Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. FM-BC Preparation
2.2. Adsorption Experiments
2.3. Characterization Analysis Method
2.4. Sample Analysis Method and Quality Control
2.5. Data and Statistics Analysis
3. Results and Discussion
3.1. The Peculiarities of the Adsorbent Structure
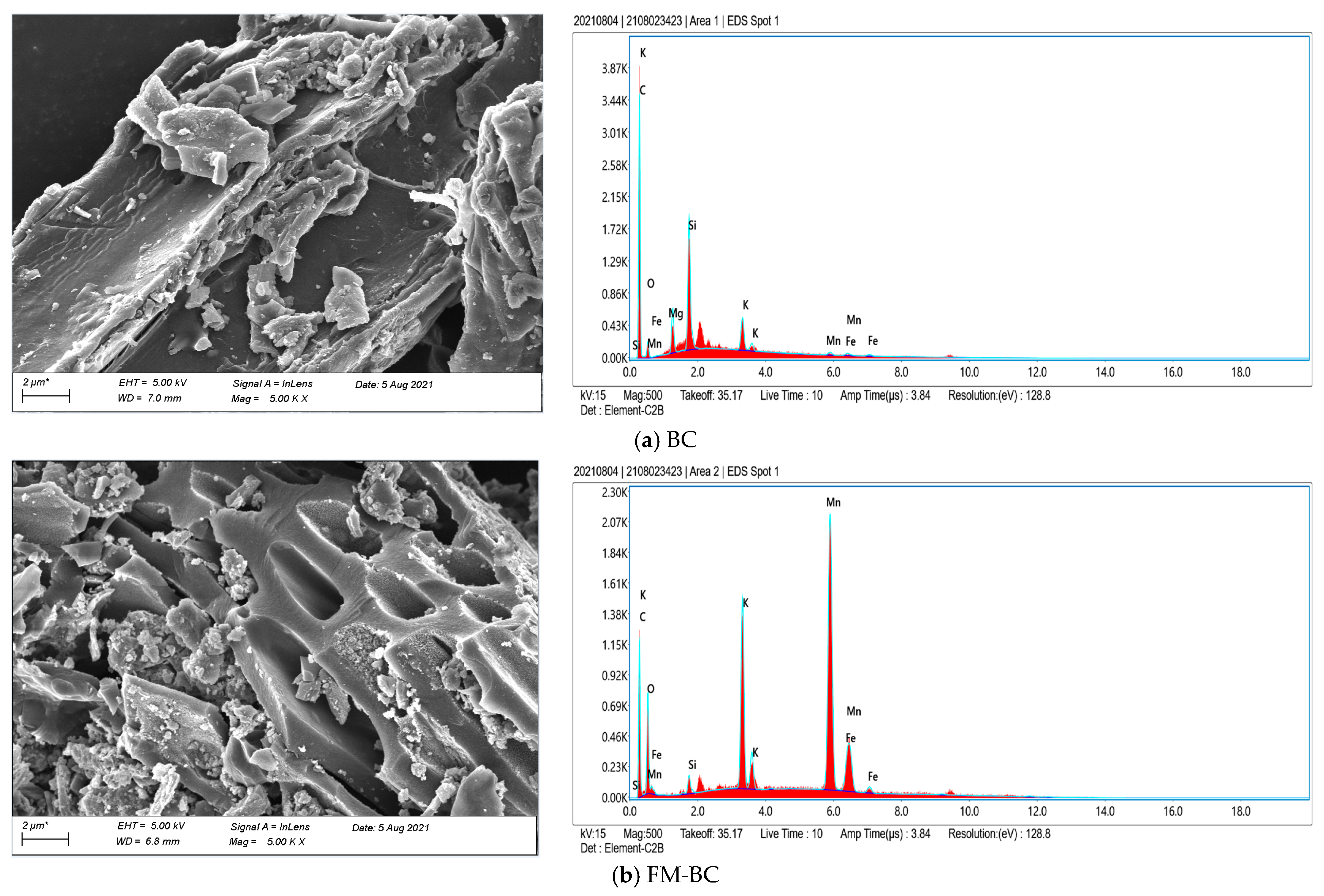
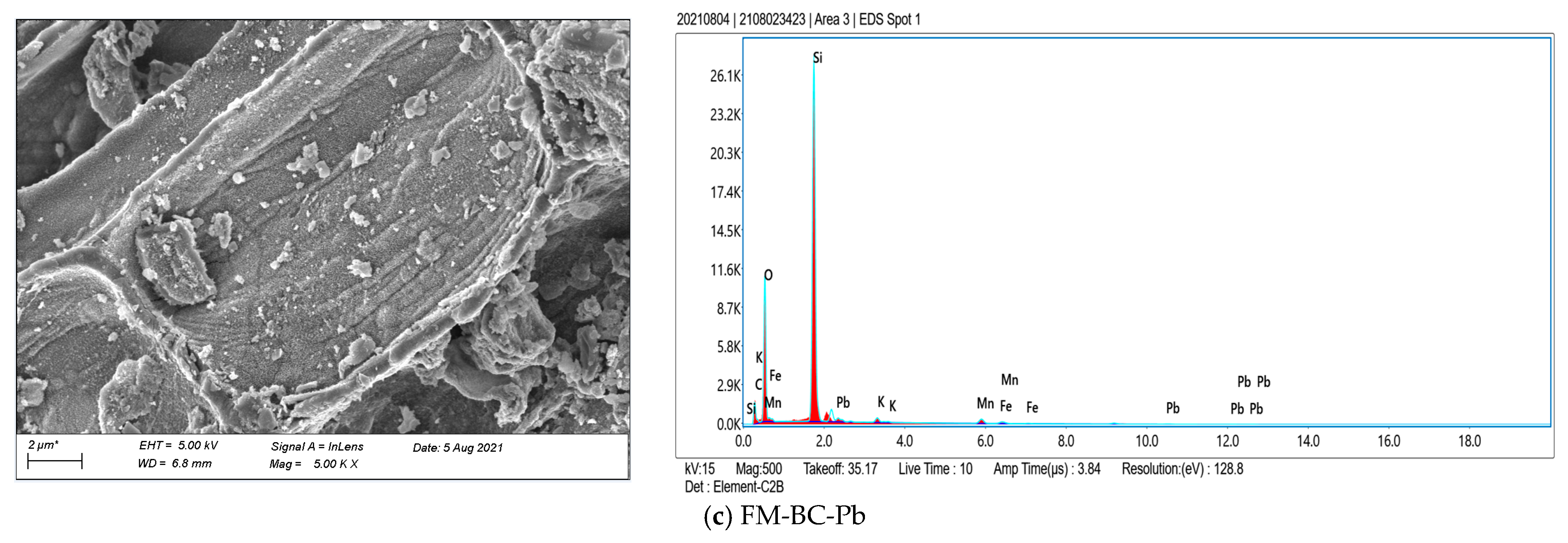
3.1.1. SEM and BET Analysis
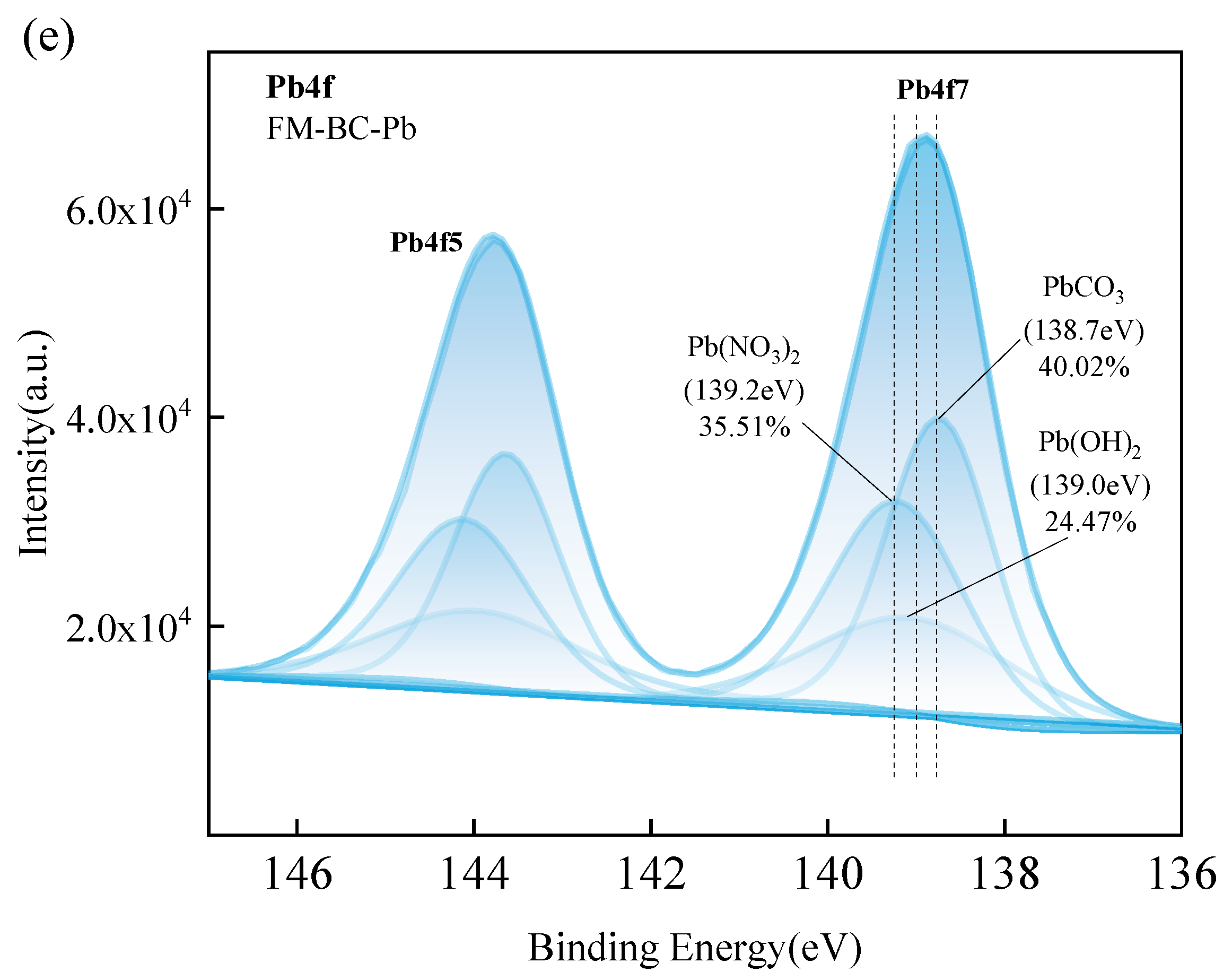
3.1.2. XPS Analysis
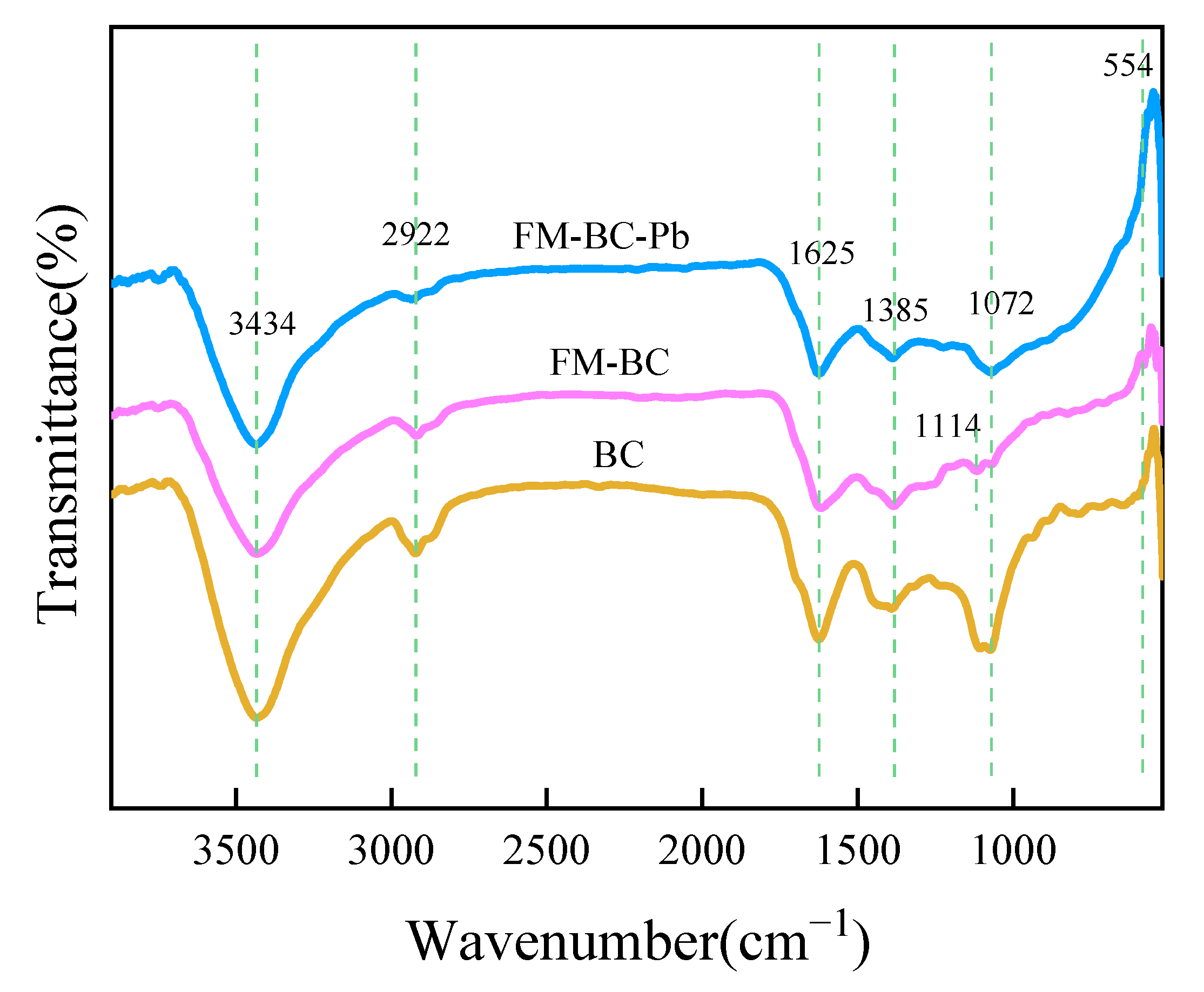
3.1.3. FTIR Analysis
3.2. Impacts of pH, Addition Dosage and Coexisting Ions on Pb(II) Adsorption by FM-BC
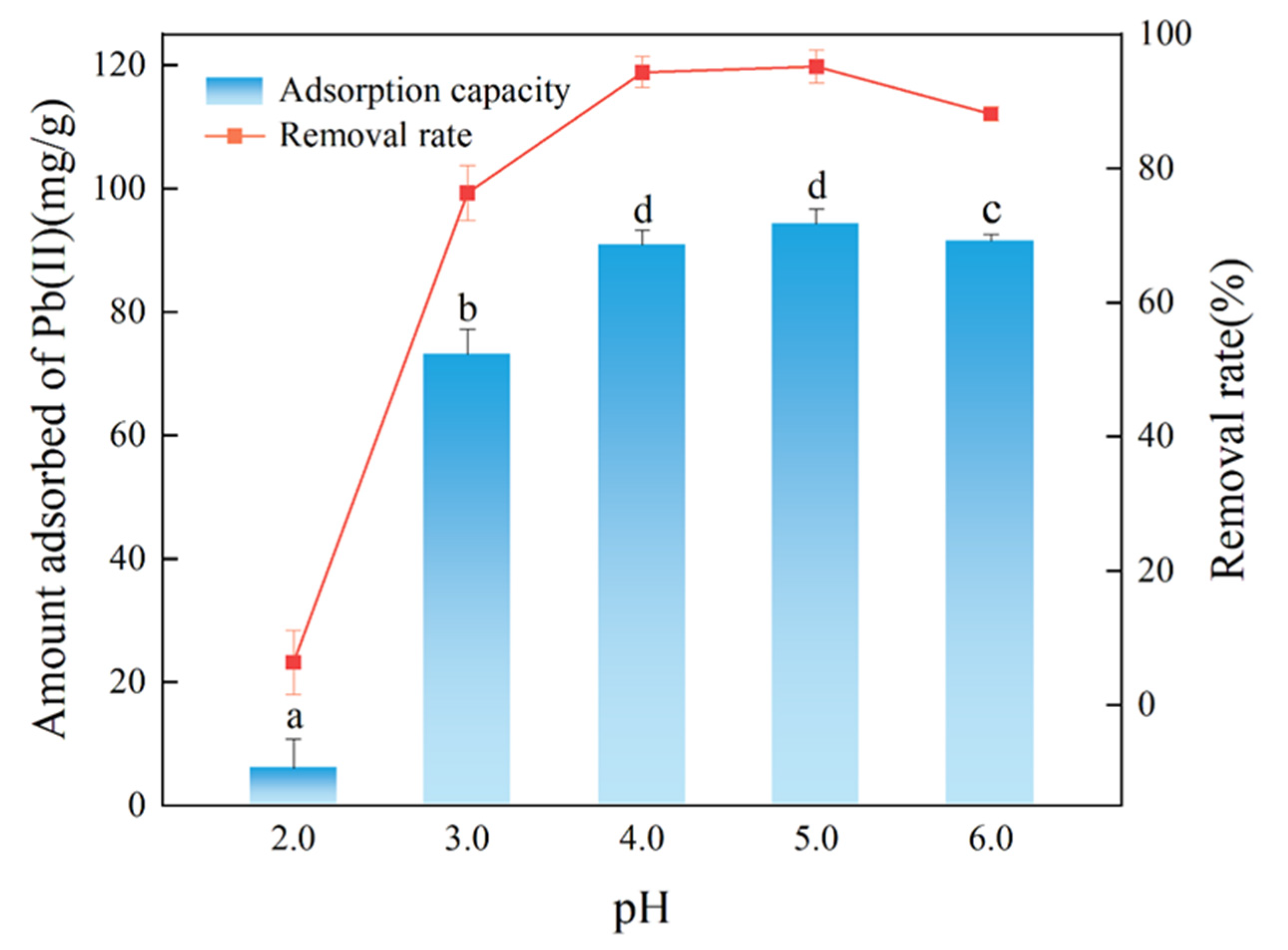
3.2.1. The Effect of pH on the Adsorption of Pb(II) by FM-BC
3.2.2. The Effect of FM-BC Addition Dosage on Pb(II) Adsorption by FM-BC
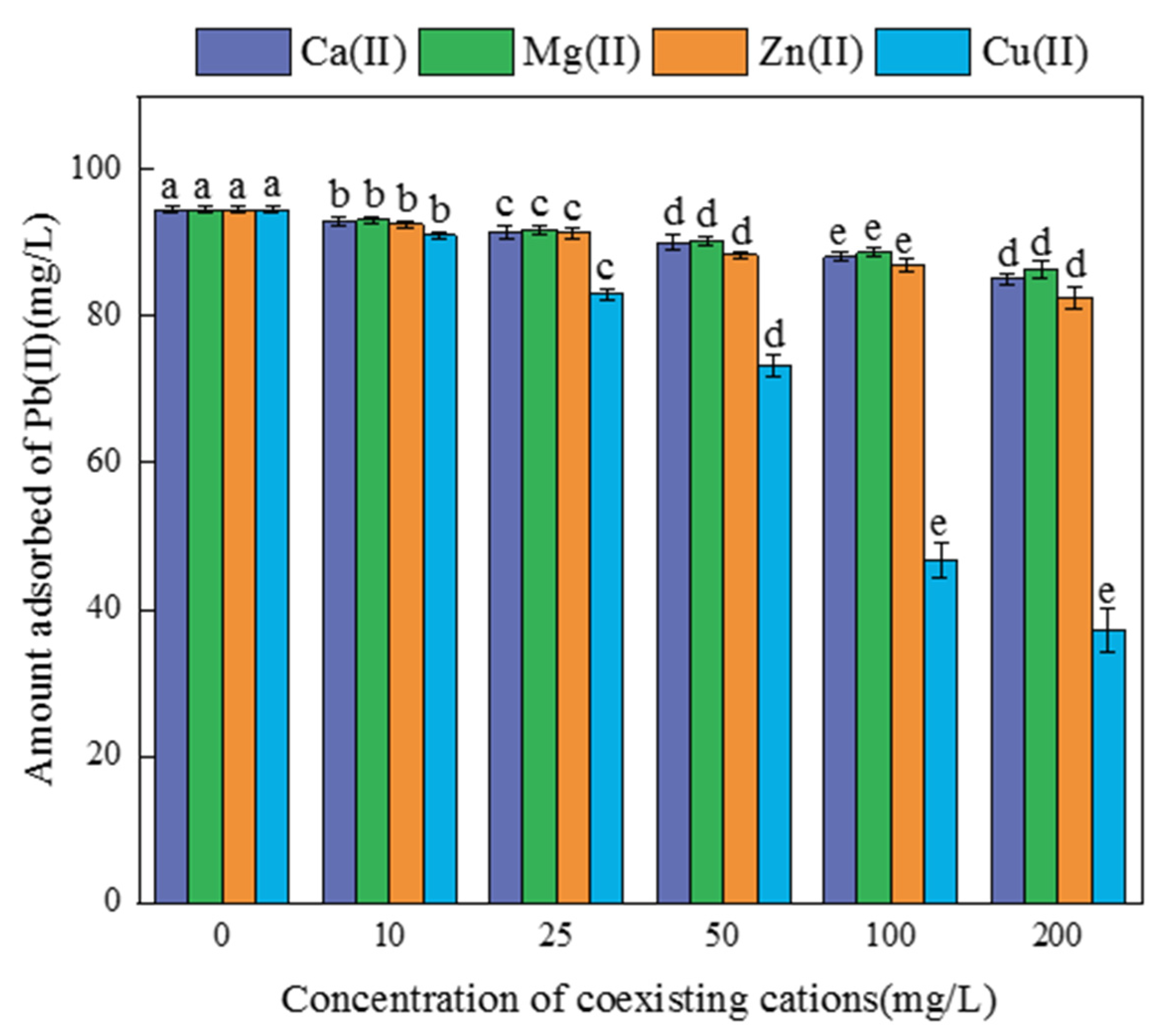
3.2.3. The Effect of Coexisting Ions on Pb(II) Adsorption by FM-BC
3.3. Adsorption Characteristics of FM-BC on Pb(II) in Water
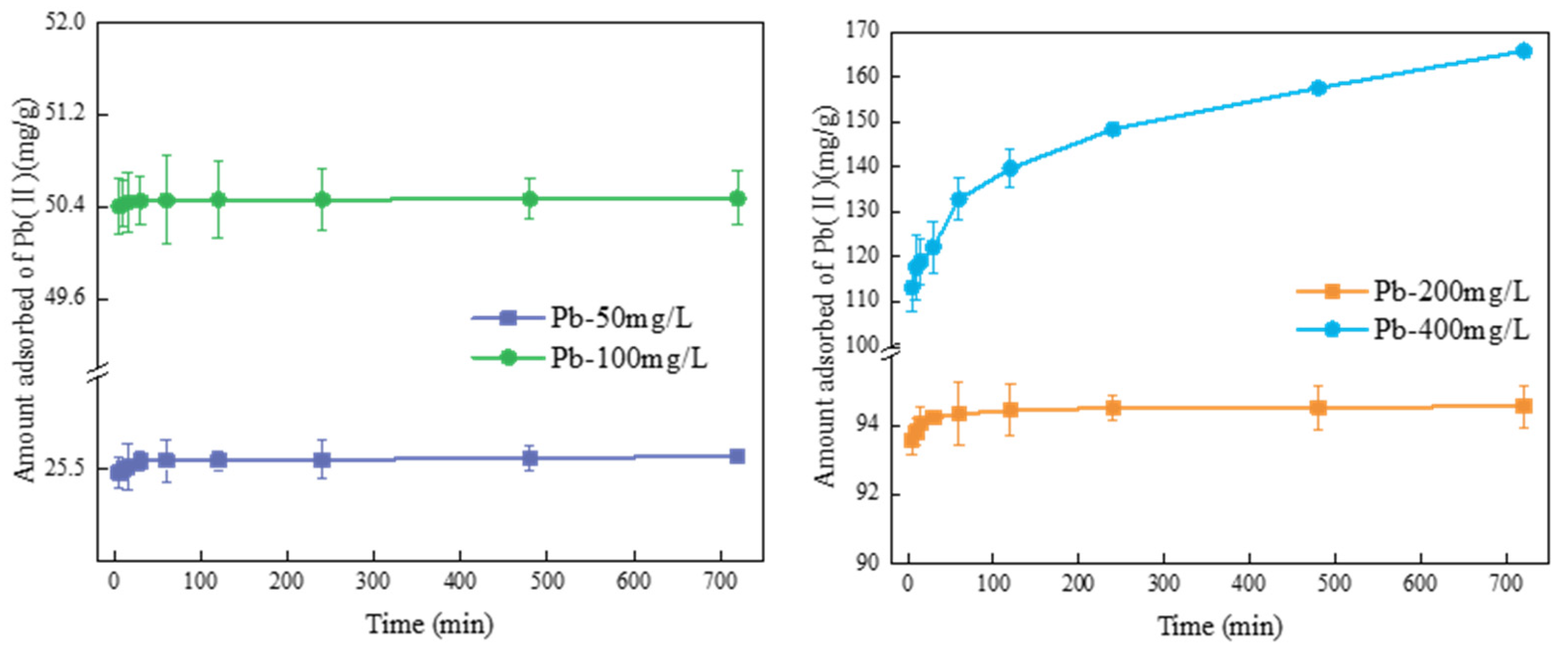
3.3.1. Adsorption Kinetics
3.3.2. Adsorption Isotherm
3.3.3. Adsorption Thermodynamics
3.4. Adsorption Mechanism of Pb(II) by FM-BC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.Y.; Luo, J.M.; Liu, C.B.; Chu, L.; Ma, J.H.; Tang, Y.H.; Zeng, Z.B.; Luo, S.L. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Yoon, M. Core-shell ferromagnetic nanorod based on amine polymer composite (Fe3O4@DAPF) for fast removal of Pb(II) from aqueous solutions. ACS Appl. Mater. Interfaces 2015, 7, 25362–25372. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.K.; Liu, H.Y.; Yang, R.Q.; Gai, X.K.; Wang, H.P.; Shan, S.D. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Escudero, E. The removal of toxic metals from liquid effluents by ion exchange resins. Part VIII: Arsenic(III)/OH-/Dowex 1x8. Rev. Metal. 2018, 54, e132. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Chen, Z.; Zhao, G.X.; Zhang, Z.B.; Xu, C.; Liu, Y.H.; Chen, J.; Zhuang, L.; Haya, T.; Wang, X.K. Enhanced adsorption of U(VI) and 241Am(III) from wastewater using Ca/Al layered double hydroxide@carbon nanotube composites. J. Hazard. Mater. 2018, 347, 67–77. [Google Scholar] [CrossRef]
- Elabbas, S.; Ouazzani, N.; Mandi, L.; Berrekhis, F.; Perdicakis, M.; Pontvianne, S.; Pons, M.N.; Lapicque, F.; Leclerc, J.P. Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. J. Hazard. Mater. 2016, 319, 69–77. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Nunez-Delgado, A.; Ali, S.; Qaswar, M.; Khan, Z.H.; Ying, H.; Chen, D.-Y. Utilization of Citrullus lanatus L. seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar. Sci. Total Environ. 2021, 771, 144955. [Google Scholar] [CrossRef]
- Yang, X.D.; Wan, Y.S.; Zheng, Y.L.; He, F.; Yu, Z.B.; Huang, J.; Wang, H.L.; Ok, Y.S.; Jiang, Y.S.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Goncalves, S.P.C.; Strauss, M.; Teodoro Martinez, D.S. The positive fate of biochar addition to soil in the degradation of PHBV-silver nanoparticle composites. Environ. Sci. Technol. 2018, 52, 13845–13853. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Kim, K.H.; Jung, J.M.; Heo, H.-S.; Kwon, E.E.; Tack, F.M.G.; Tsang, D.C.W.; Jeon, Y.J.; Ok, Y.S. Effect of biochars pyrolyzed in N2 and CO2, and feedstock on microbial community in metal(loid)s contaminated soils. Environ. Int. 2019, 126, 791–801. [Google Scholar] [CrossRef]
- Kvakic, M.; Pellerin, S.; Ciais, P.; Achat, D.L.; Augusto, L.; Denoroy, P.; Gerber, J.S.; Goll, D.; Mollier, A.; Mueller, N.D.; et al. Quantifying the limitation to world cereal production due to soil phosphorus status. Global Biogeochem. Cycles 2018, 32, 143–157. [Google Scholar] [CrossRef]
- Yoo, J.-C.; Beiyuan, J.; Wang, L.; Tsang, D.C.W.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.D. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci. Total Environ. 2018, 616, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Q.; Wu, Y.; Liu, Y.; Xiao, D. Removal of Pb(II) ions from aqueous solution by manganese oxide coated rice straw biochar-A low-cost and highly effective sorbent. J. Taiwan Inst. Chem. Eng. 2018, 84, 85–92. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.L.; Peng, T.Y.; Shen, Z.T.; Tsang, D.C.W.; Hou, D.Y. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Tan, G.C.; Sun, W.L.; Xu, Y.R.; Wang, H.Y.; Xu, N. Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Q.; Wang, Z.H.; Gao, B.; Fan, Z.X.; Li, M.; Hao, H.R.; Wei, X.N.; Zhong, M. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study. Sci. Total Environ. 2021, 758, 143584. [Google Scholar] [CrossRef]
- Yunus, Z.M.; Yashni, G.; Al-Gheethi, A.; Othman, N.; Hamdan, R.; Ruslan, N.N. Advanced methods for activated carbon from agriculture wastes; a comprehensive review. Int. J. Environ. Anal. Chem. 2022, 102, 134–158. [Google Scholar] [CrossRef]
- Li, M.X.; Liu, H.B.; Chen, T.H.; Dong, C.; Sun, Y.B. Synthesis of magnetic biochar composites for enhanced uranium (VI) adsorption. Sci. Total Environ. 2019, 651, 1020–1028. [Google Scholar] [CrossRef]
- Zhu, N.Y.; Qiao, J.; Yan, T.M. Arsenic immobilization through regulated ferrolysis in paddy field amendment with bismuth impregnated biochar. Sci. Total Environ. 2019, 648, 993–1001. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Haynes, R.J. Sorption of heavy metals by inorganic and organic components of solid wastes: Significance to use of wastes as low-cost adsorbents and immobilizing agents. Crit. Rev. Environ. Sci. Technol. 2010, 40, 909–977. [Google Scholar] [CrossRef]
- Liu, X.W.; Gao, M.L.; Qiu, W.W.; Khan, Z.H.; Liu, N.B.; Lin, L.N.; Song, Z.G. Fe-Mn-Ce oxide-modified biochar composites as efficient adsorbents for removing As(III) from water: Adsorption performance and mechanisms. Environ. Sci. Pollut. Res. 2019, 26, 17373–17382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Gu, L.Q.; Zhang, L.; Zheng, S.R.; Wan, H.Q.; Sun, J.Y.; Zhu, D.Q.; Xu, Z.Y. Removal of aqueous Pb(II) by adsorption on Al2O3-pillared layered MnO2. Appl. Surf. Sci. 2017, 406, 330–338. [Google Scholar] [CrossRef]
- Faheem; Yu, H.X.; Liu, J.; Shen, J.Y.; Sun, X.Y.; Li, J.S.; Wang, L.J. Preparation of MnOx-loaded biochar for Pb2+ removal: Adsorption performance and possible mechanism. J. Taiwan Inst. Chem. Eng. 2016, 66, 313–320. [Google Scholar] [CrossRef]
- Huang, G.X.; Wang, C.Y.; Yang, C.W.; Guo, P.C.; Yu, H.Q. Degradation of bisphenol a by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef]
- Yin, G.C.; Song, X.W.; Tao, L.; Sarkar, B.; Sarmah, A.K.; Zhang, W.X.; Lin, Q.T.; Xiao, R.B.; Liu, Q.J.; Wang, H.L. Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd(II) from aqueous solutions. Chem. Eng. J. 2020, 389, 124465. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.Q.; Yang, Y.; Han, Y.T.; Wang, T.S.; Chen, J.W.; Tsang, D.C.W. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef]
- Lin, L.N.; Qiu, W.W.; Wang, D.; Huang, Q.; Song, Z.G.; Chau, H.W. Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mirghaffari, N. A preliminary study of the preparation of porous carbon from oil sludge for water treatment by simple pyrolysis or KOH activation. New Carbon Mater. 2015, 30, 310–318. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.L.; Zhu, L.Z. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G.C.; Xing, B.S. Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: Physicochemical properties, heavy metals sorption behavior and mechanism. J. Hazard. Mater. 2020, 399, 123067. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Gao, B.; Zimmerman, A.R.; Li, Y.C.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Kong, L.; Qu, Z.; Lo, L.; Shen, G.Q. Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain. Chem. Eng. 2015, 3, 125–132. [Google Scholar] [CrossRef]
- Cao, X.D.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Zhang, L.K.; Liu, X.Y.; Huang, X.M.; Wang, W.D.; Sun, P.; Li, Y.M. Adsorption of Pb2+ from aqueous solutions using Fe-Mn binary oxides-loaded biochar: Kinetics, isotherm and thermodynamic studies. Environ. Technol. 2019, 40, 1853–1861. [Google Scholar] [CrossRef]
- Li, S.S.; Yang, F.; Li, J.S.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Ouyang, D.X.; Zhuo, Y.T.; Hu, L.; Zeng, Q.; Hu, Y.H.; He, Z.G. Research on the adsorption behavior of heavy metal ions by porous material prepared with silicate tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.B.; Ma, Y.B.; Chen, L.; Xian, K. Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: Single- and multi-metal competitive adsorption study. Geochem. J. 2010, 44, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Chowdhury, I.H.; Naskar, M.K. Nitrogen-doped nanoporous carbon nanospheroids for selective dye adsorption and Pb(II) ion removal from waste water. ACS Omega 2018, 3, 9888–9898. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Zhang, T.C.; Ouyang, L.K.; Yuan, S.J. Single-Step hydrothermal synthesis of biochar from H3PO4-activated lettuce waste for efficient adsorption of Cd(II) in aqueous solution. Molecules 2022, 27, 269. [Google Scholar] [CrossRef]
- Yang, X. Monitoring the interfacial polymerization of piperazine and trimesoyl chloride with hydrophilic interlayer or macromolecular additive by in situ FT-IR sspectroscopy. Membranes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of humic acid and suspended solids on the removal of heavy metals from water by adsorption onto granular activated carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475–10489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.C.; Zhao, H.H.; Song, X.L.; Li, Y.K.; Li, D. The adsorption characteristics and mechanism of Pb(II) onto corn straw biochar. J. Biobased Mater. Bioenergy 2021, 15, 287–295. [Google Scholar] [CrossRef]
- Feng, T.; Yi, T.; Wang, Q.B.; Li, P.W. Shrimp shells-derived biochar for efficient adsorption of Pb2+ in aqueous solutions. Desalin. Water Treat. 2021, 233, 106–117. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, F.Y.; Ouyang, X.K.; Yang, L.Y.; Wang, Y.G. Adsorption of Pb(II) from Aqueous Solution by Mussel Shell-Based Adsorbent: Preparation, Characterization, and Adsorption Performance. Materials 2021, 14, 741. [Google Scholar] [CrossRef]
- Zhao, T.C.; Ma, X.L.; Cai, H.; Ma, Z.C.; Liang, H.F. Study on the Adsorption of CuFe2O4-Loaded Corncob Biochar for Pb(II). Molecules 2020, 25, 3456. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids Surf. A 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Militaru, B.A.; Pode, R.; Lupa, L.; Schmidt, W.; Tekle-Roettering, A.; Kazamer, N. Using sewage sludge ash as an efficient adsorbent for Pb (II) and Cu (II) in single and binary systems. Molecules. 2020, 25, 2559. [Google Scholar] [CrossRef]
- Irani, M.; Amjadi, M.; Mousavian, M.A. Comparative study of lead sorption onto natural perlite, dolomite and diatomite. Chem. Eng. J. 2011, 178, 317–323. [Google Scholar] [CrossRef]
- Chen, C.G.; Qiu, M.Q. High efficiency removal of Pb(II) in aqueous solution by a biochar-supported nanoscale ferrous sulfide composite. RSC Adv. 2021, 11, 953–959. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Nunez-Delgado, A.; Ali, S.; Qaswar, M.; Shakoor, A.; Mahmood, M.; Chen, D.Y. Enhanced adsorption of aqueous Pb(II) by modified biochar produced through pyrolysis of watermelon seeds. Sci. Total Environ. 2021, 784, 147136. [Google Scholar] [CrossRef] [PubMed]
- Akpomie, K.G.; Conradie, J. Biogenic and chemically synthesized Solanum tuberosum peel-silver nanoparticle hybrid for the ultrasonic aided adsorption of bromophenol blue dye. Sci. Rep. 2020, 10, 17094. [Google Scholar] [CrossRef] [PubMed]



| Materials | Surface Area | External Surface Area | Micropore Specific Surface Area | Pore Diameter | Total Pore Volume | Micropore Volume |
|---|---|---|---|---|---|---|
| (m2/g) | (m2/g) | (m2/g) | (nm) | (cm3/g) | (cm3/g) | |
| BC | 0.754 | 0.371 | 0.383 | 8.089 | 0.0002 | 0.0001 |
| FM-BC | 13.726 | 11.296 | 2.430 | 10.404 | 0.003 | 0.0008 |
| FM-BC-Pb | 26.434 | 19.884 | 6.550 | 7.008 | 0.006 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.-F.; Zhou, H.; Tan, W.-T.; Huang, J.-G.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 8420. https://doi.org/10.3390/ijerph19148420
Tang S-F, Zhou H, Tan W-T, Huang J-G, Zeng P, Gu J-F, Liao B-H. Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater. International Journal of Environmental Research and Public Health. 2022; 19(14):8420. https://doi.org/10.3390/ijerph19148420
Chicago/Turabian StyleTang, Shang-Feng, Hang Zhou, Wen-Tao Tan, Jun-Guo Huang, Peng Zeng, Jiao-Feng Gu, and Bo-Han Liao. 2022. "Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater" International Journal of Environmental Research and Public Health 19, no. 14: 8420. https://doi.org/10.3390/ijerph19148420
APA StyleTang, S.-F., Zhou, H., Tan, W.-T., Huang, J.-G., Zeng, P., Gu, J.-F., & Liao, B.-H. (2022). Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater. International Journal of Environmental Research and Public Health, 19(14), 8420. https://doi.org/10.3390/ijerph19148420






