lesions in envenoming by cnidarians (Portuguese man-of-war and jellyfish): Etiology and severity of accidents on the Brazilian coast. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 47–50. [Google Scholar] [CrossRef]
- Sil, A.; Panigrahi, A.; Chakraborty, S. Seabather’s Eruption. Am. J. Med. Sci. 2020, 360, 81. [Google Scholar] [CrossRef]
- Ahmad, A. Jelly fish eye stings. J. Egypt. Ophthalmol. Soc. 2016, 109, 172–173. [Google Scholar] [CrossRef]
- Fu, J.; Koo, K.; Sang, A.X.; Shisler, D.C. Jellyfish envenomation in an ocean swimmer. Intern. Emerg. Med. 2014, 9, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, K.; Nichols, M.M.; Schnadig, V.; Ellis, M.D. Sudden death in a child following jellyfish envenomation by Chiropsalmus quadrumanus. Case report and autopsy findings. JAMA 1991, 266, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Wood, Y.K. Identification of Chironex fleckeri envenomation by nematocyst recovery from skin. Med. J. Aust. 1995, 162, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Thaikruea, L.; Santidherakul, S. The public health impact of a new simple practical technique for collection and transfer of toxic jellyfish specimens and for nematocyst identification. J. Public Health Policy 2018, 39, 143–155. [Google Scholar] [CrossRef]
- Hartman, K.R.; Calton, G.J.; Burnett, J.W. Use of the radioallergosorbent test for the study of coelenterate toxin-specific immunoglobulin E. Int. Arch. Allergy Appl. Immunol. 1980, 61, 389–393. [Google Scholar] [CrossRef]
- Fenner, P.J.; Lippmann, J.; Gershwin, L.A. Fatal and nonfatal severe jellyfish stings in Thai waters. J. Travel Med. 2010, 17, 133–138. [Google Scholar] [CrossRef]
- Tønseth, K.A.; Andersen, T.S.; Pripp, A.H.; Karlsen, H.E. Prophylactic treatment of jellyfish stings—A randomised trial. Idsskrift Den Nor. Legeforen. 2012, 132, 1446–1449. [Google Scholar] [CrossRef]
- Kimball, A.B.; Arambula, K.Z.; Stauffer, A.R.; Levy, V.; Davis, V.W.; Liu, M.; Rehmus, W.E.; Lotan, A.; Auerbach, P.S. Efficacy of a jellyfish sting inhibitor in preventing jellyfish stings in normal volunteers. Wilderness Environ. Med. 2004, 15, 102–108. [Google Scholar] [CrossRef]
- Ballesteros, A.; Marambio, M.; Fuentes, V.; Narda, M.; Santín, A.; Gili, J.M. Differing Effects of Vinegar on Pelagia noctiluca (Cnidaria: Scyphozoa) and Carybdea marsupialis (Cnidaria: Cubozoa) Stings-Implications for First Aid Protocols. Toxins 2021, 13, 509. [Google Scholar] [CrossRef]
- Doyle, T.K.; Headlam, J.L.; Wilcox, C.L.; MacLoughlin, E.; Yanagihara, A.A. Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In Vitro Envenomation Models. Toxins 2017, 9, 215. [Google Scholar] [CrossRef]
- Pyo, M.J.; Lee, H.; Bae, S.K.; Heo, Y.; Choudhary, I.; Yoon, W.D.; Kang, C.; Kim, E. Modulation of jellyfish nematocyst discharges and management of human skin stings in Nemopilema nomurai and Carybdea mora. Toxicon 2016, 109, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Loten, C.; Stokes, B.; Worsley, D.; Seymour, J.E.; Jiang, S.; Isbister, G.K. A randomised controlled trial of hot water (45 degrees C) immersion versus ice packs for pain relief in bluebottle stings. Med. J. Aust. 2006, 184, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.A.; Isbister, G.K. ACP Journal Club. Review: Application of heat or hot water reduces pain from jellyfish stings. Ann. Intern. Med. 2012, 157, Jc6-12. [Google Scholar] [CrossRef] [PubMed]
- Staggs, R.; Pay, J.L. Cnidaria Toxicity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wilcox, C.L.; Headlam, J.L.; Doyle, T.K.; Yanagihara, A.A. Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models. Toxins 2017, 9, 149. [Google Scholar] [CrossRef]
- Li, L.; McGee, R.G.; Isbister, G.; Webster, A.C. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst. Rev. 2013, 10, CD009688. [Google Scholar] [CrossRef]
- Li, L.; McGee, R.G.; Webster, A.C. Pain from bluebottle jellyfish stings. J. Paediatr. Child. Health 2015, 51, 734–737. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Wilcox, C.; King, R.; Hurwitz, K.; Castelfranco, A.M. Experimental Assays to Assess the Efficacy of Vinegar and Other Topical First-Aid Approaches on Cubozoan (Alatina alata) Tentacle Firing and Venom Toxicity. Toxins 2016, 8, 19. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Wilcox, C.L. Cubozoan Sting-Site Seawater Rinse, Scraping, and Ice Can Increase Venom Load: Upending Current First Aid Recommendations. Toxins 2017, 9, 105. [Google Scholar] [CrossRef]
- Hartwick, R.; Callanan, V.; Williamson, J. Disarming the box-jellyfish: Nematocyst inhibition in Chironex fleckeri. Med. J. Aust. 1980, 1, 15–20. [Google Scholar] [CrossRef]
- Little, M.; Fitzpatrick, R.; Seymour, J. Successful use of heat as first aid for tropical Australian jellyfish stings. Toxicon 2016, 122, 142–144. [Google Scholar] [CrossRef]
- Isbister, G.K.; Palmer, D.J.; Weir, R.L.; Currie, B.J. Hot water immersion v icepacks for treating the pain of Chironex fleckeri stings: A randomised controlled trial. Med. J. Aust. 2017, 206, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, N.A.; Maalouf, G.J.; Mahmassani, D.M. Jellyfish Stings: A Practical Approach. Wilderness Environ. Med. 2015, 26, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Macrokanis, C.J.; Hall, N.L.; Mein, J.K. Irukandji syndrome in northern Western Australia: An emerging health problem. Med. J. Aust. 2004, 181, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Berling, I.; Isbister, G. Marine envenomations. Aust. Fam. Phys. 2015, 44, 28–32. [Google Scholar]
- Pereira, P.; Corkeron, M.; Little, M.; Farlow, D. Irukandji Taskforce guidelines for the emergency management of Irukandji syndrome. Diving Hyperb. Med. South Pac. Underw. Med. Soc. 2007, 37, 48. [Google Scholar]
- McCullagh, N.; Pereira, P.; Cullen, P.; Mulcahy, R.; Bonin, R.; Little, M.; Gray, S.; Seymour, J. Randomised trial of magnesium in the treatment of Irukandji syndrome. Emerg. Med. Australas 2012, 24, 560–565. [Google Scholar] [CrossRef]
- Guevara, B.E.K.; Dayrit, J.F.; Haddad, V., Jr. Seabather’s eruption caused by the thimble jellyfish (Linuche aquila) in the Philippines. Clin. Exp. Dermatol. 2017, 42, 808–810. [Google Scholar] [CrossRef]
- Sonmez, B.; Beden, U.; Yeter, V.; Erkan, D. Jellyfish sting injury to the cornea. Ophthalmic Surg. Lasers Imaging Retin. 2008, 39, 415–417. [Google Scholar] [CrossRef]
- Yu Yao, H.; Cho, T.H.; Lu, C.H.; Lin, F.C.; Horng, C.T. Hypertonic saline in the treatment of corneal jellyfish stings. J. Travel Med. 2016, 23, tav030. [Google Scholar] [CrossRef]
- Olivieri, L.; Mazzarelli, D.; Cappella, A.; De Angelis, D.; Piscitelli, V.; Cattaneo, C. The importance of “secondary methods” in the identification of the victims of the 3rd of October 2013 shipwreck. La Rev. De Med. Leg. 2017, 8, 190–191. [Google Scholar] [CrossRef]
- Stein, M.R.; Marraccini, J.V.; Rothschild, N.E.; Burnett, J.W. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann. Emerg. Med. 1989, 18, 312–315. [Google Scholar] [CrossRef]
- Purcell, J.E.; Atienza, D.; Fuentes, V.; Olariaga, A.; Tilves, U.; Colahan, C.; Gili, J.-M. Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. Hydrobiologia 2012, 690, 169–180. [Google Scholar] [CrossRef]
- Widmer, C.L.; Fox, C.J.; Brierley, A.S. Effects of temperature and salinity on four species of northeastern Atlantic scyphistomae (Cnidaria: Scyphozoa). Mar. Ecol. Prog. Ser. 2016, 559, 73–88. [Google Scholar] [CrossRef]
- Schiariti, A.; Melica, V.; Kogovšek, T.; Malej, A. Density-dependent effects control the reproductive strategy and population growth of Aurelia aurita s.l. scyphistomae. Mar. Biol. 2015, 162, 1665–1672. [Google Scholar] [CrossRef]
- Sokołowski, A.; Brulińska, D.; Olenycz, M.; Wołowicz, M. Does temperature and salinity limit asexual reproduction of Aurelia aurita polyps (Cnidaria: Scyphozoa) in the Gulf of Gdańsk (southern Baltic Sea)? An experimental study. Hydrobiologia 2016, 773, 49–62. [Google Scholar] [CrossRef]
- Treible, L.; Condon, R. Temperature-driven asexual reproduction and strobilation in three scyphozoan jellyfish polyps. J. Exp. Mar. Biol. Ecol. 2019, 520, 151204. [Google Scholar] [CrossRef]
- Goldstein, J.; Steiner, U.K. Ecological drivers of jellyfish blooms—The complex life history of a ‘well-known’ medusa (Aurelia aurita). J. Anim. Ecol. 2020, 89, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Li, A.; Yu, C.; Li, P. Preparation and Neutralization Efficacy of Novel Jellyfish Antivenoms against Cyanea nozakii Toxins. Toxins 2021, 13, 165. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Li, A.; Yu, C.; Li, P. Refinement and Neutralization Evaluation of the F(ab’)2 Type of Antivenom against the Deadly Jellyfish Nemopilema nomurai Toxins. Int. J. Mol. Sci. 2021, 22, 12672. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).