Associations between the COVID-19 Pandemic and Hospital Infrastructure Adaptation and Planning—A Scoping Review
Abstract
:1. Introduction
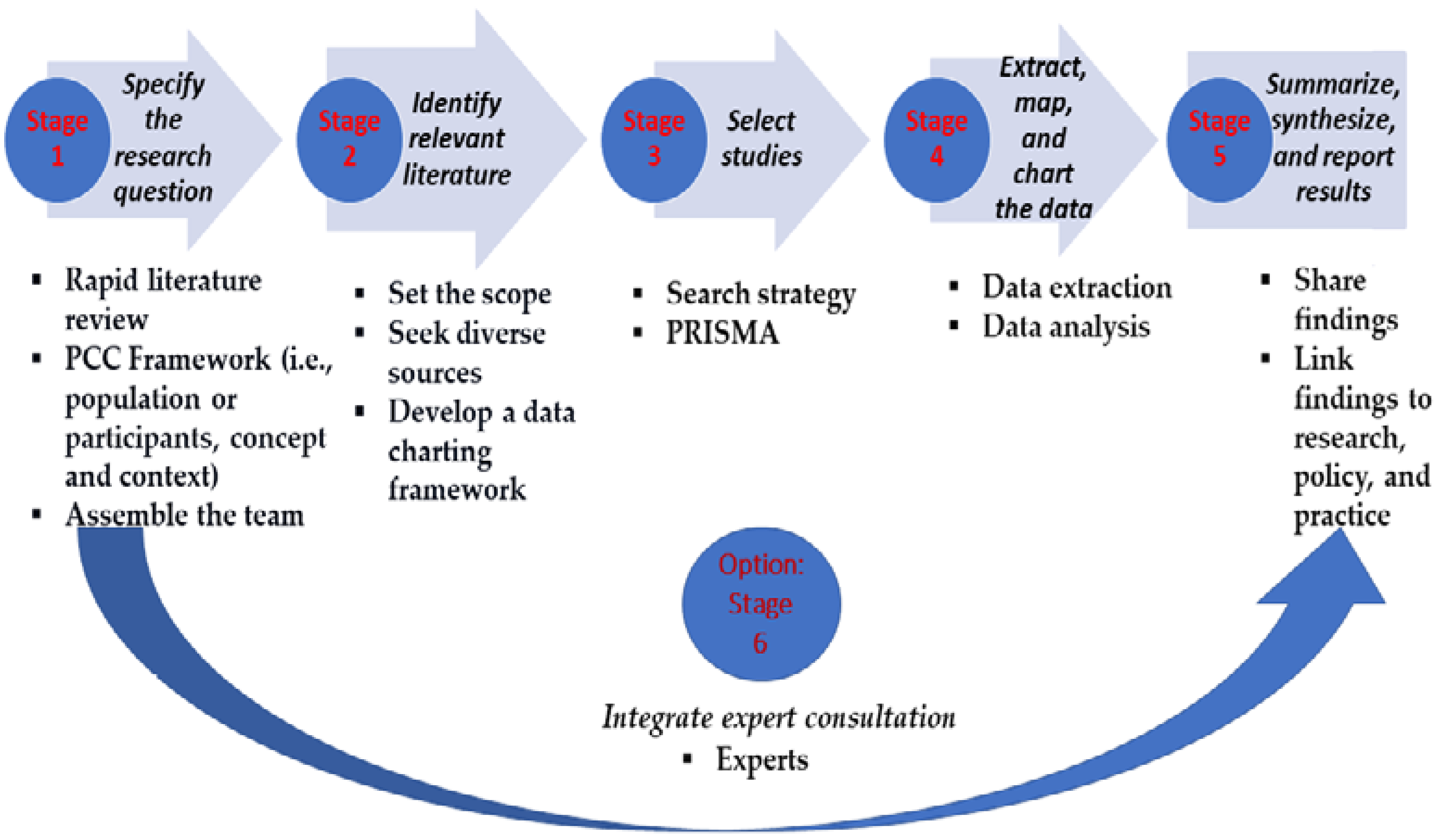
2. Materials and Methods
2.1. Definition of Research Questions (RQs)
- RQ1: What were the general characteristics of the study (authorship/title; date of publication; studies focusing on specific countries or with a regional or global approach)?
- RQ2: What was the design of the study?
- RQ3: What was (were) the objective (s)/type of association (s) assessed?
- RQ4: What was (were) the proposed solution(s), adaptation(s), concept/theoretical framework(s), or planning?
- RQ5: What limitations have been specified?
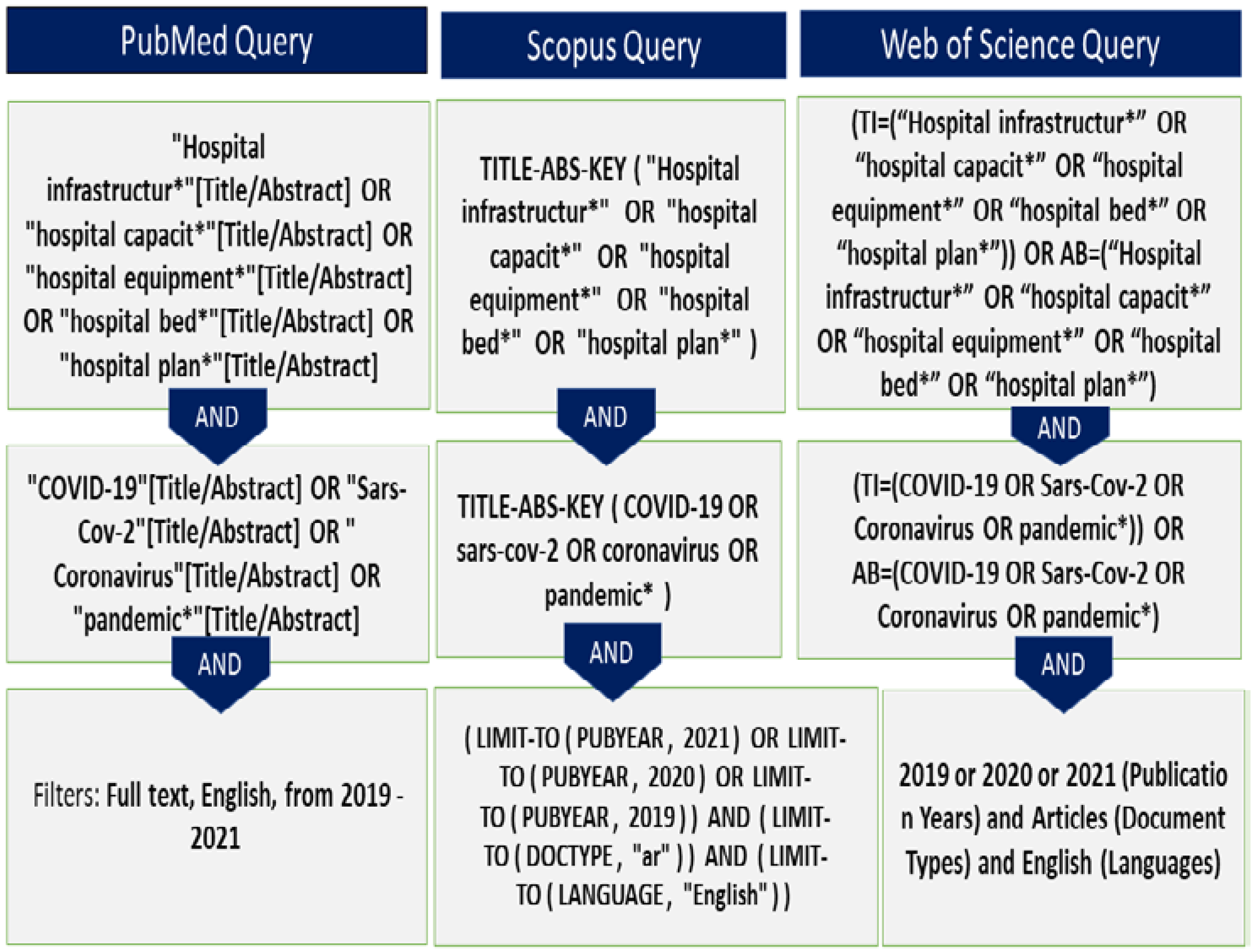
2.2. Identification of Studies
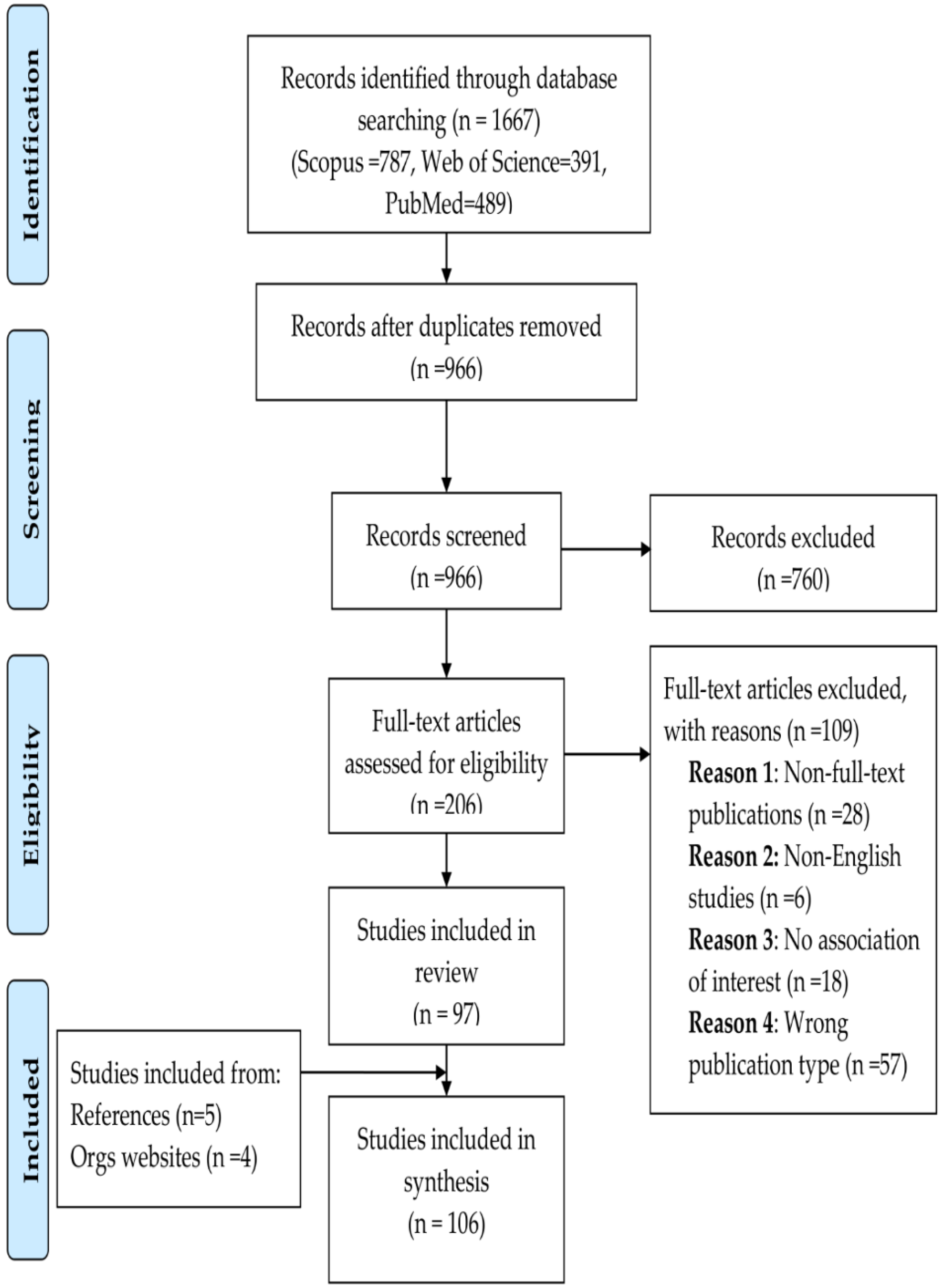
2.3. Selection of Studies
2.4. Extraction of Data and Charting
2.5. Results: Collating, Summarizing, and Reporting
2.6. Consultation
3. Results
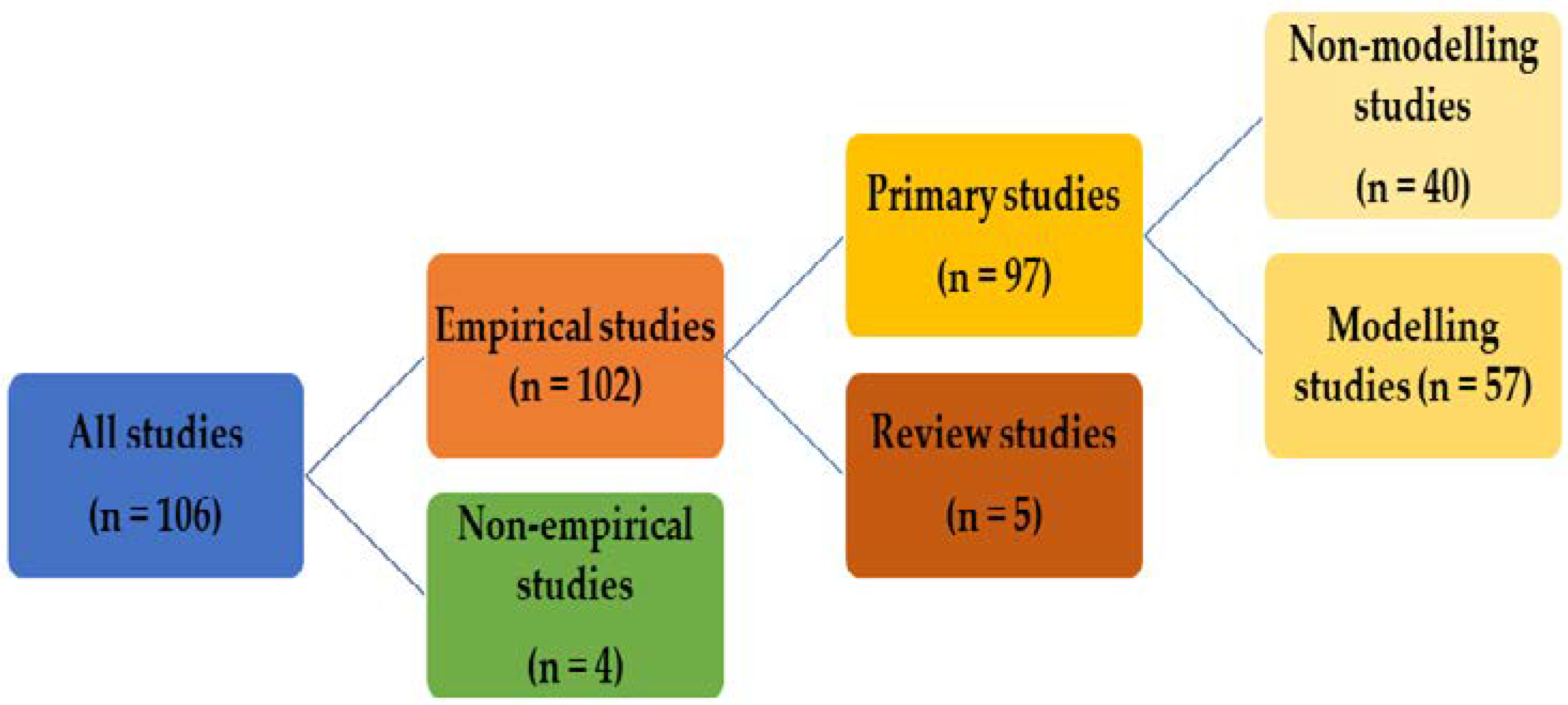
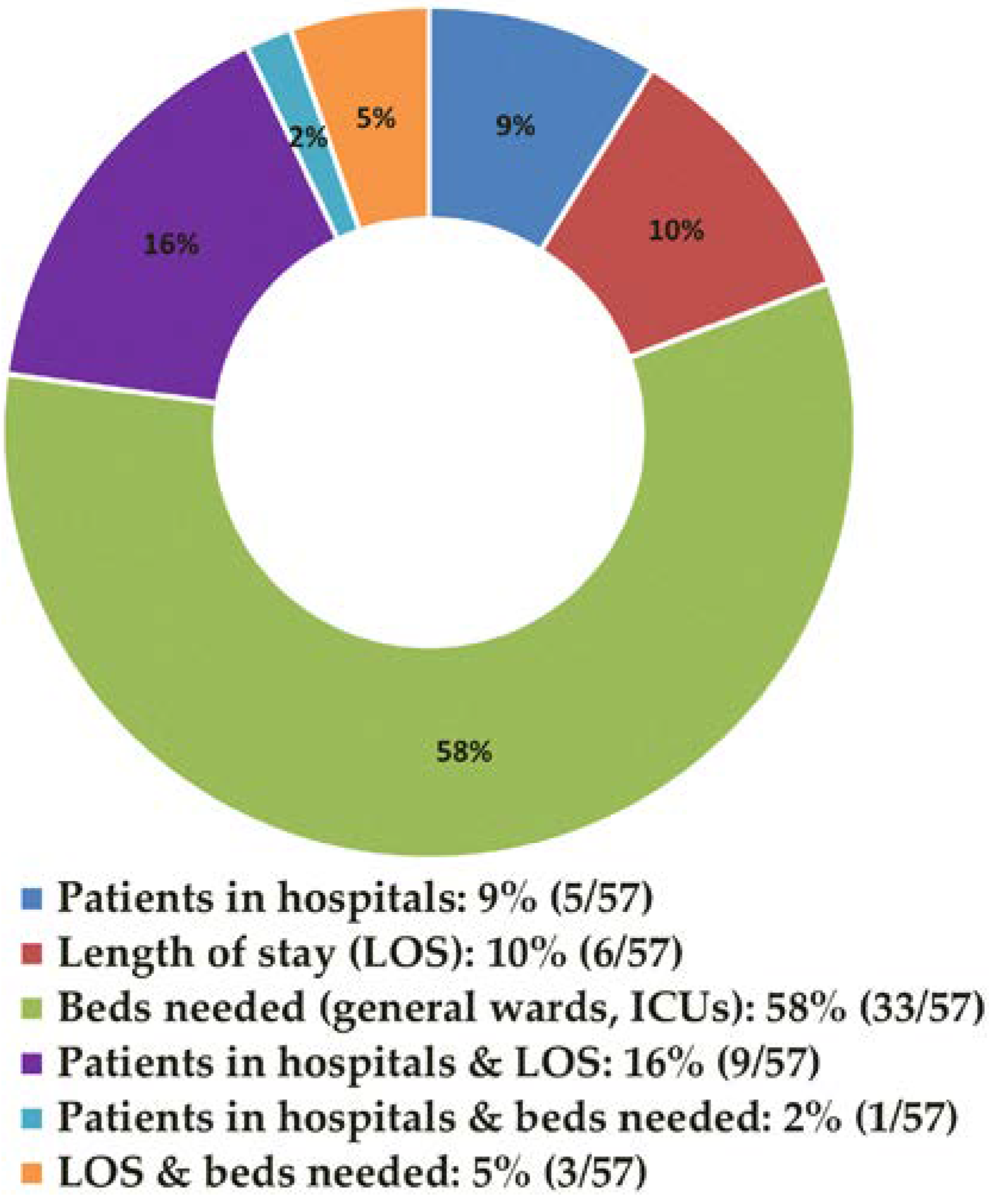
3.1. General Overview of the Findings
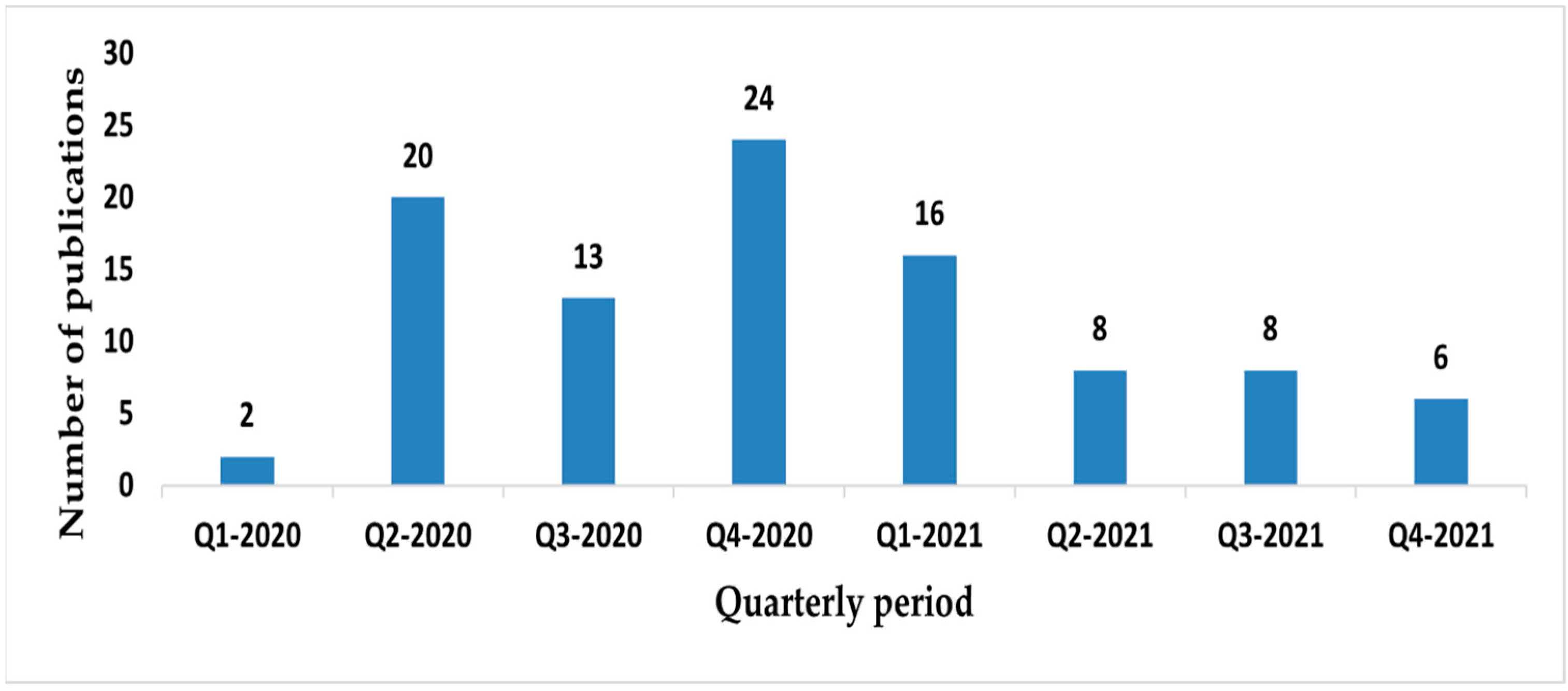
3.1.1. Number of Publications
3.1.2. Type of Publications
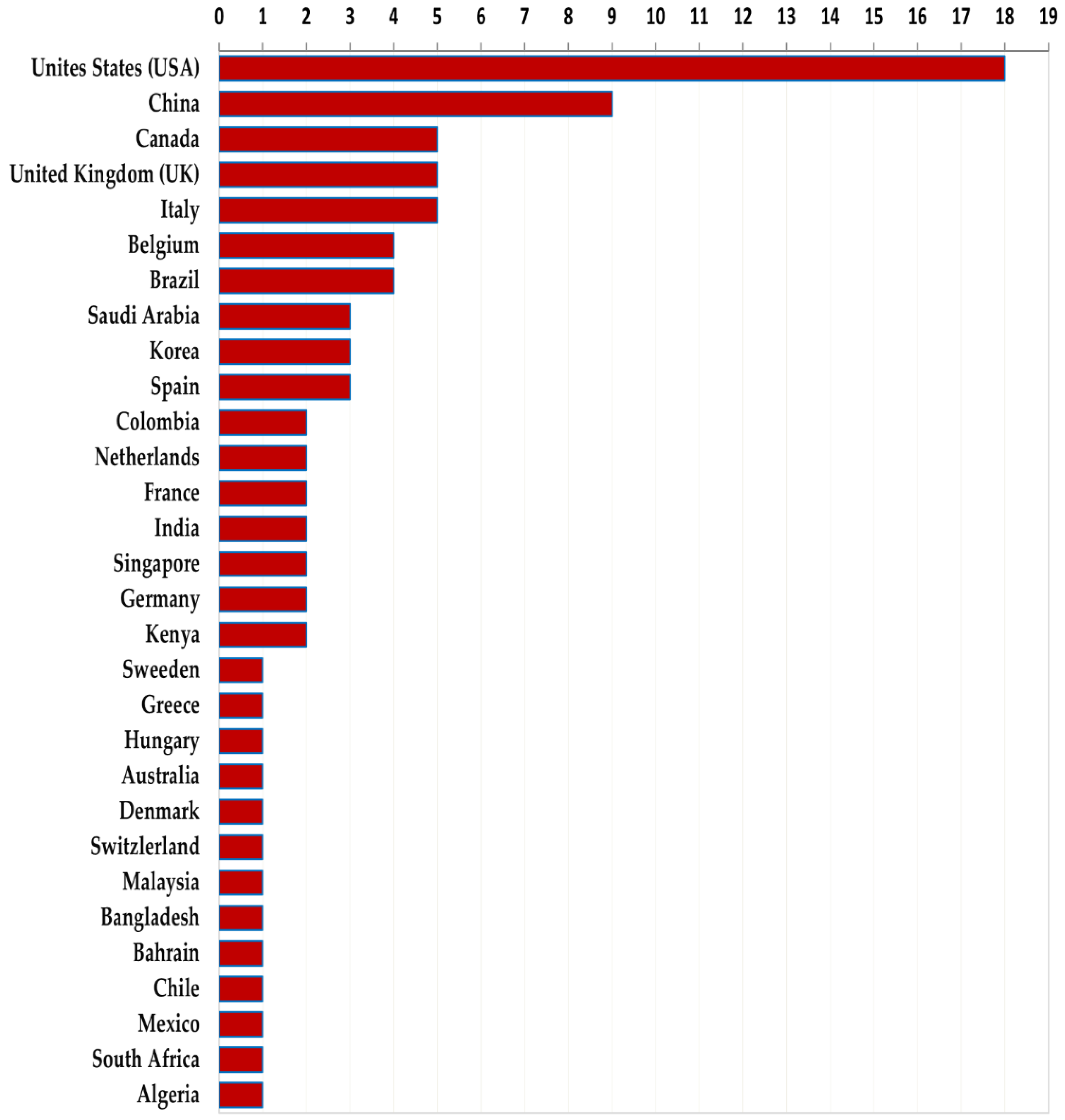
3.1.3. Geographical Distribution and Publication Period of Empirical Studies
3.2. Empirical Studies on Hospital Infrastructure Adaptation
3.3. Empirical Studies Predicting the Number of Hospital Beds (Modeling Studies)
4. Discussion
4.1. Summary of the Results
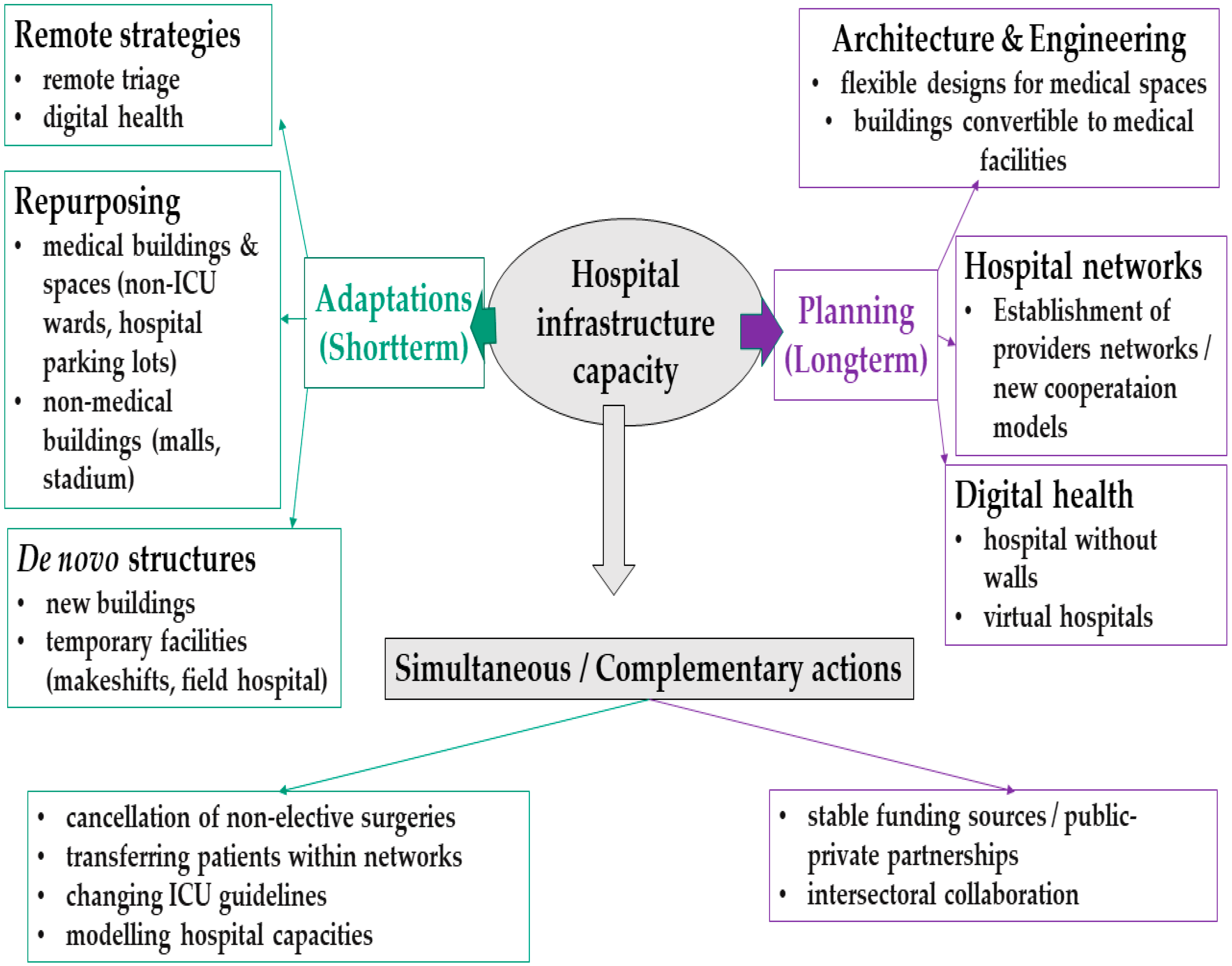
4.2. Hospital Infrastructure Adaptation Mechanisms
4.3. Hospital Infrastructure Planning
4.4. Study Limitations
4.5. Study Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aristodemou, K.; Buchhass, L.; Claringbould, D. The COVID-19 crisis in the EU: The resilience of healthcare systems, government responses and their socio-economic effects. Eurasian Econ. Rev. 2021, 11, 251–281. [Google Scholar] [CrossRef]
- Pitlik, S.D. COVID-19 compared to other pandemic diseases. Rambam Maimonides Med. J. 2020, 11, e0027. [Google Scholar] [CrossRef] [PubMed]
- Iftimie, S.; Lopez-Azcona, A.F.; Vallverdu, I.; Hernandez-Flix, S.; De Febrer, G.; Parra, S.; Hernandez-Aguilera, A.; Riu, F.; Joven, J.; Andreychuk, N.; et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS ONE 2021, 16, e0248029. [Google Scholar] [CrossRef] [PubMed]
- Cacciapaglia, G.; Cot, C.; Sannino, F. Second wave COVID-19 pandemics in Europe: A temporal playbook. Sci. Rep. 2020, 10, 15514. [Google Scholar] [CrossRef]
- Garzotto, F.; Ceresola, E.; Panagiotakopoulou, S.; Spina, G.; Menotto, F.; Benozzi, M.; Casarotto, M.; Lanera, C.; Bonavina, M.G.; Gregori, D.; et al. COVID-19: Ensuring our medical equipment can meet the challenge. Expert Rev. Med. Devices 2020, 17, 483–489. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Yang, J.; Wang, J.; Zhai, X.; Bärnighausen, T.; Wang, C. Fangcang shelter hospitals: A novel concept for responding to public health emergencies. Lancet 2020, 395, 1305–1314. [Google Scholar] [CrossRef]
- Capolongo, S.; Gola, M.; Brambilla, A.; Morganti, A.; Mosca, E.I.; Barach, P. COVID-19 and Healthcare Facilities: A Decalogue of Design Strategies for Resilient Hospitals. Acta Biomed. 2020, 91, 50–60. [Google Scholar] [CrossRef]
- Ndayishimiye, C.; Weitzel, T.; Middleton, J. What Has Been the Role of Makeshift and Mobile Health Care Facilities across Europe during COVID-19?—Cross-Country Analysis. Health System Response Monitor. 2021. Available online: https://analysis.covid19healthsystem.org/index.php/2021/02/16/what-has-been-the-role-of-makeshift-and-mobile-health-care-facilities-across-europe-during-covid-19/ (accessed on 20 December 2021).
- Winkelmann, J.; Webb, E.; Williams, G.A.; Hernández-Quevedo, C.; Maier, C.B.; Panteli, D. European countries’ responses in ensuring sufficient physical infrastructure and workforce capacity during the first COVID-19 wave. Health Policy 2021, 126, 362–372. [Google Scholar] [CrossRef]
- Tonna, J.E.; Hanson, H.A.; Cohan, J.N.; McCrum, M.L.; Horns, J.J.; Brooke, B.S.; Das, R.; Kelly, B.C.; Campbell, A.J.; Hotaling, J. Balancing revenue generation with capacity generation: Case distribution, financial impact and hospital capacity changes from cancelling or resuming elective surgeries in the US during COVID-19. BMC Health Serv. Res. 2020, 20, 1119. [Google Scholar] [CrossRef]
- Jefferson, T.; Heneghan, C. COVID-19: Fever Hospitals; Oxford University Centre for Evidence-Based Medicine: Oxford, UK, 2020. [Google Scholar]
- Oliver, D. David Oliver: Were Nightingale units and fever hospitals ever workable responses to COVID-19? BMJ 2021, 374, 10–11. [Google Scholar] [CrossRef]
- Luke, J.; Franklin, R.; Aitken, P.; Dyson, J. Safer Hospital Infrastructure Assessments for Socio-Natural Disaster–A Scoping Review. Prehospital Disaster Med. 2021, 36, 627–635. [Google Scholar] [CrossRef]
- McCabe, R.; Schmit, N.; Christen, P.; D’Aeth, J.C.; Løchen, A.; Rizmie, D.; Nayagam, S.; Miraldo, M.; Aylin, P.; Bottle, A.; et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020, 18, 329. [Google Scholar] [CrossRef]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; Group, C.W.; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef]
- Klein, M.G.; Cheng, C.J.; Lii, E.; Mao, K.; Mesbahi, H.; Zhu, T.; Muckstadt, J.A.; Hupert, N. COVID-19 Models for Hospital Surge Capacity Planning: A Systematic Review. Disaster Med. Public Health Prep. 2020, 16, 390–397. [Google Scholar] [CrossRef]
- Ravaghi, H.; Alidoost, S.; Mannion, R.; Bélorgeot, V.D. Models and methods for determining the optimal number of beds in hospitals and regions: A systematic scoping review. BMC Health Serv. Res. 2020, 20, 186. [Google Scholar] [CrossRef]
- Teare, G.; Taks, M. Extending the scoping review framework: A guide for interdisciplinary researchers. Int. J. Soc. Res. Methodol. 2020, 23, 311–315. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Open Science Framework, Open Science Framework Registries. Available online: https://osf.io/fms83 (accessed on 13 December 2021).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Platto, S.; Wang, Y.; Zhou, J.; Carafoli, E. History of the COVID-19 pandemic: Origin, explosion, worldwide spreading. Biochem. Biophys. Res. Commun. 2021, 538, 14–23. [Google Scholar] [CrossRef]
- Nebehay, S. Europe is Epicenter of Coronavirus Pandemic: WHO. Reuters. 2020. Available online: https://www.reuters.com/article/us-health-coronavirus-who-idUSKBN2102Q0 (accessed on 14 March 2021).
- British Broadcasting Corporation. Coronavirus: Europe Now Epicentre of the Pandemic, Says WHO. BBC. 2020. Available online: https://www.bbc.com/news/world-europe-51876784 (accessed on 14 March 2021).
- Johnson, N.; Phillips, M. Rayyan for systematic reviews. J. Electron. Resour. Librariansh. 2018, 30, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Akuamoa-Boateng, D.; Wegen, S.; Ferdinandus, J.; Marksteder, R.; Baues, C.; Marnitz, S. Managing patient flows in radiation oncology during the COVID-19 pandemic: Reworking existing treatment designs to prevent infections at a German hot spot area University Hospital. Strahlenther. Onkol. 2020, 196, 1080–1085. [Google Scholar] [CrossRef]
- Whiteside, T.; Kane, E.; Aljohani, B.; Alsamman, M.; Pourmand, A. Redesigning emergency department operations amidst a viral pandemic. Am. J. Emerg. Med. 2020, 38, 1448–1453. [Google Scholar] [CrossRef]
- Lacasa, L.; Challen, R.; Brooks-Pollock, E.; Danon, L. A flexible method for optimising sharing of healthcare resources and demand in the context of the COVID-19 pandemic. PLoS ONE 2020, 15, e0241027. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Aldawood, A.S.; Almatrood, A.; Burrows, V.; Naidu, B.; Alchin, J.D.; Alhumedi, H.; Tashkandi, N.; Al-Jahdali, H.; Hussain, A.; et al. Managing critical care during COVID-19 pandemic: The experience of an ICU of a tertiary care hospital. J. Infect. Public Health 2021, 14, 1635–1641. [Google Scholar] [CrossRef]
- Nogués, X.; Sánchez-Martinez, F.; Castells, X.; Díez-Pérez, A.; Sabaté, R.A.; Petit, I.; Brasé, A.; Horcajada, J.P.; Güerri-Fernández, R.; Pascual, J.; et al. Hospital-at-Home Expands Hospital Capacity during COVID-19 Pandemic. J. Am. Med. Dir. Assoc. 2021, 22, 939–942. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, K.S.; Kim, K.; Lee, J.J.; Kim, J.-Y. A brief telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea. J. Korean Med. Sci. 2020, 35, e152. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, M.; Hu, L.; Liu, X.; Zhuo, L.; Xie, Q. Emergency reconstruction of large general hospital under the perspective of new COVID-19 prevention and control. Wien. Klin. Wochenschr. 2020, 132, 677–684. [Google Scholar] [CrossRef]
- Witcher, T.R. Swift Support. Civ. Eng. Mag. Arch. 2020, 90, 76–79. [Google Scholar] [CrossRef]
- Van Goethem, N.; Vilain, A.; Wyndham-Thomas, C.; Deblonde, J.; Bossuyt, N.; Lernout, T.; Rebolledo Gonzalez, J.; Quoilin, S.; Melis, V.; Van Beckhoven, D. Rapid establishment of a national surveillance of COVID-19 hospitalizations in Belgium. Arch. Public Health 2020, 78, 121. [Google Scholar] [CrossRef]
- Aziz, S.; Arabi, Y.M.; Alhazzani, W.; Evans, L.; Citerio, G.; Fischkoff, K.; Salluh, J.; Meyfroidt, G.; Alshamsi, F.; Oczkowski, S.; et al. Managing ICU surge during the COVID-19 crisis: Rapid guidelines. Intensive Care Med. 2020, 46, 1303–1325. [Google Scholar] [CrossRef]
- Bamias, G.; Lagou, S.; Gizis, M.; Karampekos, G.; Kyriakoulis, K.G.; Pontas, C.; Mantzaris, G.J. The Greek response to COVID-19: A true success story from an IBD perspective. Inflamm. Bowel Dis. 2020, 26, 1144–1148. [Google Scholar] [CrossRef]
- Borgen, I.; Romney, M.C.; Redwood, N.; Delgado, B.; Alea, P.; George, B.H.; Puzziferro, J.; Shihabuddin, L. From Hospital to Home: An Intensive Transitional Care Management Intervention for Patients with COVID-19. Popul. Health Manag. 2021, 24, 27–34. [Google Scholar] [CrossRef]
- Tan, Y.H.; Liao, Y.; Tan, Z.; Li, K.-H.H. Application of a Machine Learning Algorithms in a Wrist-Wearable Sensor for Patient Health Monitoring during Autonomous Hospital Bed Transport. Sensors 2021, 21, 5711. [Google Scholar] [CrossRef]
- Hron, J.D.; Parsons, C.R.; Williams, L.A.; Harper, M.B.; Bourgeois, F.C. Rapid Implementation of an Inpatient Telehealth Program during the COVID-19 Pandemic. Appl. Clin. Inform. 2020, 11, 452–459. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Li, C.; Chen, K.; Zhang, M. Ultra-rapid delivery of specialty field hospitals to combat COVID-19: Lessons learned from the Leishenshan Hospital project in Wuhan. Autom. Constr. 2020, 119, 103345. [Google Scholar] [CrossRef]
- Perondi, B.; Miethke-Morais, A.; Montal, A.C.; Harima, L.; Segurado, A.C. Setting up hospital care provision to patients with COVID-19: Lessons learnt at a 2400-bed academic tertiary center in São Paulo, Brazil. Braz. J. Infect. Dis. 2020, 24, 570–574. [Google Scholar] [CrossRef]
- Af Ugglas, B.; Skyttberg, N.; Wladis, A.; Djärv, T.; Holzmann, M.J. Emergency department crowding and hospital transformation during COVID-19, a retrospective, descriptive study of a university hospital in Stockholm, Sweden. Scand. J. Trauma. Resusc. Emerg. Med. 2020, 28, 107. [Google Scholar] [CrossRef]
- Raith, E.P.; Luoma, A.M.V.; Earl, M.; Dalal, M.; Fairley, S.; Fox, F.; Hunt, K.; Willett, C.; Reddy, U. Repurposing a neurocritical care unit for the management of severely ill patients with COVID-19: A retrospective evaluation. J. Neurosurg. Anesthesiol. 2021, 33, 77–81. [Google Scholar] [CrossRef]
- Brown, D.R.; Hennecke, P.; Nottebrock, D.; Dhillon, P. Vancouver Convention Health Centre (COVID-19 Response): Planning, implementation, and four lessons learned. Am. J. Disaster Med. 2020, 15, 143–148. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Park, J.S.; Kim, H.A.; Hyun, M.; Suh, Y.-S.; Nam, S.I.; Chung, W.J.; Cho, C.-H. Lessons from a COVID-19 hospital, Republic of Korea. Bull. World Health Organ. 2020, 98, 842–848. [Google Scholar] [CrossRef]
- Louri, N.A.; Alkhan, J.A.; Isa, H.H.; Asad, Y.; Alsharooqi, A.; Alomari, K.A.; Hasan, N.K.; Al Khalifa, F.B.K.; Ahmed, G.F.; Alasmi, M.Y.; et al. Establishing a 130-Bed Field Intensive Care Unit to Prepare for COVID-19 in 7 Days in Bahrain Military Hospital. Disaster Med. Public Health Prep. 2021, 15, e34–e43. [Google Scholar] [CrossRef]
- Marcon, E.; Scotton, F.; Marcante, E.; Rigo, A.; Monticelli, J.; Buggio, M.E.; Pilerci, C.; Montemurro, D.; Benini, P. Schiavonia Hospital response to COVID-19 outbreak: A first single-center experience. Ann. Ist. Super. Sanita 2020, 56, 365–372. [Google Scholar] [CrossRef]
- Joshi, M.; Kulkarni, M. Evaluation and Planning for a 250 Bedded COVID-19 Healthcare Infrastructure in City of Gurgaon, India. Hosp. Top. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Franke, G.; Knobling, B.; Brill, F.; Becker, B.; Klupp, E.; Campos, C.B.; Pfefferle, S.; Lütgehetmann, M.; Knobloch, J. An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. J. Hosp. Infect. 2021, 112, 108–113. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.; Hwang, J. Quarantine Facility for Patients with COVID-19 with Mild Symptoms in Korea: Experience from Eighteen Residential Treatment Centers. J. Korean Med. Sci. 2020, 35, e429. [Google Scholar] [CrossRef]
- Christen, P.; D’Aeth, J.C.; Løchen, A.; McCabe, R.; Rizmie, D.; Schmit, N.; Nayagam, S.; Miraldo, M.; Aylin, P.; Bottle, A.; et al. The J-IDEA Pandemic Planner: A Framework for Implementing Hospital Provision Interventions during the COVID-19 Pandemic. Med. Care 2021, 59, 371–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Cao, Y.; Chen, H.; Wang, X.; Sun, G. Wuhan mobile cabin hospital: A critical health policy at a critical time in China. Medicine 2021, 100, e24077. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Xiao, K.; Zhang, H.; Tian, Y.; Clifford, S.P.; Xu, J.; Huang, J. Establishing and Managing a Temporary Coronavirus Disease 2019 Specialty Hospital in Wuhan, China. Anesthesiology 2020, 132, 1339–1345. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Huang, T.; Liu, X.; Xu, G. The effects of “Fangcang, Huoshenshan, and Leishenshan” hospitals and environmental factors on the mortality of COVID-19. PeerJ 2020, 8, e9578. [Google Scholar] [CrossRef]
- Barasa, E.W.; Ouma, P.O.; Okiro, E.A. Assessing the hospital surge capacity of the Kenyan health system in the face of the COVID-19 pandemic. PLoS ONE 2020, 15, e0236308. [Google Scholar] [CrossRef] [PubMed]
- Poeran, J.; Zhong, H.; Wilson, L.; Liu, J.; Memtsoudis, S.G. Cancellation of Elective Surgery and Intensive Care Unit Capacity in New York State: A Retrospective Cohort Analysis. Anesth. Analg. 2020, 131, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Lefrant, J.-Y.; Fischer, M.-O.; Potier, H.; Degryse, C.; Jaber, S.; Muller, L.; Pottecher, J.; Charboneau, H.; Meaudre, E.; Lanot, P.; et al. A national healthcare response to intensive care bed requirements during the COVID-19 outbreak in France. Anaesth. Crit. Care Pain Med. 2020, 39, 709–715. [Google Scholar] [CrossRef]
- Fang, D.; Pan, S.; Li, Z.; Yuan, T.; Jiang, B.; Gan, D.; Sheng, B.; Han, J.; Wang, T.; Liu, Z. Large-scale public venues as medical emergency sites in disasters: Lessons from COVID-19 and the use of Fangcang shelter hospitals in Wuhan, China. BMJ Glob. Health 2020, 5, e002815. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Azoulay, E.; Al-Dorzi, H.M.; Phua, J.; Salluh, J.; Binnie, A.; Hodgson, C.; Angus, D.C.; Cecconi, M.; Du, B.; et al. How the COVID-19 pandemic will change the future of critical care. Intensive Care Med. 2021, 47, 282–291. [Google Scholar] [CrossRef]
- Tadavarthy, S.N.; Finnegan, K.; Bernatowicz, G.; Lowe, E.; Coffin, S.E.; Manning, M. Developing and implementing an infection prevention and control program for a COVID-19 alternative care site in Philadelphia, PA. Am. J. Infect. Control 2021, 49, 77–81. [Google Scholar] [CrossRef]
- Hickey, S.; Mathews, K.S.; Siller, J.; Sueker, J.; Thakore, M.; Ravikumar, D.; Olmedo, R.E.; McGreevy, J.; Kohli-Seth, R.; Carr, B.; et al. Rapid deployment of an emergency department-intensive care unit for the COVID-19 pandemic. Clin. Exp. Emerg. Med. 2020, 7, 319–325. [Google Scholar] [CrossRef]
- Emmanuel, U.; Osondu, E.D.; Kalu, K.C. Architectural design strategies for infection prevention and control (IPC) in health-care facilities: Towards curbing the spread of COVID-19. J. Environ. Health Sci. Eng. 2020, 18, 1699–1707. [Google Scholar] [CrossRef]
- Rivera-Rodriguez, C.; Urdinola, B.P. Predicting Hospital Demand during the COVID-19 Outbreak in Bogotá, Colombia. Front. Public Health 2020, 8, 582706. [Google Scholar] [CrossRef]
- Gitto, S.; Di Mauro, C.; Ancarani, A.; Mancuso, P. Forecasting national and regional level intensive care unit bed demand during COVID-19: The case of Italy. PLoS ONE 2021, 16, e0247726. [Google Scholar] [CrossRef]
- Gombos, K.; Herczeg, R.; Erőss, B.; Kovács, S.Z.; Uzzoli, A.; Nagy, T.; Kiss, S.; Szakács, Z.; Imrei, M.; Szentesi, A.; et al. Translating Scientific Knowledge to Government Decision Makers Has Crucial Importance in the Management of the COVID-19 Pandemic. Popul. Health Manag. 2021, 24, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, T.; Tirtha, S.D.; Iraganaboina, N.C.; Eluru, N. A comprehensive analysis of COVID-19 transmission and mortality rates at the county level in the United States considering socio-demographics, health indicators, mobility trends and health care infrastructure attributes. PLoS ONE 2021, 16, e0249133. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.H. Locally Informed Simulation to Predict Hospital Capacity Needs during the COVID-19 Pandemic. Ann. Intern. Med. 2020, 173, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.M.; Duval, A.; Pouwels, K.; Guillemot, D.; Fernandes, J.; Huynh, B.-T.; Temime, L.; Opatowski, L. Optimizing COVID-19 surveillance in long-term care facilities: A modelling study. BMC Med. 2020, 18, 386. [Google Scholar] [CrossRef]
- Qian, Z.; Alaa, A.M.; van der Schaar, M. CPAS: The UK’s national machine learning-based hospital capacity planning system for COVID-19. Mach. Learn. 2020, 110, 15–35. [Google Scholar] [CrossRef]
- de Barros Braga, M.; da Silva Fernandes, R.; de Souza, G.N.; da Rocha, J.E.C.; Dolácio, C.J.F.; da Silva Tavares, I.; Pinheiro, R.R.; Noronha, F.N.; Rodrigues, L.L.S.; Ramos, R.T.J.; et al. Artificial neural networks for short-term forecasting of cases, deaths, and hospital beds occupancy in the COVID-19 pandemic at the Brazilian Amazon. PLoS ONE 2021, 16, e0248161. [Google Scholar] [CrossRef]
- Guzzi, P.H.; Tradigo, G.; Veltri, P. Spatio-temporal resource mapping for intensive care units at regional level for COVID-19 emergency in Italy. Int. J. Environ. Res. Public Health 2020, 17, 3344. [Google Scholar] [CrossRef]
- Salles Neto, L.L.; Martins, C.B.; Chaves, A.A.; Konstantyner, T.C.R.O.; Yanasse, H.H.; Campos, C.B.L.; Bellini, A.J.O.; Butkeraites, R.B.; Correia, L.; Magro, I.L.; et al. Forecast UTI: Application for predicting intensive care unit beds in the context of the COVID-19 pandemic. Epidemiol. Serv. Saude Rev. Sist. Unico Saude Bras. 2020, 29, e2020391. [Google Scholar] [CrossRef]
- Barrett, K.; Khan, Y.A.; Mac, S.; Ximenes, R.; Naimark, D.M.; Sander, B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. Can. Med. Assoc. J. 2020, 192, E640–E646. [Google Scholar] [CrossRef]
- Iragorri, N.; Gómez-Restrepo, C.; Barrett, K.; Herrera, S.; Hurtado, I.; Khan, Y.; Mac, S.; Naimark, D.; Pechlivanoglou, P.; Rosselli, D.; et al. COVID-19: Adaptation of a model to predict healthcare resource needs in valle del Cauca, Colombia|COVID-19: Adaptación de un modelo para predecir las necesidades de recursos de salud en el valle del cauca, Colombia. Colomb. Med. 2020, 51, e204534. [Google Scholar] [CrossRef]
- Zhao, C.; Tepekule, B.; Criscuolo, N.G.; Wendel Garcia, P.D.; Hilty, M.P.; Risc-Icu Consortium Investigators In Switzerland; Fumeaux, T.; Van Boeckel, T. icumonitoring.ch: A platform for short-term forecasting of intensive care unit occupancy during the COVID-19 epidemic in Switzerland. Swiss Med. Wkly. 2020, 150, w20277. [Google Scholar] [CrossRef] [PubMed]
- Akakba, A.; Lahmar, B. The use of geocoding for home healthcare application and management an epidemic situation: Case of COVID-19 virus outbreak. Geogr. Pannonica 2020, 24, 285–293. [Google Scholar] [CrossRef]
- Presanis, A.M.; Kunzmann, K.; Grosso, F.M.; Jackson, C.H.; Corbella, A.; Grasselli, G.; Salmoiraghi, M.; Gramegna, M.; De Angelis, D.; Cereda, D. Risk factors associated with severe hospital burden of COVID-19 disease in Regione Lombardia: A cohort study. BMC Infect. Dis. 2021, 21, 1041. [Google Scholar] [CrossRef]
- Calabuig, J.M.; Jiménez-Fernández, E.; Sánchez-Pérez, E.A.; Manzanares, S. Modeling Hospital Resource Management during the COVID-19 Pandemic: An Experimental Validation. Econometrics 2021, 9, 38. [Google Scholar] [CrossRef]
- Weissman, G.E.; Crane-Droesch, A.; Chivers, C.; Luong, T.; Hanish, A.; Levy, M.Z.; Lubken, J.; Becker, M.; Draugelis, M.E.; Anesi, G.L.; et al. Locally Informed Simulation to Predict Hospital Capacity Needs during the COVID-19 Pandemic. Ann. Intern. Med. 2020, 173, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; McDonald, S.; Bale, S.; Luteijn, M.; Sarkar, R. Construction of a demand and capacity model for intensive care and hospital ward beds, and mortality from COVID-19. BMC Med. Inform. Decis. Mak. 2021, 21, 138. [Google Scholar] [CrossRef]
- Berta, P.; Paruolo, P.; Verzillo, S.; Lovaglio, P.G. A bivariate prediction approach for adapting the health care system response to the spread of COVID-19. PLoS ONE 2020, 15, e0240150. [Google Scholar] [CrossRef]
- Lorenzen, S.S.; Nielsen, M.; Jimenez-Solem, E.; Petersen, T.S.; Perner, A.; Thorsen-Meyer, H.-C.; Igel, C.; Sillesen, M. Using machine learning for predicting intensive care unit resource use during the COVID-19 pandemic in Denmark. Sci. Rep. 2021, 11, 18959. [Google Scholar] [CrossRef]
- Shoukat, A.; Wells, C.R.; Langley, J.M.; Singer, B.H.; Galvani, A.P.; Moghadas, S.M. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. Can. Med Assoc. J. 2020, 192, E489–E496. [Google Scholar] [CrossRef] [Green Version]
- Locey, K.J.; Webb, T.A.; Khan, J.; Antony, A.K.; Hota, B. An interactive tool to forecast US hospital needs in the coronavirus 2019 pandemic. JAMIA Open 2020, 3, 506–512. [Google Scholar] [CrossRef]
- Bhandari, S.; Tak, A.; Singhal, S.; Shukla, J.; Shaktawat, A.S.; Gupta, J.; Patel, B.; Kakkar, S.; Dube, A.; Dia, S.; et al. Patient Flow Dynamics in Hospital Systems during Times of COVID-19: Cox Proportional Hazard Regression Analysis. Front. Public Health 2020, 8, 585850. [Google Scholar] [CrossRef] [PubMed]
- Capistran, M.A.; Capella, A.; Christen, J.A. Forecasting hospital demand in metropolitan areas during the current COVID-19 pandemic and estimates of lockdown-induced 2nd waves. PLoS ONE 2021, 16, e0245669. [Google Scholar] [CrossRef] [PubMed]
- McCabe, R.; Kont, M.D.; Schmit, N.; Whittaker, C.; Løchen, A.; Baguelin, M.; Knock, E.; Whittles, L.K.; Lees, J.; Brazeau, N.F.; et al. Modelling intensive care unit capacity under different epidemiological scenarios of the COVID-19 pandemic in three Western European countries. Int. J. Epidemiol. 2021, 50, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Endres-Dighe, S.; Jones, K.; Hadley, E.; Preiss, A.; Kery, C.; Stoner, M.; Eversole, S.; Rhea, S. Lessons learned from the rapid development of a statewide simulation model for predicting COVID-19′s impact on healthcare resources and capacity. PLoS ONE 2021, 16, e0260310. [Google Scholar] [CrossRef]
- Fort, D.; Seoane, L.; Unis, G.D.; Price-Haywood, E.G. Locally Informed Modeling to Predict Hospital and Intensive Care Unit Capacity during the COVID-19 Epidemic. Ochsner J. 2020, 20, 285–292. [Google Scholar] [CrossRef]
- Ebinger, J.; Wells, M.; Ouyang, D.; Davis, T.; Kaufman, N.; Cheng, S.; Chugh, S. A Machine Learning Algorithm Predicts Duration of hospitalization in COVID-19 patients. Intell. Med. 2021, 5, 100035. [Google Scholar] [CrossRef]
- Verelst, F.; Kuylen, E.; Beutels, P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID-19) cases, March 2020. Eurosurveillance 2020, 25, 2000323. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Song, K.; Yang, B.; Gao, Y.; Gao, X. Preliminary Assessment of the COVID-19 Outbreak Using 3-Staged Model e-ISHR. J. Shanghai Jiaotong Univ. 2020, 25, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.M.; McWilliams, C.J.; Thomas, M.J.; Bourdeaux, C.P.; Vasilakis, C. COVID-19 scenario modelling for the mitigation of capacity-dependent deaths in intensive care. Health Care Manag. Sci. 2020, 23, 315–324. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, A.; Wang, X.; Cheke, R.A.; Xiao, Y.; Tang, S. Impact of Hospital Bed Shortages on the Containment of COVID-19 in Wuhan. Int. J. Environ. Res. Public Health 2020, 17, 8560. [Google Scholar] [CrossRef]
- Alqahtani, F.; Khan, A.; Alowais, J.; Alaama, T.; Jokhdar, H. Bed Surge Capacity in Saudi Hospitals during the COVID-19 Pandemic. Disaster Med. Public Health Prep. 2021, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Wang, L.; Ma, H.; Yiu, K.C.; Paterson, J.M.; Kim, E.; Schull, M.J.; Pequegnat, V.; Lee, A.; Ishiguro, L.; et al. Estimated surge in hospital and intensive care admission because of the coronavirus disease 2019 pandemic in the Greater Toronto Area, Canada: A mathematical modelling study. CMAJ Open 2020, 8, E593–E604. [Google Scholar] [CrossRef] [PubMed]
- Faes, C.; Hens, N.; Gilbert, M. On the timing of interventions to preserve hospital capacity: Lessons to be learned from the Belgian SARS-CoV-2 pandemic in 2020. Arch. Public Health 2021, 79, 164. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Heng, K.; Shawon, S.R.; Goh, G.; Okonofua, D.; Ochoa-Rosales, C.; Gonzalez-Jaramillo, V.; Bhuiya, A.; Reidpath, D.; Prathapan, S.; et al. Dynamic interventions to control COVID-19 pandemic: A multivariate prediction modelling study comparing 16 worldwide countries. Eur. J. Epidemiology 2020, 35, 389–399. [Google Scholar] [CrossRef]
- Deschepper, M.; Eeckloo, K.; Malfait, S.; Benoit, D.; Callens, S.; Vansteelandt, S. Prediction of hospital bed capacity during the COVID− 19 pandemic. BMC Health Serv. Res. 2021, 21, 468. [Google Scholar] [CrossRef]
- Chin, V.; Samia, N.I.; Marchant, R.; Rosen, O.; Ioannidis, J.P.A.; Tanner, M.A.; Cripps, S. A case study in model failure? COVID-19 daily deaths and ICU bed utilisation predictions in New York state. Eur. J. Epidemiol. 2020, 35, 733–742. [Google Scholar] [CrossRef]
- Goic, M.; Bozanic-Leal, M.S.; Badal, M.; Basso, L.J. COVID-19: Short-term forecast of ICU beds in times of crisis. PLoS ONE 2021, 16, e0245272. [Google Scholar] [CrossRef]
- Baas, S.; Dijkstra, S.; Braaksma, A.; van Rooij, P.; Snijders, F.J.; Tiemessen, L.; Boucherie, R.J. Real-time forecasting of COVID-19 bed occupancy in wards and Intensive Care Units. Health Care Manag. Sci. 2021, 24, 402–419. [Google Scholar] [CrossRef]
- Cheng, F.-Y.; Joshi, H.; Tandon, P.; Freeman, R.; Reich, D.L.; Mazumdar, M.; Kohli-seth, R.; Levin, M.; Timsina, P.; Kia, A. Using machine learning to predict ICU transfer in hospitalized COVID-19 patients. J. Clin. Med. 2020, 9, 1668. [Google Scholar] [CrossRef]
- Manca, D.; Caldiroli, D.; Storti, E. A simplified math approach to predict ICU beds and mortality rate for hospital emergency planning under COVID-19 pandemic. Comput. Chem. Eng. 2020, 140, 106945. [Google Scholar] [CrossRef]
- COVID, I.; Murray, C.J.L. Forecasting the impact of the first wave of the COVID-19 pandemic on hospital demand and deaths for the USA and European Economic Area countries. MedRxiv 2020, preprint. [Google Scholar] [CrossRef] [Green Version]
- Vekaria, B.; Overton, C.; Wiśniowski, A.; Ahmad, S.; Aparicio-Castro, A.; Curran-Sebastian, J.; Eddleston, J.; Hanley, N.A.; House, T.; Kim, J.; et al. Hospital length of stay for COVID-19 patients: Data-driven methods for forward planning. BMC Infect. Dis. 2021, 21, 700. [Google Scholar] [CrossRef] [PubMed]
- López-Cheda, A.; Jácome, M.-A.; Cao, R.; De Salazar, P.M. Estimating lengths-of-stay of hospitalized COVID-19 patients using a non-parametric model: A case study in Galicia (Spain). Epidemiol. Infect. 2021, 149, e102. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Wood, J.; Brown, D.; Shearer, F.M.; Black, A.J.; Glass, K.; Cheng, A.C.; McCaw, J.M.; McVernon, J. Coronavirus Disease Model to Inform Transmission-Reducing Measures and Health System Preparedness, Australia. Emerg. Infect. Dis. 2020, 26, 2844–2853. [Google Scholar] [CrossRef]
- Bentout, S.; Tridane, A.; Djilali, S.; Touaoula, T.M. Age-Structured Modeling of COVID-19 Epidemic in the USA, UAE and Algeria. Alex. Eng. J. 2021, 60, 401–411. [Google Scholar] [CrossRef]
- Bhowmik, T.; Eluru, N. A comprehensive county level model to identify factors affecting hospital capacity and predict future hospital demand. Sci. Rep. 2021, 11, 23098. [Google Scholar] [CrossRef]
- Bekker, R.; Broek, M.U.H.; Koole, G. Modeling COVID-19 hospital admissions and occupancy in the Netherlands. Eur. J. Oper. Res. 2022, in press. [Google Scholar] [CrossRef]
- Knafo, W. COVID-19: Monitoring the propagation of the first waves of the pandemic. 4Open 2020, 3, 5. [Google Scholar] [CrossRef]
- Alqahtani, R.T. Mathematical model of SIR epidemic system (COVID-19) with fractional derivative: Stability and numerical analysis. Adv. Differ. Equ. 2021, 2021, 2. [Google Scholar] [CrossRef]
- Wells, C.M.; Zhang, Z.; Spano-Szekely, L.; Siller, J.; Brannon, H.; Schulz, K.; Scott, C.; Dolphy, M.; Hughes, E.; Kohli-Seth, R. Tiered model of nurse staffing for critical care and emergency departments in the wake of a pandemic. J. Nurs. Adm. 2021, 51, E1–E5. [Google Scholar] [CrossRef]
- Aborujilah, A.; Elsebaie, A.-E.F.M.; Mokhtar, S.A. IoT MEMS: IoT-Based Paradigm for Medical Equipment Management Systems of ICUs in Light of COVID-19 Outbreak. IEEE Access 2021, 9, 131120–131133. [Google Scholar] [CrossRef]
- Tembine, H. COVID-19: Data-Driven Mean-Field-Type Game Perspective. Games 2020, 11, 51. [Google Scholar] [CrossRef]
- Gel, E.S.; Jehn, M.; Lant, T.; Muldoon, A.R.; Nelson, T.; Ross, H.M. COVID-19 healthcare demand projections: Arizona. PLoS ONE 2020, 15, e0242588. [Google Scholar] [CrossRef]
- Bayraktar, Y.; Özyılmaz, A.; Toprak, M.; Işık, E.; Büyükakın, F.; Olgun, M.F. Role of the Health System in Combating COVID-19: Cross-Section Analysis and Artificial Neural Network Simulation for 124 Country Cases. Soc. Work Public Health 2020, 36, 178–193. [Google Scholar] [CrossRef]
- Mokhele, T.; Sewpaul, R.; Sifunda, S.; Weir-Smith, G.; Dlamini, S.; Manyaapelo, T.; Naidoo, I.; Parker, W.-A.; Dukhi, N.; Jooste, S.; et al. Spatial Analysis of Perceived Health System Capability and Actual Health System Capacity for COVID-19 in South Africa. Open Public Health J. 2021, 14, 388–398. [Google Scholar] [CrossRef]
- Sim, S.S.; Yip, M.Y.T.; Wang, Z.; Tan, A.C.S.; Tan, G.S.W.; Cheung, C.M.G.; Chakravarthy, U.; Wong, T.Y.; Teo, K.Y.C.; Ting, D.S.W. Digital technology for amd management in the post-COVID-19 new normal. Asia-Pac. J. Ophthalmol. 2021, 10, 39–48. [Google Scholar] [CrossRef]
- Cancino, A.; Castillo, C.; De Wolff, T.; Gajardo, P.; Lecaros, R.; Munoz, C.; Ortega, J.; Ramırez, H.; Valenzuela, N.; Marıa, S. Report#4: Estimation of Maximal ICU Beds Demand for COVID-19 Outbreak in Some Chilean Regions and the Effects of Different Mitigation Strategies, Technical Report, CMM-AM2V-CEPS, Chilean, Chile, March 2020. Available online: http://covid-19.cmm.uchile.cl (accessed on 14 March 2021).
- Van de Voorde, C.; Lefevre, M.; Mistiaen, P.; Detollenaere, J.; Kohn, L.; Van den Heede, K. Assessing the Management of Hospital Surge Capacity in the First Wave of the COVID-19 Pandemic in Belgium; KCE: Brussels, Belgium, 2020. [Google Scholar]
- Rentschler, J.; Klaiber, C.; Tariverdi, M.; Desjonqueres, C.; Mercadante, J. Frontline: Preparing Healthcare Systems for Shocks from Disasters to Pandemics; World Bank: Hong Kong, China, 2021. [Google Scholar]
- Sagan, A.; Thomas, S.; McKee, M.; Karanikolos, M.; Azzopardi-Muscat, N.; de la Mata, I.; Figueras, J.; World Health Organization. COVID-19 and health systems resilience: Lessons going forwards. Eurohealth 2020, 26, 20–24. [Google Scholar]
- Deep, A.; Knight, P.; Kernie, S.G.; D’Silva, P.; Sobin, B.; Best, T.; Zorrilla, M.; Carson, L.; Zoica, B.; Ahn, D. A Hybrid Model of Pediatric and Adult Critical Care during the Coronavirus Disease 2019 Surge: The Experience of Two Tertiary Hospitals in London and New York. Pediatr. Crit. Care Med. 2020, 22, e125–e134. [Google Scholar] [CrossRef]
- Centers for Medicare & Medicaid Services (CMS). CMS Announces Comprehensive Strategy to Enhance Hospital Capacity Amid COVID-19 Surge. Hospitals, Innovation Models. 2020. Available online: https://www.cms.gov/newsroom/press-releases/cms-announces-comprehensive-strategy-enhance-hospital-capacity-amid-COVID-19-surge (accessed on 21 February 2022).
- Budhiraja, S.; Indrayan, A.; Aggarwal, M.; Jha, V.; Jain, D.; Tarai, B.; Das, P.; Aggarwal, B.; Mishra, R.S.; Bali, S. Differentials in the characteristics of COVID-19 cases in Wave-1 and Wave-2 admitted to a network of hospitals in North India. Medrxiv 2021, preprint. [Google Scholar] [CrossRef]
- Webster, C.; Wordsworth, M.; Marsden, M.; Navaratne, L.; Bowley, D.; Chaloner, E.; O’Brien, B.; Schilling, R. C4: ‘Command, control, coordination and communication’ at NHS Nightingale, London: Introducing the tactical commander. J. R. Soc. Med. 2020, 113, 299–301. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: All non-urgent elective surgery is suspended for at least three months in England. BMJ 2020, 368, m1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhao, B. Makeshift hospitals for COVID-19 patients: Where health-care workers and patients need sufficient ventilation for more protection. J. Hosp. Infect. 2020, 105, 98–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HSU, J. How the COVID-19 Pandemic May Reshape U.S. Hospital Design. 2020. Available online: https://undark.org/2020/04/16/covid-19-modified-hospital-design/ (accessed on 11 February 2022).
- Nicolás, D.; Coloma, E.; Pericàs, J.M. Alternatives to conventional hospitalisation that enhance health systems’ capacity to treat COVID-19. Lancet. Infect. Dis. 2021, 21, 591–593. [Google Scholar] [CrossRef]
- Pilosof, N.P. Building for change: Comparative case study of hospital architecture. HERD Health Environ. Res. Des. J. 2021, 14, 47–60. [Google Scholar] [CrossRef]
- Siegel-Itzkovich, J. Rambam Parking Lot Will Serve as Hospital. The Jerusalem Post, 3 June 2012. Available online: https://www.jpost.com/health-and-science/rambam-parking-lot-will-serve-as-hospital (accessed on 16 March 2021).
- Health Transformation Lab; RMIT University; Cisco. Dynamic Health Capacity: Towards Adaptable Health Systems in Times of Crisis; Cisco: San Jose, CA, USA, 2020. [Google Scholar]
- Headley, M. Hospitals and Health Systems Adapt to Fight COVID-19. ASHE, 12 May 2020. Available online: https://www.hfmmagazine.com/ (accessed on 15 March 2021).
- Mannix, L. Australia’s First Virtual Hospital Rolls out for COVID-19 Patients. The Sydney Morning Herald, 29 March 2020. Available online: https://www.smh.com.au/national/australia-s-first-virtual-hospital-rolls-out-for-covid-19-patients-20200329-p54ezj.html (accessed on 16 March 2021).
- Mageit, S. Doccla and Northampton NHS trust trial virtual wards for vulnerable patients. EMEA Digital Health, 2020. [Google Scholar]
- Das, R. The Boom of Telehealth in UAE: Is It Here to Stay? Omnia Health. 2020. Available online: https://insights.omnia-health.com/technology/boom-telehealth-uae-it-here-stay (accessed on 19 March 2021).
- Peyroteo, M.; Ferreira, I.A.; Elvas, L.B.; Ferreira, J.C.; Lapão, L.V. Remote Monitoring Systems for Patients With Chronic Diseases in Primary Health Care: Systematic Review. JMIR Mhealth Uhealth 2021, 9, e28285. [Google Scholar] [CrossRef]
- NeJhaddadgar, N.; Ziapour, A.; Zakkipour, G.; Abbas, J.; Abolfathi, M.; Shabani, M. Effectiveness of telephone-based screening and triage during COVID-19 outbreak in the promoted primary healthcare system: A case study in Ardabil province, Iran. J. Public Health 2020, 30, 1301–1306. [Google Scholar] [CrossRef]
- Illman, J. NHS Block Books Almost All Private Hospital Sector Capacity to Fight COVID-19. Policy and Regulation, 21 March 2020. Available online: https://www.hsj.co.uk/policy-and-regulation/nhs-block-books-almost-all-private-hospital-sector-capacity-to-fight-covid-19/7027196.article (accessed on 22 March 2022).
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based. Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [Green Version]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Reference | Area of Focus | Strategy | |
|---|---|---|---|
| [126] | Adaptations to increase ICU bed capacity | (a) Within ICUs | • Use of non-operational ICU beds • Making large ICU rooms into double rooms for two or more patients. • Moving low-acuity patients to beds in the wards |
| [34,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58] | (b) Within hospitals | • Repurposing other non-ICU beds to ICUs (e.g., EDs, ORs, etc.) • Establishing de novo ICUs | |
| [34,52] | (c) Outside hospitals | • Field hospitals • Prediction models • Surveillance discharges vs. admission match | |
| [7,28,34,43,47,48,62] | Adaptations in emergency department (ED) | • Screening patients using outside pods or tents. • Restricting the number of visitors • Separating contagious patients in waiting rooms • Infection hotspots’ designation, identification, and separation • Changes to air systems to achieve negative pressurization • Patients shift from overcrowded EDs to offsite venues for telehealth • To execute mass triage, modular or movable structures were deployed • Converting external spaces into temporary emergency care units (e.g., garages and parking spaces) | |
| [30,40] | Adaptations in outpatient department (OPD) | • Considerable shift to telemedicine owing to: (i) temporary shutdown of outpatient clinics; (ii) use of outpatient facilities as centers for COVID-19 purposes, such as hospitalizations, treatments, isolations, testing, etc. • Adoption of the strategy known as “Hospitals Without Walls *” • Patient’s symptoms and temperature checking, preferably upon arrival to OPD; occasionally remotely |
| Reference | Function of a De Novo Structure |
|---|---|
| [6,54,59] |
|
| [6,32,43,49,50,51,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127] |
|
| [6,36,54] |
|
| [6,54] |
|
| [6,30,54] |
|
| [6,47,54,56] |
|
| [6,54] |
|
| [6,54,127] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndayishimiye, C.; Sowada, C.; Dyjach, P.; Stasiak, A.; Middleton, J.; Lopes, H.; Dubas-Jakóbczyk, K. Associations between the COVID-19 Pandemic and Hospital Infrastructure Adaptation and Planning—A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8195. https://doi.org/10.3390/ijerph19138195
Ndayishimiye C, Sowada C, Dyjach P, Stasiak A, Middleton J, Lopes H, Dubas-Jakóbczyk K. Associations between the COVID-19 Pandemic and Hospital Infrastructure Adaptation and Planning—A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(13):8195. https://doi.org/10.3390/ijerph19138195
Chicago/Turabian StyleNdayishimiye, Costase, Christoph Sowada, Patrycja Dyjach, Agnieszka Stasiak, John Middleton, Henrique Lopes, and Katarzyna Dubas-Jakóbczyk. 2022. "Associations between the COVID-19 Pandemic and Hospital Infrastructure Adaptation and Planning—A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 13: 8195. https://doi.org/10.3390/ijerph19138195
APA StyleNdayishimiye, C., Sowada, C., Dyjach, P., Stasiak, A., Middleton, J., Lopes, H., & Dubas-Jakóbczyk, K. (2022). Associations between the COVID-19 Pandemic and Hospital Infrastructure Adaptation and Planning—A Scoping Review. International Journal of Environmental Research and Public Health, 19(13), 8195. https://doi.org/10.3390/ijerph19138195






