Metformin and the Development of Asthma in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Source
2.2. Study Design and Participants
2.3. Procedures
2.4. Main Outcomes
2.5. Statistical Analysis
3. Results
3.1. Participants
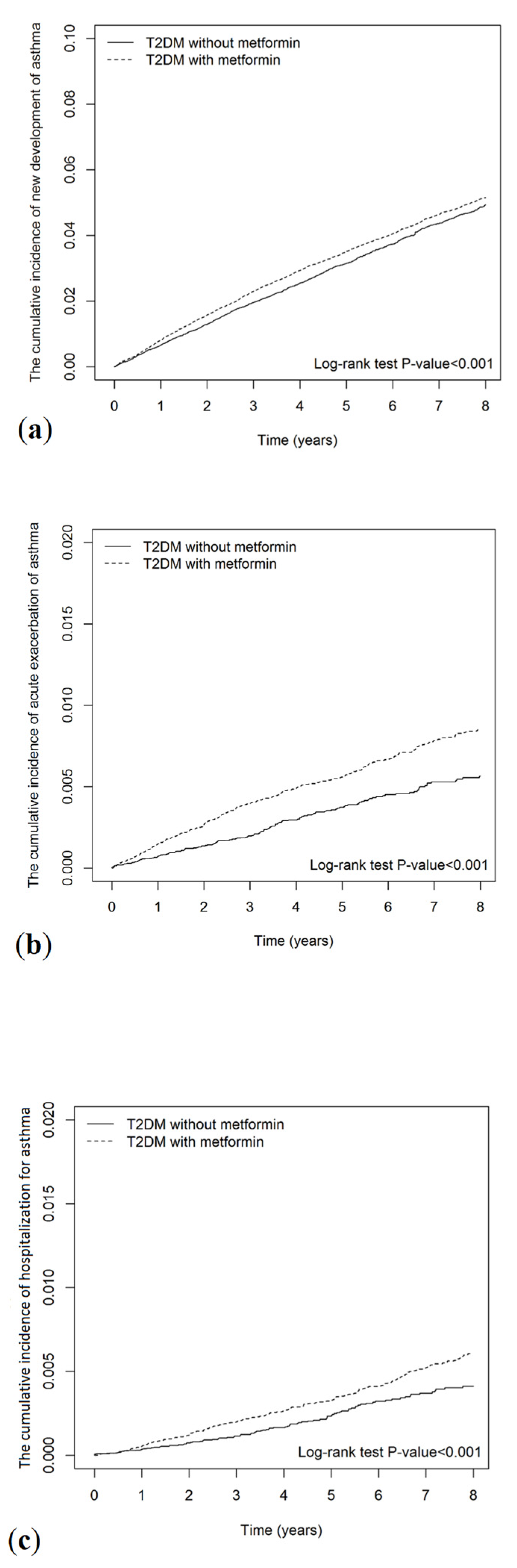
3.2. Main Outcomes
3.3. Cumulative Duration of Metformin Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, G.P. Asthma, chronic bronchitis and emphysema. The use of intermittent positive pressure breathing with inspiratory flow rate control. A review of the literature. Calif. Med. 1963, 98, 212–229. [Google Scholar] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- GBD. Institute for Health Metrics and Evaluation, Global Health Data Exchange, Global Burden of Disease Study 2019 (GBD 2019) Data Resources, GBD Results Tool, Terms and Conditions (2019). Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 19 November 2021).
- Beuther, D.A.; Sutherland, E.R. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.F.; Quesenberry, C.P., Jr.; Van Den Eeden, S.K.; Shan, J.; Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Duffy, D.L.; Kyvik, K.O.; Skytthe, A.; Backer, V. Risk of asthma in adult twins with type 2 diabetes and increased body mass index. Allergy 2011, 66, 562–568. [Google Scholar] [CrossRef]
- Kuschnir, F.C.; Felix, M.M.R.; Caetano Kuschnir, M.C.; Bloch, K.V.; Azevedo de Oliveira Costa Jordão, E.; Solé, D.; da Cunha, A.J.L.; Szklo, M. Severe asthma is associated with metabolic syndrome in Brazilian adolescents. J. Allergy Clin. Immunol. 2018, 141, 1947–1949.e4. [Google Scholar] [CrossRef]
- Ma, B.; Athari, S.S.; Mehrabi Nasab, E.; Zhao, L. PI3K/AKT/mTOR and TLR4/MyD88/NF-κB signaling inhibitors attenuate pathological mechanisms of allergic asthma. Inflammation 2021, 44, 1895–1907. [Google Scholar] [CrossRef]
- Wang, H.C.; Huang, S.K. Metformin inhibits IgE- and aryl hydrocarbon receptor-mediated mast cell activation in vitro and in vivo. Eur. J. Immunol. 2018, 48, 1989–1996. [Google Scholar] [CrossRef]
- Calixto, M.C.; Lintomen, L.; André, D.M.; Leiria, L.O.; Ferreira, D.; Lellis-Santos, C.; Anhê, G.F.; Bordin, S.; Landgraf, R.G.; Antunes, E. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS ONE 2013, 8, e76786. [Google Scholar] [CrossRef]
- Ratnovsky, A.; Mellema, M.; An, S.S.; Fredberg, J.J.; Shore, S.A. Airway smooth muscle proliferation and mechanics: Effects of AMP kinase agonists. Mol. Cell. Biomech. 2007, 4, 143–157. [Google Scholar]
- Chen, C.Z.; Hsu, C.H.; Li, C.Y.; Hsiue, T.R. Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort. J. Asthma 2017, 54, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Rayner, L.H.; Mcgovern, A.; Sherlock, J.; Gatenby, P.; Correa, A.; Creagh-Brown, B.; de Lusignan, S. The impact of therapy on the risk of asthma in type 2 diabetes. Clin. Respir. J. 2019, 13, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Erickson, S.R.; Wu, C.H. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology 2016, 21, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Keet, C.A.; Fawzy, A.; Segal, J.B.; Brigham, E.P.; McCormack, M.C. Association of metformin initiation and risk of asthma exacerbation. A claims-based cohort study. Ann. Am. Thorac. Soc. 2019, 16, 1527–1533. [Google Scholar] [CrossRef]
- Cheng, T.M. Taiwan’s new national health insurance program: Genesis and experience so far. Health Aff. 2003, 22, 61–76. [Google Scholar] [CrossRef]
- Lin, C.C.; Lai, M.S.; Syu, C.Y.; Chang, S.C.; Tseng, F.Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 2005, 104, 157–163. [Google Scholar]
- Meduru, P.; Helmer, D.; Rajan, M.; Tseng, C.L.; Pogach, L.; Sambamoorthi, U. Chronic illness with complexity: Implications for performance measurement of optimal glycemic control. J. Gen. Intern. Med. 2007, 22, 408–418. [Google Scholar] [CrossRef]
- Young, B.A.; Lin, E.; Von Korff, M.; Simon, G.; Ciechanowski, P.; Ludman, E.J.; Everson-Stewart, S.; Kinder, L.; Oliver, M.; Boyko, E.J.; et al. Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am. J. Manag. Care 2008, 14, 15–23. [Google Scholar]
- Wang, Y.T.; Tsai, M.C.; Wang, Y.H.; Wei, J.C. Association between proton pump inhibitors and asthma: A population-based cohort study. Front. Pharmacol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Reddel, H.K.; Taylor, D.R.; Bateman, E.D.; Boulet, L.P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009, 180, 59–99. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Shrank, W.H.; Patrick, A.R.; Brookhart, M.A. Healthy user and related biases in observational studies of preventive interventions: A primer for physicians. J. Gen. Intern. Med. 2011, 26, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhong, W.; Chai, Y.; Zhong, Q.; Gao, J.; Guan, L.; Mengzhi, Z.; Huaiquan, L.; Haiyang, Y.; Qingxue, W.; et al. Association of metformin use with asthma exacerbation in patients with concurrent asthma and diabetes: A systematic review and meta-analysis of observational studies. Can. Respir. J. 2020, 2020, 9705604. [Google Scholar] [CrossRef] [PubMed]
- Saenwongsa, W.; Nithichanon, A.; Chittaganpitch, M.; Buayai, K.; Kewcharoenwong, C.; Thumrongwilainet, B.; Butta, P.; Palaga, T.; Takahashi, Y.; Ato, M.; et al. Metformin-induced suppression of IFN-α via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Sci. Rep. 2020, 10, 3229. [Google Scholar] [CrossRef]
- Shore, S.A.; Williams, E.S.; Zhu, M. No effect of metformin on the innate airway hyperresponsiveness and increased responses to ozone observed in obese mice. J. Appl. Physiol. 1985, 105, 1127–1133. [Google Scholar] [CrossRef]
- Shen, T.C.; Lin, C.L.; Wei, C.C.; Tu, C.Y.; Li, Y.F. The risk of asthma in rheumatoid arthritis: A population-based cohort study. QJM 2014, 107, 435–442. [Google Scholar] [CrossRef][Green Version]
- Leung, J.M.; Sin, D.D. Asthma-COPD overlap syndrome: Pathogenesis, clinical features, and therapeutic targets. BMJ 2017, 358, j3772. [Google Scholar] [CrossRef]
- Huang, C.C.; Chan, W.L.; Chen, Y.C.; Chen, T.J.; Chou, K.T.; Lin, S.J.; Chen, J.W.; Leu, H.B. Statin use in patients with asthma: A nationwide population-based study. Eur. J. Clin. Investig. 2011, 41, 507–512. [Google Scholar] [CrossRef]
- Iribarren, C.; Tolstykh, I.V.; Miller, M.K.; Sobel, E.; Eisner, M.D. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: A prospective study of 2 matched cohorts. Am. J. Epidemiol. 2012, 176, 1014–1024. [Google Scholar] [CrossRef]
- Huang, H.L.; Ho, S.Y.; Li, C.H.; Chu, F.Y.; Ciou, L.P.; Lee, H.C.; Chen, W.L.; Tzeng, N.S. Bronchial asthma is associated with increased risk of chronic kidney disease. BMC Pulm. Med. 2014, 14, 80. [Google Scholar] [CrossRef]
- Hung, S.C.; Chang, Y.K.; Liu, J.S.; Kuo, K.L.; Chen, Y.H.; Hsu, C.C.; Tarng, D.C. Metformin use and mortality in patients with advanced chronic kidney disease: National, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015, 3, 605–614. [Google Scholar] [CrossRef]
- Edwards, C.M.; Barton, M.A.; Snook, J.; David, M.; Mak, V.H.; Chowdhury, T.A. Metformin-associated lactic acidosis in a patient with liver disease. QJM 2003, 96, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Wei, J.C.; Yang, Y.C.; Hsu, C.C.; Hwu, C.M. Respiratory outcomes of metformin use in patients with type 2 diabetes and chronic obstructive pulmonary disease. Sci. Rep. 2020, 10, 10298. [Google Scholar] [CrossRef]
- Larsen, S.; Rabøl, R.; Hansen, C.N.; Madsbad, S.; Helge, J.W.; Dela, F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 2012, 55, 443–449. [Google Scholar] [CrossRef]
- Paulin, F.V.; Zagatto, A.M.; Chiappa, G.R.; Muller, P.T. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: A randomized and controlled study. Respir. Med. 2017, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]

| Variables | T2D without Metformin | T2D with Metformin | SMD | ||
|---|---|---|---|---|---|
| (n = 57,743) | (n = 57,743) | ||||
| n | % | n | % | ||
| Sex | |||||
| female | 29,164 | 50.51 | 29,117 | 50.43 | 0.002 |
| male | 28,579 | 49.49 | 28,626 | 49.57 | 0.002 |
| Age | |||||
| 20–40 | 6633 | 11.49 | 6958 | 12.05 | 0.017 |
| 41–60 | 26,487 | 45.87 | 27,258 | 47.21 | 0.027 |
| 61–80 | 24,623 | 42.64 | 23,527 | 40.74 | 0.039 |
| Obesity | 1196 | 2.07 | 1242 | 2.15 | 0.006 |
| Smoking status | 1389 | 2.41 | 1435 | 2.49 | 0.005 |
| Comorbidities | |||||
| Hypertension | 31,946 | 55.32 | 33,796 | 58.53 | 0.065 |
| Dyslipidemia | 33,858 | 58.64 | 35,323 | 61.17 | 0.052 |
| Coronary artery disease | 16,168 | 28.00 | 16,191 | 28.04 | 0.001 |
| Stroke | 5879 | 10.18 | 5664 | 9.81 | 0.012 |
| Atrial fibrillation | 71 | 0.12 | 62 | 0.11 | 0.005 |
| PAOD | 2162 | 3.74 | 2095 | 3.63 | 0.006 |
| CKD | 3978 | 6.89 | 3530 | 6.11 | 0.031 |
| Rheumatoid arthritis | 1108 | 1.92 | 1085 | 1.88 | 0.003 |
| Systemic lupus erythematous | 157 | 0.27 | 124 | 0.21 | 0.012 |
| Liver cirrhosis | 1395 | 2.42 | 1424 | 2.47 | 0.003 |
| Cancer | 2899 | 5.02 | 2761 | 4.78 | 0.011 |
| Psychosis | 1219 | 2.11 | 1239 | 2.15 | 0.002 |
| Depression | 20,088 | 34.79 | 19,806 | 34.30 | 0.01 |
| Dementia | 1946 | 3.37 | 1775 | 3.07 | 0.017 |
| COPD | 11,241 | 19.47 | 11,176 | 19.35 | 0.003 |
| Heart failure | 2921 | 5.06 | 2917 | 5.05 | 0 |
| Alcohol-related disorders | 3219 | 5.57 | 3396 | 5.88 | 0.013 |
| CCI | |||||
| 1 | 11,989 | 20.76 | 11,214 | 19.42 | 0.034 |
| 2–3 | 28,937 | 50.11 | 30,508 | 52.83 | 0.054 |
| >3 | 16,817 | 29.12 | 16,021 | 27.75 | 0.031 |
| DCSI | |||||
| 0 | 20,352 | 35.25 | 19,974 | 34.59 | 0.014 |
| 1 | 10,012 | 17.34 | 10,589 | 18.34 | 0.026 |
| ≥2 | 27,379 | 47.42 | 27,180 | 47.07 | 0.007 |
| Medication | |||||
| SU | 5919 | 10.25 | 5982 | 10.36 | 0.004 |
| TZD | 522 | 0.90 | 503 | 0.87 | 0.004 |
| DPP-4 inhibitor | 585 | 1.01 | 600 | 1.04 | 0.003 |
| AGI | 1366 | 2.37 | 1418 | 2.46 | 0.006 |
| SGLT2i | 16 | 0.03 | 4 | 0.01 | 0.016 |
| Number of oral antidiabetic drugs | |||||
| 1 | 56,805 | 98.38 | 56,861 | 98.47 | 0.039 |
| 2–3 | 924 | 1.60 | 865 | 1.50 | 0.039 |
| >3 | 14 | 0.02 | 17 | 0.03 | 0.039 |
| Insulin | 21,207 | 36.73 | 21,239 | 36.78 | 0.001 |
| Immunosuppressants | 322 | 0.56 | 305 | 0.53 | 0.004 |
| Statin | 18,150 | 31.43 | 18,491 | 32.02 | 0.013 |
| Aspirin | 21,304 | 36.89 | 21,627 | 37.45 | 0.012 |
| Influenza vaccination | 12,121 | 20.99 | 12,369 | 21.42 | 0.011 |
| Adult health examination | 28,976 | 50.18 | 29,612 | 51.28 | 0.022 |
| HbA1C > 2 times per year | 88 | 0.15 | 91 | 0.16 | 0.001 |
| Variables | New Development of Asthma | ||||||
|---|---|---|---|---|---|---|---|
| n | PY | IR | cHR | (95% CI) | aHR 1 | (95% CI) | |
| T2D without metformin | 1440 | 235,580 | 6.11 | 1.00 | (Reference) | 1.00 | (Reference) |
| T2D with metformin | 2505 | 376,385 | 6.66 | 1.12 | (1.05, 1.19) *** | 1.13 | (1.06, 1.2) *** |
| Sex | |||||||
| female | 2273 | 324,306 | 7.01 | 1.00 | (Reference) | 1.00 | (Reference) |
| male | 1672 | 287,660 | 5.81 | 0.82 | (0.77, 0.88) *** | 0.85 | (0.79, 0.9) *** |
| Age | |||||||
| 20–40 | 347 | 83,608 | 4.15 | 1.00 | (Reference) | 1.00 | (Reference) |
| 41–60 | 1571 | 293,585 | 5.35 | 1.28 | (1.14, 1.44) *** | 1.27 | (1.12, 1.43) *** |
| 61–80 | 2027 | 234,772 | 8.63 | 2.05 | (1.83, 2.3) *** | 1.77 | (1.55, 2.01) *** |
| Obesity | 58 | 9665 | 6.00 | 0.9 | (0.7, 1.17) | 1.02 | (0.79, 1.33) |
| Smoking | 48 | 9542 | 5.03 | 0.75 | (0.56, 0.99) * | 0.88 | (0.62, 1.24) |
| Comorbidities | |||||||
| Hypertension | 2363 | 328,017 | 7.20 | 1.28 | (1.2, 1.36) *** | 1.02 | (0.95, 1.1) |
| Dyslipidemia | 2180 | 348,665 | 6.25 | 0.92 | (0.86, 0.98) ** | 0.9 | (0.84, 0.97) ** |
| Coronary artery disease | 1418 | 165,550 | 8.57 | 1.51 | (1.41, 1.61) *** | 1.25 | (1.15, 1.36) *** |
| Stroke | 432 | 53,914 | 8.01 | 1.26 | (1.14, 1.39) *** | 1.05 | (0.94, 1.18) |
| Atrial fibrillation | 7 | 400 | 17.51 | 2.62 | (1.25, 5.51) * | 2.18 | (1.03, 4.58) * |
| PAOD | 158 | 19,645 | 8.04 | 1.24 | (1.06, 1.45) ** | 1.06 | (0.9, 1.25) |
| CKD | 245 | 32,030 | 7.65 | 1.18 | (1.04, 1.34) * | 1.01 | (0.88, 1.16) |
| RA | 112 | 10,952 | 10.23 | 1.59 | (1.32, 1.92) *** | 1.37 | (1.13, 1.66) ** |
| SLE | 5 | 1518 | 3.30 | 0.51 | (0.21, 1.23) | 0.45 | (0.19, 1.09) |
| Liver cirrhosis | 74 | 12,202 | 6.07 | 0.93 | (0.74, 1.17) | 0.88 | (0.7, 1.12) |
| Cancer | 155 | 22,538 | 6.88 | 1.05 | (0.9, 1.24) | 0.98 | (0.82, 1.16) |
| Psychosis | 81 | 11,822 | 6.85 | 1.06 | (0.85, 1.32) | 1.08 | (0.86, 1.36) |
| Depression | 1532 | 201,906 | 7.59 | 1.28 | (1.2, 1.36) *** | 1.09 | (1.02, 1.18) * |
| Dementia | 149 | 15,206 | 9.80 | 1.51 | (1.28, 1.78) *** | 1.1 | (0.93, 1.31) |
| COPD | 1280 | 110,472 | 11.59 | 2.17 | (2.03, 2.31) *** | 1.96 | (1.82, 2.12) *** |
| Heart failure | 327 | 26,773 | 12.21 | 1.95 | (1.74, 2.19) *** | 1.45 | (1.28, 1.64) *** |
| Alcohol-related disorders | 139 | 26,686 | 5.21 | 0.78 | (0.66, 0.93) ** | 0.91 | (0.74, 1.12) |
| CCI | |||||||
| 1 | 762 | 150,986 | 5.05 | 1 | - | ||
| 2–3 | 1998 | 317,901 | 6.29 | 1.23 | (1.13, 1.34) *** | 0.95 | (0.86, 1.04) |
| >3 | 1185 | 143,079 | 8.28 | 1.6 | (1.46, 1.76) *** | 0.88 | (0.78, 0.99) * |
| DCSI | |||||||
| 0 | 1314 | 239,845 | 5.48 | 1 | - | ||
| 1 | 716 | 114,542 | 6.25 | 1.13 | (1.03, 1.24) ** | 0.96 | (0.87, 1.05) |
| ≥2 | 1915 | 257,579 | 7.44 | 1.34 | (1.24, 1.43) *** | 0.94 | (0.85, 1.03) |
| Medications | |||||||
| SU | 527 | 71,531 | 7.37 | 1.18 | (1.08, 1.29) *** | 1.1 | (1, 1.22) * |
| TZD | 42 | 5412 | 7.76 | 1.2 | (0.89, 1.63) | 1.36 | (0.95, 1.96) |
| DPP-4 inhibitor | 10 | 2846 | 3.51 | 0.51 | (0.27, 0.94) * | 0.56 | (0.29, 1.05) |
| AGI | 80 | 12,851 | 6.23 | 0.95 | (0.76, 1.19) | 0.96 | (0.75, 1.24) |
| Insulin | 1567 | 211,239 | 7.42 | 1.24 | (1.16, 1.32) *** | 1.07 | (1, 1.14) |
| Immunosuppressants | 17 | 2407 | 7.06 | 1.07 | (0.67, 1.72) | 1.07 | (0.66, 1.74) |
| Statin | 996 | 162,800 | 6.12 | 0.91 | (0.85, 0.98) * | 0.8 | (0.74, 0.87) *** |
| Aspirin | 1553 | 208,758 | 7.44 | 1.24 | (1.16, 1.32) *** | 0.96 | (0.89, 1.04) |
| Outcome | T2DM without Metformin | T2DM with Metformin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PY | IR | n | PY | IR | cHR | (95% CI) | p-Value | aHR 1 | (95% CI) | p-Value | |
| New development of asthma | 1440 | 235,580 | 6.11 | 2505 | 376,385 | 6.66 | 1.12 | (1.05, 1.19) | <0.001 | 1.13 | (1.06, 1.2) | <0.001 |
| Acute exacerbation of asthma | 163 | 241,618 | 0.67 | 393 | 389,209 | 1.01 | 1.59 | (1.32, 1.91) | <0.001 | 1.62 | (1.35, 1.95) | <0.001 |
| Hospitalization for asthma | 124 | 241,947 | 0.51 | 328 | 390,263 | 0.84 | 1.52 | (1.23, 1.87) | <0.001 | 1.5 | (1.22, 1.85) | <0.001 |
| Variables | New Development of Asthma | ||||||
| n | PY | IR | cHR | (95% CI) | aHR 1 | (95% CI) | |
| Non-use of metformin | 1440 | 235,580 | 6.11 | 1.00 | (Reference) | 1.00 | (Reference) |
| Metformin of drug days | |||||||
| 28–364 | 593 | 91,935 | 6.45 | 1.05 | (0.95, 1.15) | 1.03 | (0.93, 1.13) |
| 364–728 | 364 | 65,236 | 5.58 | 0.91 | (0.81, 1.02) | 0.93 | (0.83, 1.05) |
| >728 | 1548 | 219,214 | 7.06 | 1.23 | (1.14, 1.32) *** | 1.24 | (1.16, 1.34) *** |
| Variables | Acute Exacerbation of Asthma | ||||||
| n | PY | IR | cHR | (95% CI) | aHR 1 | (95% CI) | |
| Non-use of metformin | 163 | 241,618 | 0.67 | 1.00 | (Reference) | 1.00 | (Reference) |
| Metformin of drug days | |||||||
| 28–364 | 97 | 94,395 | 1.03 | 1.51 | (1.17, 1.94) ** | 1.56 | (1.21, 2.01) *** |
| 364–728 | 53 | 66,695 | 0.79 | 1.16 | (0.85, 1.58) | 1.26 | (0.93, 1.73) |
| >728 | 243 | 228,118 | 1.07 | 1.78 | (1.46, 2.18) *** | 1.77 | (1.44, 2.17) *** |
| Variables | Hospitalization for Asthma | ||||||
| n | PY | IR | cHR | (95% CI) | aHR 1 | (95% CI) | |
| Non-use of metformin | 124 | 241,947 | 0.51 | 1.00 | (Reference) | 1.00 | (Reference) |
| Metformin of drug days | |||||||
| 28–364 | 76 | 94,594 | 0.80 | 1.59 | (1.19, 2.12) ** | 1.59 | (1.2, 2.12) ** |
| 364–728 | 43 | 66,792 | 0.64 | 1.28 | (0.91, 1.82) | 1.36 | (0.96, 1.92) |
| >728 | 209 | 228,877 | 0.91 | 1.55 | (1.24, 1.95) *** | 1.5 | (1.19, 1.89) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, F.-S.; Hsu, C.-C.; Shih, Y.-H.; Pan, W.-L.; Wei, J.C.-C.; Hwu, C.-M. Metformin and the Development of Asthma in Patients with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2022, 19, 8211. https://doi.org/10.3390/ijerph19138211
Yen F-S, Hsu C-C, Shih Y-H, Pan W-L, Wei JC-C, Hwu C-M. Metformin and the Development of Asthma in Patients with Type 2 Diabetes. International Journal of Environmental Research and Public Health. 2022; 19(13):8211. https://doi.org/10.3390/ijerph19138211
Chicago/Turabian StyleYen, Fu-Shun, Chih-Cheng Hsu, Ying-Hsiu Shih, Wei-Lin Pan, James Cheng-Chung Wei, and Chii-Min Hwu. 2022. "Metformin and the Development of Asthma in Patients with Type 2 Diabetes" International Journal of Environmental Research and Public Health 19, no. 13: 8211. https://doi.org/10.3390/ijerph19138211
APA StyleYen, F.-S., Hsu, C.-C., Shih, Y.-H., Pan, W.-L., Wei, J. C.-C., & Hwu, C.-M. (2022). Metformin and the Development of Asthma in Patients with Type 2 Diabetes. International Journal of Environmental Research and Public Health, 19(13), 8211. https://doi.org/10.3390/ijerph19138211







