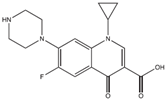
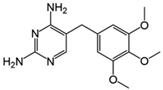
Abstract
The current research focuses on the adsorption/desorption characteristics of the antibiotics ciprofloxacin (CIP) and trimethoprim (TRI) taking place in 17 agricultural soils, which are studied by means of batch-type experiments. The results show that adsorption was higher for CIP, with Freundlich KF values ranging between 1150 and 5086 Ln µmol1−n kg−1, while they were between 29 and 110 Ln µmol1−n kg−1 in the case of TRI. Other parameters, such as the Langmuir maximum adsorption capacity (qm(ads)), as well as the Kd parameter in the linear model and also the adsorption percentages, follow the same trend as KF. Desorption was lower for CIP (with KF(des) values in the range 1089–6234 Ln µmol1−n kg−1) than for TRI (with KF(des) ranging between 26 and 138 Ln µmol1−n kg−1). The higher irreversibility of CIP adsorption was also confirmed by its lower nF(des)/nF(ads) ratios, compared to TRI. Regarding soil characteristics, it was evidenced that nitrogen and carbon contents, as well as mineral fractions, had the highest influence on the adsorption/desorption process. These results can be considered relevant as regards the fate of both antibiotics when they reach the environment as pollutants and therefore could be considered in assessment procedures focused on environmental and public health aspects.
1. Introduction
Soils are complex and heterogeneous environment compartments, with multiple functions that depend on the processes taking place inside them, which influence the fate of compounds such as antibiotics reaching these media as contaminants. In fact, these antibiotics are considered within the group of emerging pollutants. In recent years, these substances have been detected in increasing concentrations in the environment, and it has been facilitated due to their increased use related to comorbidities associated with the COVID-19 pandemic [1]. According to Ezzariai et al. [2] it can be estimated that the amount of antibiotics used annually worldwide is between 100,000 and 200,000 t.
Between 30 and 90% of the amount of antibiotics ingested by humans is excreted in feces and urine, in their original form or as metabolites [1,3]. In wastewater treatment plants, antibiotics are not completely eliminated, with variate percentage removals, such as 13% in the case of TRI and 87% in the case of fluoroquinolones [4,5]. Although purification methods have been improved as regards antibiotics removal, their total elimination is not achieved, causing the study of adsorption methods using different materials such as polymers [6], activated carbon [7] or biochar [8] to still be clearly interesting and very abundant.
These emerging pollutants reach the soil after the use of wastewater for irrigation as well as due to the application of sludge as agricultural soil amendments [2], with both pathways being the main inputs of antibiotics into the environment, along with the application of slurries as soil amendments [9]. In this sense, in different European countries, including Spain, 50% of the sewage sludge produced is used as an agricultural amendment, since it has high organic matter and nutrient contents, including nitrogen and phosphorus [1,4]. Soils devoted to corn and vineyard production are among those most modified with sludge and manure to increase their fertility, since they are two of the ten most important products derived from agriculture in Spain [10].
Once antibiotics reach the soil, they can pose a serious risk to both human and ecological health. Among their associated environmental and public health risks, they can give rise to the appearance of resistant genes in bacteria, with relevant levels found in sediments, soils and waters [11]. Antibiotic resistance genes exist naturally in the chromosomes of bacteria present in different environmental compartments, but currently, due to the pressure exerted by the high presence of antibiotic pollutants, resistant genes are also found in plasmids [3], which can increase their transmission to other organisms, both pathogenic and non-pathogenic. In addition, different studies suggest that the combination of antibiotics with other contaminants, such as heavy metals, taking place in soils can favor the proliferation of antibiotic resistance [3,12]. An additional problem is the bioaccumulation of these compounds, which leads to high concentrations in various plants, favoring their entry into the food chain [13,14]. Antibiotics can also move through the soil and contaminate surface, subsurface and groundwater, causing increasingly high concentrations of these contaminants to be found in water bodies [15]. This depends on the characteristics of the specific antibiotic molecule, as well as on those of the soil and the environmental conditions [16]. Their fate will largely depend on the chemical form of the antibiotics present in the soil, since they are molecules which can behave as neutral and/or charged species (in the form of zwitterion, negatively or positively charged), and will also depend on the multiple processes that take place in the soil, such as degradation, transport (for example by runoff and leaching), plant uptake, as well as adsorption/desorption.
Among the most widely used antibiotics are ciprofloxacin (CIP), which belongs to the family of fluoroquinolones, and trimethoprim (TRI), a diaminopyrimidine, which are characterized by being broad-spectrum biocides. Fluoroquinolones are among the families most present in sewage sludge, along with tetracyclines and sulfonamides [2,17], with concentrations of ciprofloxacin found in soils reaching between 0.57 µg kg−1 and 0.35 mg kg−1 [9]. In the case of TRI, its presence in soils has been reported to be between 0.64 and 2.15 µg kg−1 [4]. The possibility for these antibiotics to reach water bodies, as well as crops, and the food chain, will be clearly affected by adsorption-desorption. A high adsorption and low desorption will favor the retention of these compounds in the soil, this process being conditioned by molecular characteristics of the antibiotics, as well as by soil physicochemical properties (such as pH, mineral concentration, cation exchange capacity, organic matter content and structure [16]).
In view of the above, the main objective of this study is to elucidate the main characteristics of the adsorption-desorption processes affecting CIP and TRI antibiotics which contact agricultural soils with different edaphic properties, since both antibiotics have not been widely studied in soils up to now. Therefore, this study was carried out to determine the probable time-course evolution of these antibiotics and their fate once they reach the environment as pollutants, taking into account that retention/release will be key as regards their mobility and its possible impact on water bodies, the food chain and ultimately on environmental and human health.
2. Materials and Methods
2.1. Soil Samples
In this study, 17 agricultural soils were used: 10 vineyard soils (soils 1–10) and 7 soils dedicated to corn cultivation (soils 11–17), located at different areas of the northwest of Spain, specifically in Galicia. These crops are two of the most widely cultivated in the world, and the soils that were sampled for this study were selected due to the variability in their pH values and organic matter contents. Soil samples were taken with an Edelman probe at a depth of 0–20 cm, air-dried, sieved by 2 mm and stored in polyethylene bottles until analysis. In each of the soils, the final sample resulted from mixing and homogenizing 10 subsamples, randomly taken in each respective area.
The particle size distribution was determined in the <2 mm fractions, by means of the pipette method, carried out after a previous treatment with H2O2 (6%) to eliminate organic matter, and with 0.1 M HCl to eliminate Fe and Al oxides [18]. As a result, 3 different fractions were quantified: sand (2–0.05 mm), silt (0.05–0.002 mm) and clay (<0.002 mm). Soil pH was measured in water and in 0.1 M KCl (using 1:2.5 as soil:solution ratio). Total organic carbon and total organic nitrogen were determined by means of an elemental analyzer (1112 Series NC, Thermo Finnigan, Amsterdam, The Netherlands).
The effective cation exchange capacity (eCEC) was estimated as the sum of the basic exchangeable cations (Nae, Ke, Cae and Mge), which were extracted with 0.2 M NH4Cl [19], and the exchangeable Al (Ale), which was extracted with 1 M KCl [20]. Ca, Mg and Al were quantified via flame atomic absorption spectrophotometry, while Na and K were determined via atomic emission spectrophotometry (with an AAnalyst 200 spectrophotometer, Perkin Elmer, Boston, MA, USA).
2.2. Chemicals and Reagents
The antibiotics ciprofloxacin (CIP) and trimethoprim (TRI) were supplied by Sigma-Aldrich (Barcelona, Spain), both with a purity of 98%. Their main characteristics are shown in Table 1. All the reagents used were of high purity analytical grade, supplied by Panreac (Barcelona, Spain) and by Fisher Scientific (Madrid, Spain) in the case of acetonitrile.

Table 1.
Main characteristics of the antibiotics studied. KOW: coefficient of partition octanol/water.
2.3. Adsorption/Desorption Experiments
To carry out adsorption experiments, aliquots of 0.5 g of soil were weighed in 50 mL centrifuge tubes (Deltalab, Spain) and suspended in 40 mL of solution of each of the antibiotics (CIP and TRI), at 7 different concentrations (between 2.5–50 µM for TRI and between 5–400 µM for CIP), all of these containing 0.005 M CaCl2 as the ionic background electrolyte.
The suspensions were shaken for 48 h in the dark, at 50 rpm, on a rotary shaker and at room temperature (25 ± 1 °C). Adsorption kinetic studies were previously carried out, indicating that 48 h is enough time to reach equilibrium. Once shaken, the samples were centrifuged for 15 min at 4000 rpm using a Rotina 35R centrifuge (Hettich Zentrifugen, Tuttlingen, Germany). Then, these samples were filtered using nylon syringe filters (0.45 µm pore size). Antibiotic concentrations were quantified via HPLC using 2 mL capacity Eppendorf propylene vials (Fisherbrand, Madrid, Spain). The pH of the samples was also measured using a combined glass micro-electrode (Crison, Barcelona, Spain). The amount of antibiotic adsorbed in the soils was calculated as the difference between the amount initially added and that remaining in the solution once equilibrium is reached (48 h).
To study desorption, the soil samples previously used in the adsorption experiments were weighed to determine the amount of solution left in the soil, then the soil was re-suspended in 40 mL of 0.005 M CaCl2. These samples were then shaken, centrifuged, filtered and analyzed in the same way as in the adsorption phase.
All determinations were made in triplicate.
2.4. Quantification of the Antibiotics CIP and TRI
The quantification of the antibiotics was performed following the methodology previously detailed in Rodríguez-López et al. [24]. Briefly, the analysis was carried out in Ultimate 3000 HPLC equipment (Thermo Fisher Scientific, Madrid, Spain), with a quaternary pump, an auto-sampler, a thermostatted column compartment and an Ultimate 3000 series UV detector. This equipment was connected to a computer with version 7 of the Chromeleon software (Thermo Fisher Scientific, Madrid, Spain). Chromatographic separations were carried out with a C18 analytical column (150 mm long; 4.6 mm internal diameter; 5 μm particle size) from Phenomenex (Madrid, Spain) and a security column (4 mm long; 3 mm internal diameter; 5 μm particle size), packed with the same material as the column.
The quantification limits were 0.1 µM for CIP and 0.05 µM for TRI while the detection limits were 0.03 µM and 0.015 µM, respectively. The injection volume was 50 µL and the flow rate was 1.5 mL min−1. Phase A was acetonitrile and phase B was 0.01 M phosphoric acid (pH = 2). The dilution gradient was from 5 to 32% for phase A and from 95 to 68% for phase B, in a time of 10.5 min. The initial conditions were resumed after 2 min, with a total analysis time of 15 min and retention times of 6.5 min for CIP and 5.6 min for TRI. The wavelength used was 212 nm for both antibiotics.
2.5. Statistical Analysis and Data Treatment
The data obtained in the adsorption and desorption experiments were described using the Freundlich, Langmuir and linear models, which correspond to the following equations, respectively:
where qa (µmol kg−1) is the amount of antibiotic adsorbed at equilibrium, Ceq (µmol L−1) is the concentration of antibiotic that remains in the equilibrium solution, KF (Ln µmol1−n kg−1) is the Freundlich affinity coefficient; n (dimensionless) is the Freundlich linearity index, KL (L µmol−1) is the Langmuir constant related to the adsorption energy, qm (µmol kg−1) is the maximum adsorption capacity of the soil and Kd (L kg−1) is the distribution coefficient in the linear model.
The adjustment of the different models to the experimental data was carried out using IBM SPSS Statistics 21.0 software (New York, NY, USA). In addition, bivariate Pearson correlations and multiple linear regressions were performed between the adsorption and desorption parameters and the different soil properties, using the same software.
3. Results
3.1. Soil Characteristics
Table 2 shows the physicochemical characteristics of the soils studied. These soils have a pH in water in the range 5.0 to 8.0 and a pH in KCl from 4.2 to 7.8. The lower values of pH in KCl with respect to those in water are indicative of a predominance of negative charge in these soils. Regarding eCEC values, they show high variability, ranging between 5.43 and 42.81 cmol(c)kg−1, with Ca being the predominant element in most cases. Soil organic carbon (SOC) also shows high variability, with values between 1.0 and 7.7%, while total soil nitrogen (TSN) has values between 0.11 and 0.63%. Sand is the predominant textural fraction in all soils, with percentages between 43 and 69%, followed by silt (between 10 and 34%) and clay (between 12 and 25%), with which the textures are sandy-loam (soils 3, 4, 9, 15 and 16), loam (soils 1, 2, 5, 11, 12 and 13) and sandy-clay-loam (soils 6, 7, 8, 10, 14 and 17).

Table 2.
Main physicochemical properties of the soils studied. pHw: pH in water; pHKCl: pH in 0.1 M KCl; Cae: exchangeable calcium; Mge: exchangeable magnesium; Nae: exchangeable sodium; Ke: exchangeable potassium; Ale: exchangeable aluminum; eCEC: effective cation exchange capacity; TSN: total soil nitrogen; SOC: soil organic carbon.
3.2. CIP and TRI Adsorption
Figure 1 and Figure 2 show the adsorption curves for the antibiotics CIP and TRI, respectively, plotting the amount adsorbed in the soil (qa, in µmol kg−1) against the concentration present at equilibrium (Ceq, in µmol L−1), for the 17 soils studied.
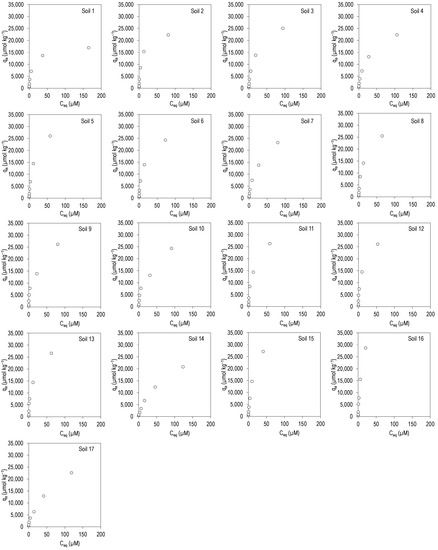
Figure 1.
Adsorption curves for CIP in the 17 soils studied. qa: CIP adsorbed onto the soil; Ceq: CIP concentration in the equilibrium solution. Average values (n = 3), with coefficients of variation always lower than 5%.
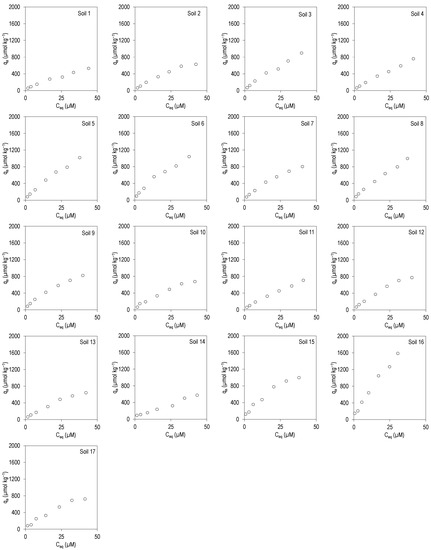
Figure 2.
Adsorption curves for TRI in the 17 soils studied. qa: TRI adsorbed onto the soil; Ceq: TRI concentration in the equilibrium solution. Average values (n = 3), with coefficients of variation always lower than 5%.
In the case of CIP (Figure 1), the curves are L-type (as per Giles at al. [25]), indicating that the antibiotic has a high affinity for the adsorption sites. These are non-linear and concave curves, which indicates that at low concentrations of antibiotic there is a high affinity for the soil, with almost all the amount of pollutant being adsorbed. In fact, the adsorption percentages are greater than 90% for concentrations added ranging from 5 to 100 µM, while adsorption percentages ranged between 56 and 97% for added concentrations going from 200 to 400 µM (Table S1, Supplementary Material). Median values ranged from 97% to 79%, being slightly lower for added concentrations of 220–400 µM (Table S1, Supplementary Material). These high adsorption percentages are a confirmation of the affinity of this antibiotic for the soils studied.
Table 3 shows that, judging by the R2 values, the fits were satisfactory for the three adsorption models assessed (Freundlich, with R2 values between 0.937 and 0.998, Langmuir, with R2 values between 0.946 and 0.998, and linear, with R2 values between 0.840 and 0.976). Among these R2 values, those corresponding to the Langmuir model are slightly higher, indicating that it is the model that best fits the experimental data. KF values ranged between 1150 and 5086 Ln µmol1−n kg−1, with a mean value of 3334 Ln µmol1−n kg−1. The values of the n parameter were lower than 1, indicating a certain concavity of the adsorption curves. Maximum adsorption calculated using the Langmuir equation (qm) ranged between 17,264 and 40,722 µmol kg−1, with a mean value of 30,236 µmol kg−1, and KL ranged between 0.01 and 0.14 L µmol−1. Kd values were lower than those of KF, ranging between 90 and 1322 L kg−1, with a mean score of 364 L kg−1 (Table 3).

Table 3.
Minimum, maximum, mean and median of the parameters resulting from the fits of the experimental adsorption data to the Freundlich, Langmuir and linear models, for ciprofloxacin (CIP) and trimethoprim (TRI). KF (Ln µmol1−n kg−1) is the Freundlich affinity coefficient; n (dimensionless) is the Freundlich linearity index; KL (L µmol−1) is the Langmuir constant related to adsorption energy; qm (µmol kg−1) is the maximum soil adsorption capacity; Kd (L kg−1) is the distribution constant in the linear model; R2: coefficient of determination.
Figure 2 shows that, in the case of TRI, adsorption curves have a higher tendency to linearity, although they can also be considered type L, but with a much lower slope compared to those of CIP. In fact, the fits are satisfactory for the three models, judging by the values of R2, and for CIP, the Langmuir model is the one that best describes the experimental data, since its R2 values are the highest.
Adsorption percentages in this case were much lower, with mean values of 22–40% and with a median of 20–38% for the different initial concentrations added (Table S2, Supplementary Material). KF values ranged between 29 and 125 Ln µmol1−n kg−1, while the values of the n parameter were in this case closer to 1, ranging between 0.62 and 0.84. Regarding Kd, its values were very similar to those of KF, ranging between 10 and 48 L kg−1. The values of n and Kd confirm the greater linearity in the adsorption curves corresponding to TRI, compared to those of CIP. The qm values ranged between 1297 and 4460 µmol kg−1, with a mean of 2209 µmol kg−1, and those of KL ranged between 0.007 and 0.036 L µmol−1, being lower than those obtained for CIP (Table 3).
3.3. Desorption of CIP and TRI
Figures S1 and S2 (Supplementary Material) show desorption curves for CIP and TRI, respectively, corresponding to the 17 soils under study. These figures plot the amount of CIP (Figure S1) and TRI (Figure S2) that remained adsorbed to the soil after a desorption cycle (qm(des), µmol kg−1) against the concentration of CIP or TRI in the equilibrium solution (Ceq(des), µmol L−1).
For CIP, the slopes of the desorption curves were greater than those of adsorption, indicating that an important part of CIP remains adsorbed to the soil after a desorption cycle. In addition, it was noted that as the amount of CIP initially added was increased, desorption was higher (Table 4). The desorption percentages were low, in the range of 0.0–22.4%, with a mean value of 3.0–9.2% for all concentrations used (5–400 µM) and a median of 2.9–6.9% (Table 4).

Table 4.
Ciprofloxacin (CIP) desorbed in the equilibrium, expressed in µmol kg−1 (and in percentage into brackets) for each of the initial concentrations added (C0) and for each of the 17 soils studied. Mean, median, maximum and minimum refer to desorption percentages. nd: not determined.
Table 5 shows values of the desorption parameters related to the fits of the experimental data to the Freundlich, Langmuir and linear equations. The R2 values were above 0.921 for all the models, with the Freundlich model being the one that best fits the antibiotic CIP due to its higher values, while for TRI the best fit corresponded to the linear model. KF(des) ranged between 1089 and 6234 Ln µmol1−n kg−1, with a mean value of 3688 Ln µmol1−n kg−1, which are similar to those obtained for adsorption (Table 3). The n(des) values were higher than those of adsorption, in the range of 0.42–0.89. The qm(des) parameter ranged between 18,154 and 99,941 µmol kg−1, with a mean of 40,902 µmol kg−1, while KL ranged between 0.01 and 0.21 L µmol−1. Both parameters are indicative of low desorption taking place, since their values are similar to those of qm and KL obtained for adsorption. Kd values for desorption are higher than those of adsorption, ranging between 270 and 2337 L kg−1, with a mean of 946 L kg−1 (Table 5).

Table 5.
Minimum, maximum, mean and median of the parameters resulting from the fits of the experimental desorption data to the Freundlich, Langmuir and linear models, for ciprofloxacin (CIP) and trimethoprim (TRI). KF(des) (Ln µmol1−n kg−1) is the Freundlich affinity coefficient; n(des) (dimensionless) is the Freundlich linearity index; KL(des) (L µmol−1) is the Langmuir constant related to adsorption energy; qm(des) (µmol kg−1) is the maximum soil adsorption capacity; Kd(des) (L kg−1) is the distribution constant in the linear model; R2: coefficient of determination.
For TRI, KF(des) ranged between 26 and 138 Ln µmol1−n kg−1, while n(des) values were in the range 0.60–1.28 (Table 5), scores similar to those obtained for adsorption. In addition, Kd values were also very similar to those of KF, ranging between 29 and 109 L kg−1. The values of qm(des) ranged between 353 and 1558 µmol kg−1, with a mean of 903 µmol kg−1, which are lower than those obtained for adsorption. KL ranged between 0.047 and 0.315 L µmol−1, which are higher than those obtained for adsorption. Both parameters indicate that the reversibility of adsorption is higher for TRI than for CIP.
Table 6 shows that, in the case of TRI, the slopes of the desorption curves were similar to those of adsorption, which indicates a higher reversibility in the adsorption process. This is confirmed by the fact that desorption percentages were higher for TRI than for CIP. In the case of TRI they were in the range 28.8–74.9%, with a mean value between 49.0 and 59.7% and a median similar to the mean (varying between 51.0 and 60.4%).

Table 6.
Trimethoprim (TRI) desorbed in the equilibrium, expressed in µmol kg−1 (and in percentage into brackets) for each of the initial concentrations added (C0) and for each of the 17 soils studied. Mean, median, maximum and minimum refer to desorption percentages.
4. Discussion
This discussion will be carried out focusing on two fundamental aspects: on the one hand, the comparison of the adsorption/desorption results for CIP and TRI and, on the other hand, the relations between the adsorption/desorption parameters and the edaphic variables, studied by means of Pearson’s correlation and multiple regression analyses.
The values of the adsorption parameters indicate that CIP is more adsorbed than TRI, judging by its higher Kd, KF and qm scores (Table 3). In addition, adsorption percentages are higher for CIP (Tables S1 and S2, Supplementary Material).
Different studies have shown a high variety of values for Kd for CIP, some of them being higher than those of the present work, as is the case of Leal et al. [26], who obtained Kd values in the range of 727–1277,874 L kg−1 for a set of 13 soils with different physical-chemical properties, or those provided by Conkle et al. [27], reaching 4844 L kg−1, as referenced in a review paper by Riaz et al. [9]. Values of the same order as those obtained in the present work (between 410–11,290 L kg−1) have also been reported by Uslu et al. [28], corresponding to three soils studied in Germany, or values of 430 L kg−1 also in soils from Germany [29,30], and between 300–45,000 L kg−1 reported by Vasudevan et al. [31], derived from a study of 30 soils in the USA.
KF data are also reported in the literature. Specifically, Rath et al. [17] found values of 230–1366 mLn µg1−n g−1, which (although expressed in different units) are of a similar order to those obtained in the present work. These authors also observed that the adsorption of CIP is mainly due to the electrostatic interaction between the protonated part of the antibiotic and the negative charges of the soil. Other authors, such as Movasaghi et al. [32], who studied CIP adsorption in oat hulls, reported the influence of pH, and specifically that at low pH the positive charges of the antibiotic and the positive charges of the adsorbent surface give rise to a certain electrostatic repulsion, and therefore, adsorption is lower, while at a higher pH electrostatic attraction and greater adsorption take place. These authors also studied how mechanisms such as hydrogen bonding or electron donor acceptor (EDA) interactions can take place, since, for example, the organic compounds of the soil which have aromatic rings will present interactions as donor, with the benzene ring of CIP behaving as an acceptor. In the case of hydrogen bonds, it can also be one of the CIP adsorption mechanisms, since functional groups such as hydroxyl or carboxyl found on the surface of soil organic matter favor these bonds with carbonyl groups or/and hydroxyl groups of CIP [32,33]. In the case of soils with a pH around neutrality, the CIP molecule has a certain hydrophobicity, which leads to a low solubility of the antibiotic and therefore the adsorption process is facilitated, but these interactions may not explain the high adsorption that has been reported, since fluoroquinolones in general have low log KOW values [28,32]. In the study by Movasaghi et al. [32], KF values of 19,000–32,000 mLn µg1−n g−1 are referenced for oat hulls, while Sidhu et al. [34] reported Kd values of 357 L kg−1 for other adsorbents, such as bio-solids.
In addition, the fact of obtaining lower adsorption values for TRI than for CIP coincides with what is reported in the literature. Specifically, Kd(ads) values for TRI were in the range of 9–311 L kg−1 in different soils in Australia [35], while in Chinese soils they were in the range of 9.28–10.24 L kg−1 [36] and 5.88–21.8 L kg−1 [37]. Other researchers also found KF(ads) values confirming the low levels of TRI adsorption [23,38,39]. However, some bio-adsorbents, such as activated carbon [7], biochard [8] and bentonite [40], show high TRI adsorption. The low adsorption of TRI in soils can be associated with the amphoteric nature of the molecule. Specifically, at pH 4–5 (which is the pH of the soils that are the object of this study), authors such as Peng et al. [37] observed that the TRI molecule behaves as anionic, which gives rise to an electrostatic repulsion with the negative charges of the soil, which makes adsorption relatively low, as confirmed by other studies, such as the one carried out by Kodesová et al. [23]. Other authors such as Berges et al. [7] concluded that TRI adsorption is due to π-π interactions between the two aromatic rings of the molecule and the surface of the adsorbate.
Comparing with other families of antibiotics, it has been reported that CIP (a fluoroquinolone) has high adsorption values, although lower than those of groups such as tetracyclines, showing KF scores of up to 11,000 Ln µmol1−n kg−1 (chlortetracycline), which have a high affinity for soils [41]. Furthermore, TRI, which has low adsorption compared to CIP, presents adsorption values similar to groups such as sulfonamides, with KF lower than 22 Ln µmol1−n kg−1 for sulfachloropyridazine [42], or to the group of beta-lactams, with antibiotics such as amoxicillin showing KF below 150 Ln µmol1−n kg−1 [43]. This indicates that the family of fluoroquinolones, together with that of tetracyclines, would be among those with the highest adsorption in soils, and the family of diaminopyrimidines, beta-lactams and sulfonamides, among those with low affinity for soils.
Regarding desorption, CIP desorption percentages were lower than those of TRI, and the desorption parameters are higher for CIP, also indicative of low desorption. These kind of results had been reported in previous studies, as in the case of Conkle et al. [27], with Kd(des) values between 2788 and 5431 cm3 g−1 for CIP, being slightly higher than those obtained in the present study, which may be due to the different edaphic characteristics of the soils used. In the case of KF(des) values, Rath et al. [17] reported values in the range 537–3293 µg1−1/n (cm3)1/n g−1 for CIP, similar to the mean obtained in the current study.
As for TRI, its desorption percentages are higher, and its desorption parameter values are lower, compared to those of CIP. Regarding the Freundlich affinity coefficient, KF(des), an average of 56.8 L kg−1 was obtained, while Peng et al. [37] reported values in the range 7.0–36.0 L kg−1 for CIP in three Chinese soils with different edaphic characteristics. Franklin et al. [39] reported values of 300–1700 µg1−1/n Ln kg−1, which, despite being in different units, indicate a medium desorption. Regarding Kd(des), Zhang et al. [36] reported values between 12.5 and 15.0 L kg−1, confirming the low scores for that parameter, which correspond to a high desorption. It should be noted that, in general, desorption studies are fewer than those dealing with adsorption, for both antibiotics.
When studying the reversibility of adsorption through the n Freundlich parameter, using the expression n(des)/n(ads), the values are much lower for CIP than for TRI, which indicates a greater irreversibility of adsorption in the case of CIP.
To evaluate to what extent the adsorption/desorption results of both antibiotics are related to the different physical-chemical characteristics of the soils, a statistical study was carried out, and as a result, Table 7 shows the correlation matrix relating edaphic variables with the adsorption parameters, while Table 8 shows the correlation matrix relating edaphic variables with desorption parameters.

Table 7.
Relations among adsorption parameters for ciprofloxacin (CIP) and trimethoprim (TRI) and edaphic variables (n = 17). KF(ads) (Ln µmol1−n kg−1) is the Freundlich affinity coefficient; n(ads) (dimensionless) is the Freundlich linearity index; KL(ads) (L µmol−1): Langmuir parameter related to adsorption energy; qm(ads) (µmol kg−1): maximum adsorption capacity; Kd(ads) (L kg−1): distribution constant; pHw: pH in water; pHKCl: pH in 0.1 M KCl; Cae: exchangeable calcium; Mge: exchangeable magnesium; Nae: exchangeable sodium; Ke: exchangeable potassium; Ale: exchangeable aluminum; eCEC: effective cation exchange capacity; TSN: total soil nitrogen; SOC: soil organic carbon.

Table 8.
Relations among desorption parameters for ciprofloxacin (CIP) and trimethoprim (TRI) and edaphic variables (n = 17). KF(des) (Ln µmol1−n kg−1) is the Freundlich affinity coefficient; n(des) (dimensionless) is the Freundlich linearity index; KL(des) (L µmol−1): Langmuir parameter related to adsorption energy; qm(des) (µmol kg−1): maximum adsorption capacity; Kd(des) (L kg−1): distribution constant; pHw: pH in water; pHKCl: pH in 0.1 M KCl; Cae: exchangeable calcium; Mge: exchangeable magnesium; Nae: exchangeable sodium; Ke: exchangeable potassium; Ale: exchangeable aluminum; eCEC: effective cation exchange capacity; TSN: total soil nitrogen; SOC: soil organic carbon.
Regarding the Freundlich parameters, KF(ads) of CIP did not correlate significantly with any edaphic variable analyzed (Table 7), but n(ads) was significantly and positively correlated with variables of the change complex, such as Cae (r = 0.640, p < 0.01), Mge (r = 0.540, p < 0.05) and eCEC (r = 0.484, p < 0.01), and also with variables related to soil organic matter, such as SOC (r = 0.738, p < 0.01) and TSN (r = 0.643, p < 0.01). Authors such as Rath et al. [17] and Vasudevan et al. [31] have obtained similar results regarding the influence of the cation exchange capacity on CIP adsorption, that is, a higher cation exchange capacity favors the adsorption of this compound in the soil. The KL(ads) Langmuir parameter was positively correlated with the silt fraction (r = 0.632, p < 0.01) and negatively correlated with the sand fraction (r = −0.593, p < 0.05), while the adsorption maximum qm(ads) was positively correlated with Mge (r = 0.519, p < 0.05), SOC (r = 0.729, p < 0.01) and TSN (r = 0.736, p < 0.01). Finally, Kd(ads) correlated positively with SOC (r = 0.605, p < 0.05) and with TSN (r = 0.708, p < 0.01). The correlations between the soil organic matter (SOC) and different adsorption parameters indicate that the adsorption onto this fraction is very important, as shown by authors such us Teixidó et al. [44] and Uslu and Yediler [28], who related this adsorption to the mechanisms of cation bridging, electrostatic interactions or hydrogen bonding.
From the results of the multiple regression analysis between CIP adsorption parameters and edaphic variables, the following considerations can be made:
(a) No equation with significant fit was found relating KF(ads) and the soil variables analyzed.
(b) For Langmuir’s KL(ads) and qm(ads), the following significant equations were obtained:
KL(ads) = −0.005 ± 0.026 + Silt * 0.003 ± 0.001
R2 corrected: 0.360
F: 9.990, p = 0.006
qm(ads) = 22,076 ± 2125 + TSN * 26937 ± 6393
R2 corrected: 0.512
F: 17.7540, p = 0.0008
(c) For Kd(ads), the following significant equation was obtained via linear regression fits:
Kd(ads) = −57.3 ± 119.0 + TSN * 1390.5 ± 357.9
R2 corrected: 0.468
F: 15.095, p = 0.001
From these equations it can be stated that TSN is the edaphic variable most involved in CIP adsorption, explaining 51% of the variation of qm(ads) and 47% of Kd(ads). On the other hand, the silt fraction explains 36% of the variation of KL(ads).
Regarding TRI, KF(ads) was significantly and positively correlated with SOC (r = 0.641, p < 0.01) and with TSN (r = 0.745, p < 0.01), which is different from CIP. In addition, n(ads) was correlated with Ke (r = 0.557, p < 0.05), while Langmuir’s KL(ads) was positively correlated with Cae (r = 0.558, p < 0.05) and negatively correlated with Ke (r = −0.552, p < 0.05), and the adsorption maximum qm(ads) was positively correlated with Mge (r = 0.588, p < 0.05), Ke (r = 0.518, p < 0.05) and eCEC (r = 0.677, p < 0.01). Kd(ads) was only significantly and positively correlated with TSN (r = 0.579, p < 0.05). Peng et al. [37] reported that high organic matter contents and a high exchange capacity are positively related to a greater adsorption of TRI, as we found in the current work.
Using multiple regression analysis, the following significant equations were obtained for TRI:
KF(ads) = 16.3 ± 10.4 + TSN * 135.0 ± 31.3
R2 corrected: 0.525
F: 18.6, p < 0.0006
KL(ads) = 0.0151 ± 0.0029 + Cae * 0.0007 ± 0.0003 + Ke * −0.0008 ± 0.0003
R2 corrected: 0.462
F: 7.4, p = 0.0070
qm(ads) = 1243.0 ± 332.3 + eCEC * 70.0 ± 20.3
R2 corrected: 0.420
F: 11.9, p = 0.004
Kd(ads) = 8.98 ± 4.36 + TSN * 36.04 ± 13.10
R2 corrected: 0.291
F: 7.6, p = 0.015
Also for TRI, the edaphic variable TSN has relevance, explaining 52% of the variation of KF(ads) and 29% of Kd(ads). Furthermore, in this case edaphic variables related to ion exchange are important, with Cae and Ke explaining 46% of the variation of KL(ads), while eCEC explains 42% of qm(ads).
CIP desorption parameters were only significantly correlated in the following cases (Table 8): KL(des) correlated negatively with the sand fraction (r = −0.501, p < 0.05) and positively with the silt fraction (r = 0.553, p < 0.05); qm(des) correlated positively with Ale (r = 0.589, p < 0.05) and negatively with the silt fraction (r = −0.494, p < 0.05); finally, Kd(des) was positively correlated with SOC (r = 0.499, p < 0.05) and TSN (r = 0.621, p < 0.01).
For CIP desorption, the following significant equations were obtained:
KL(des) = −0.0051 ± 0.0451 + Silt * 0.0047 ± 0.0018
R2 corrected: 0.260
F: 6.6, p = 0.021
qm(des) = 30,710 ± 6088 + Ale * 46893 ± 16592
R2 corrected: 0.304
F: 8.0, p = 0.013
Kd(des) = 305 ± 229 + TSN * 2114 ± 689
R2 corrected: 0.345
F: 9.4, p = 0.008
As in the case of adsorption, CIP desorption is fundamentally affected by TSN, which explains 34% of Kd(des), while the silt fraction explains 26% of the variation in KL(des), and Ale explains 30% of the variation of qm(des).
In the case of TRI desorption, a significant and positive correlation of KF(des) with the sand fraction was found (r = 0.508, p < 0.05) and a negative correlation with the silt fraction (r = −0.502, p < 0.05). The parameter n(des) was positively correlated with pH in water (r = 0.482, p < 0.05) and with pH in KCl (r = 0.611, p < 0.01), as well as with exchange parameters such as Cae (r = 0.712, p < 0.01), Mge (r = 0.640, p < 0.01) and eCEC (r = 0.595, p < 0.05). Langmuir’s KL(des) was negatively correlated with Mge (r = −0.664, p < 0.01), while qm(des) was positively correlated with Cae (r = 0.625, p < 0.05), Mge (r = 0.852, p < 0.01) and eCEC (r = 0.845, p < 0.01), as well as with SOC (r = 0.614, p < 0.05) and TSN (r = 0.660, p < 0.05). Kd(des) was positively correlated with SOC (r = 0.751, p < 0.05) and TSN (r = 0.725, p < 0.05), and also with the sand fraction (r = 0.517, p < 0.05), and negatively correlated with the clay fraction (r = −0.554, p < 0.05).
Through multiple regression analysis, using the TRI desorption parameters as dependent variables, the following significant equations were obtained:
KF(des) = −7.50 ± 38.15 + Sand * 1.52 ± 0.67
R2 corrected: 0.208
F: 5.2, p = 0.037
KL(des) = 0.221 ± 0.038 + Mge * − 0.121 ± 0.041
R2 corrected: 0.390
F: 8.7, p = 0.013
qm(des) = 265.1 ± 132.1 + Mge * 770.7 ± 142.6
R2 corrected: 0.702
F: 29.2, p = 0.0002
Kd(des) = 22.13 ± 7.93 + SOC * 8.48 ± 1.93
R2 corrected: 0.534
F: 19.3501, p = 0.0005
As was the case for adsorption, the most relevant variables related to TRI desorption are some grain size fractions (specifically the sand fraction, which explains 21% of KF(des) variation), and variables related to the exchange complex (such as Mge, which explains 39% of the variation of KL(des) and 70% of the variation of qm(des)), and, finally, SOC, which explains 53% of the variation of Kd(des).
5. Conclusions
The antibiotics included in this study show a clearly differentiated adsorption/desorption behavior in the soils under examination. Specifically, on the one hand, CIP shows more affinity (56–100%) for the 17 agricultural soils used than TRI, which shows lower adsorption (13.3–74.3%). In the case of the data obtained for desorption, the behavior of both antibiotics is also very different, the values of TRI are clearly higher (28.8–74.9%) than the values of desorption of CIP (0.0–22.4%). The results of both antibiotics fit satisfactorily to the three models used, Freundlich, Langmuir and linear, with values of the different constants being similar to those obtained in previous studies. Overall, the adsorption/desorption process presents high irreversibility for CIP, contrary to what happens for TRI. The edaphic properties that most condition the adsorption processes are carbon and nitrogen contents, in the case of CIP, while they are nitrogen, potassium and cation exchange capacity when focusing on TRI. Regarding desorption, in the case of TRI it depends largely on nitrogen content, cation exchange capacity and soil texture, while for CIP it is largely dependent on silt, magnesium and nitrogen contents. These results are relevant, since determining the variables that influence the adsorption/desorption of both antibiotics, as well as the reversibility of the adsorption process, facilitates taking appropriate decisions to face contamination due to these antibiotics affecting agricultural soils. In fact, it would be key to know what parameters can favor the adsorption of these pollutants and which alternatives could be appropriate to achieve their retention/removal, preventing transfer to other environmental compartments, such as water bodies, as well as their entry into the food chain. With this in mind, the set of results provided by this research can be considered relevant in terms of risk assessment, regarding environmental and public health aspects linked to pollution of agricultural areas that receive the application of solid materials (such as organic fertilizers) or liquids (such as sewage) containing these antibiotics. For future studies, it would be interesting to include additional soils, with characteristics clearly different from those evaluated in this research, as well as to evaluate the possible mitigating or remedial effect of adding low-cost bio-adsorbents (and especially by-products) to agricultural soils susceptible to this type of contamination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19148426/s1, Table S1: Ciprofloxacin (CIP) adsorbed in the equilibrium, expressed in µmol kg−1 (and in percentage, into brackets) for each of the initial concentrations added (C0) and for each of the 17 soils studied. Mean, median, maximum and minimum values refer to adsorption percentages; Table S2: Trimethoprim (TRI) adsorbed in the equilibrium, expressed in µmol kg−1 (and in percentage, into brackets) for each of the initial concentrations added (C0) and for each of the 17 soils studied. Mean, median, maximum and minimum values refer to adsorption percentages; Figure S1: Desorption curves for Ciprofloxacin (CIP) in the 17 soils studied. qa: CIP adsorbed onto the soil after a desorption cycle; Ceq: CIP concentration in the equilibrium solution; Figure S2: Desorption curves for trimethoprim (TRI) in the 17 soils studied. qa: TRI adsorbed onto the soil after a desorption cycle; Ceq: CIP concentration in the equilibrium solution.
Author Contributions
Conceptualization, M.A.-E., E.Á.-R., P.P.-R., A.N.-D. and V.S.-M.; methodology, M.A.-E., E.Á.-R., P.P.-R., A.N.-D. and V.S.-M.; software, L.R.-L., R.C.-D. and V.S.-M.; validation, M.A.-E., E.Á.-R., P.P.-R., A.N.-D. and V.S.-M.; formal analysis, L.R.-L., R.C.-D. and V.S.-M.; investigation, L.R.-L., R.C.-D. and V.S.-M.; data curation, M.A.-E., E.Á.-R., P.P.-R., A.N.-D. and V.S.-M.; writing—original draft preparation, L.R.-L., M.A.-E., P.P.-R. and V.S.-M.; writing—review and editing, A.N.-D.; visualization, M.A.-E., E.Á.-R., A.N.-D. and V.S.-M.; supervision, M.A.-E., E.Á.-R., P.P.-R., A.N.-D. and V.S.-M.; project administration, E.Á.-R.; funding acquisition, E.Á.-R. and M.A.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness [grant numbers RTI2018-099574-B-C21 and RTI2018-099574-B-C22], with European Regional Development Funds (FEDER in Spain). L. Rodríguez-López holds a pre-doctoral FPU contract (FPU19/03758) (Ministry of Universities). P. Pérez-Rodríguez is supported by a postdoctoral fellowship (Galician Regional Government) (ED481D-2021/016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aydın, S.; Ulvi, A.; Bedük, F.; Aydın, M.E. Pharmaceutical residues in digested sewage sludge: Occurrence, seasonal variation and risk assessment for soil. Sci. Total Environ. 2022, 817, 152864. [Google Scholar] [CrossRef] [PubMed]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Fels, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: A review on their distribution and environmental risk assessment. Trends Environ. Anal. Chem. 2021, 30, e00125. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [Green Version]
- Palacio, D.A.; Urbano, B.F.; Rivas, B.L. Water-soluble polymers with the ability to remove amoxicillin as emerging pollutant from water. Environ. Technol. Innov. 2021, 23, 101589. [Google Scholar] [CrossRef]
- Berges, J.; Moles, S.; Ormad, M.P.; Mosteo, R.; Gómez, J. Antibiotics removal from aquatic environments: Adsorption of enrofloxacin, trimethoprim, sulfadiazine, and amoxicillin on vegetal powdered activated carbon. Environ. Sci. Pollut. Res. 2020, 28, 8442–8452. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Adsorption and removal of seven antibiotic compounds present in water with the use of biochar derived from the pyrolysis of organic waste feed-stocks. J. Environ. Chem. Eng. 2021, 9, 105868. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Siddique, M.B.A.; Kamal, A.; Coyne, M. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 104–720. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/faostat/en/#rankings/commodities_by_country (accessed on 16 June 2022).
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, X.; Zhao, Y.; Liu, J.; Zhou, J.; Sun, B.; Liang, Y. Antibiotic resistance genes in manure-amended paddy soils across eastern China: Occurrence and influencing factors. Front. Environ. Sci. Eng. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Piña, B. Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis. Environ. Res. 2019, 177, 108608. [Google Scholar] [CrossRef] [PubMed]
- Prosser, R.S.; Sibley, P.K. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ. Int. 2015, 75, 223–233. [Google Scholar] [CrossRef]
- Yu, C.; Pang, H.; Wang, J.-H.; Chi, Z.-Y.; Zhang, Q.; Kong, F.-T.; Xu, Y.-P.; Li, S.-Y.; Che, J. Occurrence of antibiotics in waters, removal by microalgae-based systems, and their toxicological effects: A review. Sci. Total Environ. 2021, 813, 151891. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, S.; Fostier, A.H.; Pereira, L.A.; Dioniso, A.C.; Ferreira, F.D.O.; Doretto, K.M.; Peruchi, L.M.; Viera, A.; Neto, O.F.D.O.; Bosco, S.M.D.; et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere 2018, 214, 111–122. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3. Chemical Methods; Bingham, J.M., Ed.; ASA-SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Bertsch, P.M.; Bloom, P.R. Aluminium. In Methods of Soil Analysis Part 3. Chemical Methods; Sparks, D.L., Ed.; ASA-SSSA: Madison, WI, USA, 1996; pp. 517–550. [Google Scholar]
- Gad-Allah, T.A.; Ali, M.E.; Badawy, M.I. Photocatalytic oxidation of ciprofloxacin under simulated sunlight. J. Hazard. Mater. 2010, 186, 751–755. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2019, 707, 136118. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, O. Pharmaceuticals’ sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Cela-Dablanca, R.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of pH and Humic Acids. Molecules 2021, 26, 3080. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Alleoni, L.R.F.; Tornisielo, V.L.; Regitano, J.B. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 2013, 92, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conkle, J.L.; Lattao, C.; White, J.R.; Cook, R.L. Competitive sorption and desorption behavior for the three fluoroquinolone antibiotics in a wastewater treatment wetland soil. Chemosphere 2010, 80, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Uslu, M.; Yediler, A.; Balcıoğlu, I.A.; Schulte-Hostede, S. Analysis and Sorption Behavior of Fluoroquinolones in Solid Matrices. Water Air Soil Pollut. 2007, 190, 55–63. [Google Scholar] [CrossRef]
- Nowara, A.; Burhenne, A.J.; Spiteller, M. Binding of Fluoroquinolone Carboxylic Acid Derivatives to Clay Minerals. J. Agric. Food Chem. 1997, 45, 1459–1463. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H. Adsorption behavior of antibiotic in soil environment: A critical review. Front. Environ. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, V.G.; MacKay, A.A. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Yan, B.; Niu, C. Adsorption of ciprofloxacin from water by pretreated oat hulls: Equilibrium, kinetic, and thermodynamic studies. Ind. Crop. Prod. 2018, 127, 237–250. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Lam, F.L.-Y.; Wang, Y.; Liu, Z.; Dai, H. Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon. J. Colloid Interface Sci. 2015, 460, 349–360. [Google Scholar] [CrossRef]
- Sidhu, H.; D’Angelo, E.; O’Connor, G. Retention-release of ciprofloxacin and azithromycin in biosolids and biosolids-amended soils. Sci. Total Environ. 2019, 650, 173–183. [Google Scholar] [CrossRef]
- Williams, M.; Ong, P.L.; Williams, D.B.; Kookana, R.S. Estimating the sorption of pharmaceuticals based on their pharmacological distribution. Environ. Toxicol. Chem. 2009, 28, 2572–2579. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Lin, S.-S.; Dai, C.-M.; Shi, L.; Zhou, X.-F. Sorption-desorption and transport of trimethoprim and sulfonamide antibiotics in agricultural soil: Effect of soil type, dissolved organic matter, and pH. Environ. Sci. Pollut. Res. 2014, 21, 5827–5835. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.-J.; Ying, G.-G.; Liu, Y.-S.; Su, H.-C.; He, L.-Y. Joint antibacterial activity of soil-adsorbed antibiotics trimethoprim and sulfamethazine. Sci. Total Environ. 2015, 506–507, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kocárek, M.; Kodesová, R.; Vondrácková, L.; Golovko, O.; Fér, M.; Klement, A.; Nikodem, A.; Jaksík, O.; Grabic, R. Sim-ultaneous sorption of four ionisable pharmaceuticals in different horizons of three soil types. Environ. Pollut. 2016, 218, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.M.; Williams, C.; Andrews, D.M.; Watson, J.E. Sorption and desorption behavior of four antibiotics at concentrations simulating wastewater reuse in agricultural and forested soils. Chemosphere 2022, 289, 133038. [Google Scholar] [CrossRef]
- Salihi, E.C.; Mahramanlıoğlu, M. Equilibrium and kinetic adsorption of drugs on bentonite: Presence of surface active agents effect. Appl. Clay Sci. 2014, 101, 381–389. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Estimation of adsorption/desorption Freundlich’s affinity coefficients for oxytetracycline and chloro-tetracycline from soil properties: Experimental data and pedotransfer functions. Ecotox. Environ. Safe. 2020, 196, 110584. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/desorption and transport of sulfadiazine, sulfachloropyridazine, and sulfamethazine, in acid agricultural soils. Chemosphere 2019, 234, 978–986. [Google Scholar] [CrossRef]
- Cela-Dablanca, R.; Barreiro, A.; López, L.R.; Santás-Miguel, V.; Arias-Estévez, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Relevance of sorption in bio-reduction of amoxicillin taking place in forest and crop soils. Environ. Res. 2022, 208, 112753. [Google Scholar] [CrossRef]
- Teixidó, M.; Medeiros, J.; Beltran, J.L.; Prat, M.-D.; Granados, M. Sorption of Enrofloxacin and Ciprofloxacin in Agricultural Soils: Effect of Organic Matter. Adsorpt. Sci. Technol. 2014, 32, 153–163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).