The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Sampling
2.2. Data Management and Statistical Analysis
3. Results
3.1. Response Rates
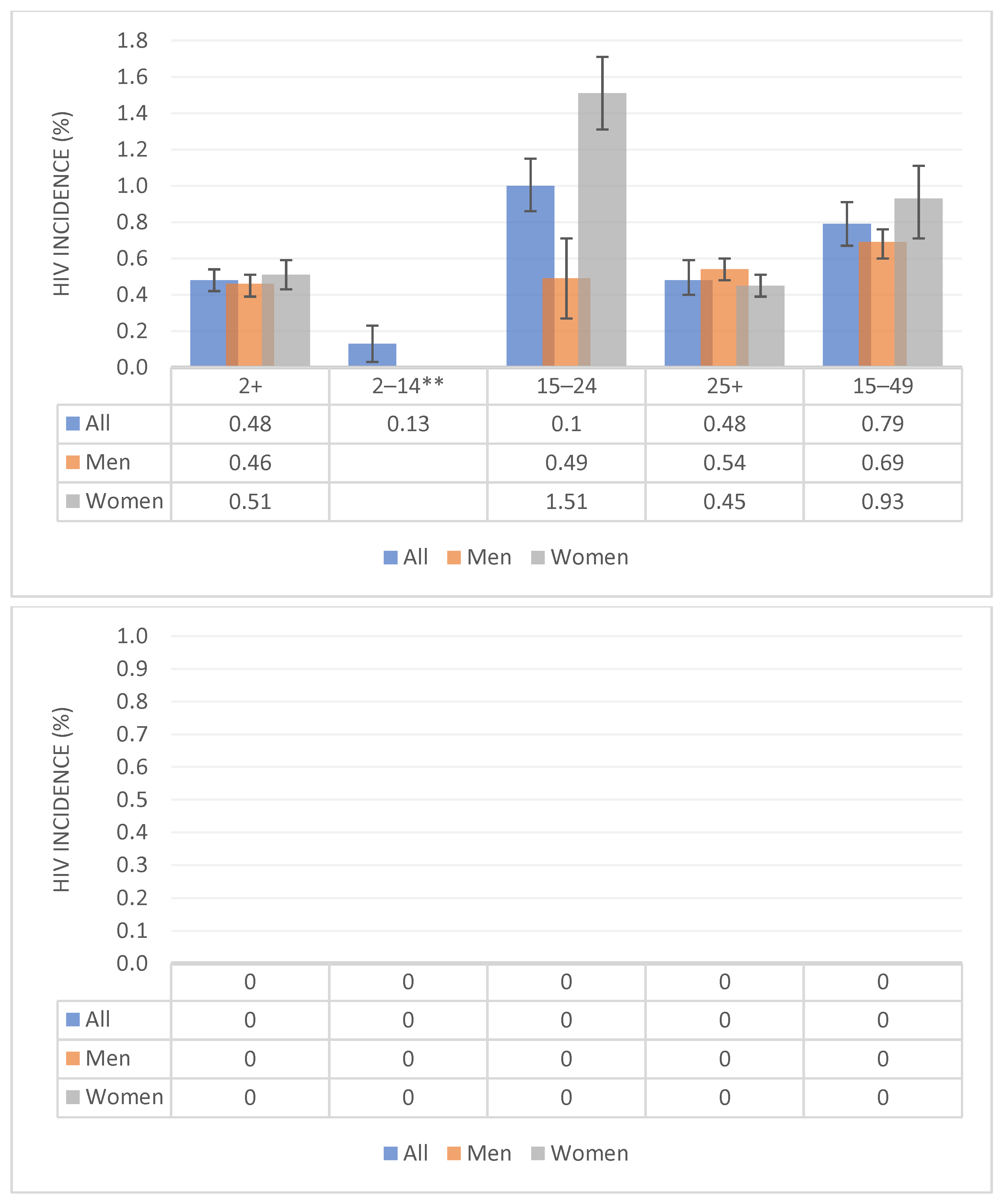
3.2. HIV Incidence
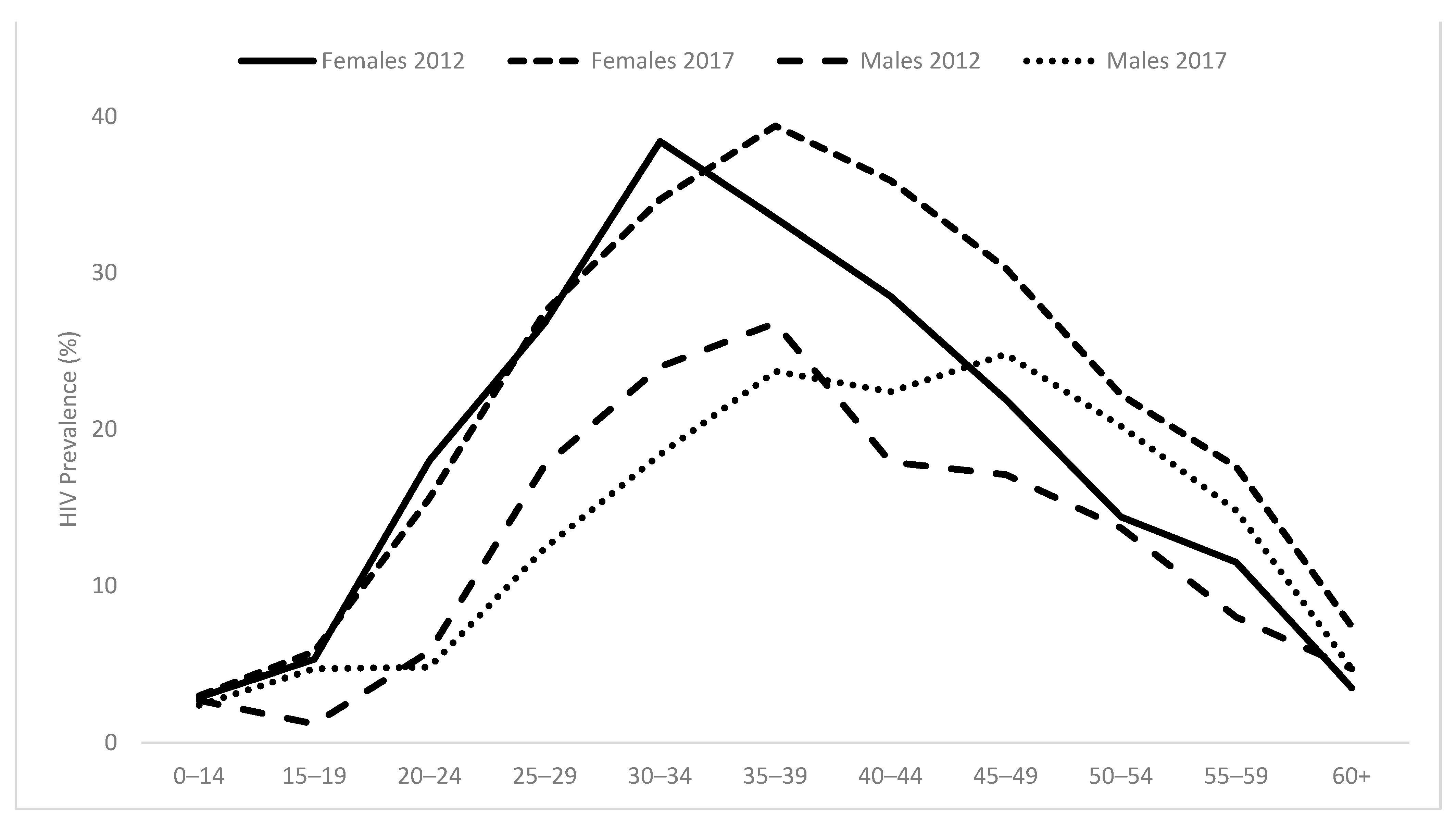
3.3. HIV Prevalence
3.4. Exposure to Antiretroviral (ARV) and HIV Viral Load Suppression
3.5. UNAIDS 90-90-90 Targets
3.6. Related Behavioural Determinants
3.7. HIV Testing
3.8. Male Circumcision
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shisana, O.; Simbayi, L. The Nelson Mandela/HSRC Study of HIV/AIDS: South African National HIV Prevalence, Behavioural Risks and Mass Media: Household Survey 2002; HSRC Press: Cape Town, South Africa, 2002. [Google Scholar]
- Shisana, O.; Rehle, T.; Simbayi, L.; Parker, W.; Zuma, K.; Bhana, A.; Connolley, C.; Jooste, S. South African National HIV Prevalence, HIV Incidence, Behaviour and Communication Survey, 2005; HSRC Press: Cape Town, South Africa, 2005. [Google Scholar]
- Shisana, O.; Rehle, T.; Simbayi, L.C.; Zuma, K.; Jooste, S.; Pillay-Van Wyk, V.; Mbelle, N.; Van Zyl, J.; Parker, W.; Zungu, N.P.; et al. South African National HIV Prevalence, Incidence and Communication Survey, 2008: A Turning Tide among Teenagers; HSRC Press: Cape Town, South Africa, 2009. [Google Scholar]
- Shisana, O.; Rehle, T.; Simbayi, L.C.; Zuma, K.; Jooste, S.; Zungu, N.; Labadarios, D.; Onoya, D. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012; HSRC Press: Cape Town, South Africa, 2014. [Google Scholar]
- Simbayi, L.C.; Zuma, K.; Zungu, N.; Moyo, S.; Marinda, E.; Jooste, S.; Mabaso, M.; Ramlagan, S.; North, A.; van Zyl, J.; et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017; HSRC Press: Cape Town, South Africa, 2019. [Google Scholar]
- Rehle, T.; Johnson, L.; Hallett, T.; Mahy, M.; Kim, A.; Odido, H.; Onoya, D.; Jooste, S.; Shisana, O.; Puren, A.; et al. A Comparison of South African National HIV Incidence Estimates: A Critical Appraisal of Different Methods. PLoS ONE 2015, 10, e0133255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuma, K.; Shisana, O.; Rehle, T.M.; Simbayi, L.C.; Jooste, S.; Zungu, N.; Labadarios, D.; Onoya, D.; Evans, M.; Moyo, S.; et al. New insights into HIV epidemic in South Africa: Key findings from the National HIV Prevalence, Incidence and Behaviour Survey, 2012. Afr. J. AIDS Res. 2016, 15, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Colebunders, R.; Kenyon, C. Behaviour, not mobility, is a risk factor for HIV. Lancet HIV 2015, 2, E223–E224. [Google Scholar] [CrossRef] [Green Version]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Madiba, S.; Ngwenya, N. Cultural practices, gender inequality and inconsistent condom use increase vulnerability to HIV infection: Narratives from married and cohabiting women in rural communities in Mpumalanga province, South Africa. Glob. Health Action 2017, 10, 1341597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, N.; Eaton, J.W.; Newell, M.-L.; Hosegood, V. Migration, sexual behaviour, and HIV risk: A general population cohort in rural South Africa. Lancet HIV 2015, 2, e252–e259. [Google Scholar] [CrossRef] [Green Version]
- Pitpitan, E.V.; Smith, L.R.; Goodman-Meza, D.; Torres, K.; Semple, S.J.; Strathdee, S.A.; Patterson, T.L. “Outness” as a moderator of the association between syndemic conditions and HIV risk-taking behaviour among men who have sex with men in Tijuana, Mexico. AIDS Behav. 2016, 20, 431–438. [Google Scholar] [CrossRef] [Green Version]
- SANAC. National WHO Working Group on HIV Incidence Measurement and Data Use; SANAC: Pretoria, South Africa, 2017. [Google Scholar]
- UNAIDS. Addressing a Blind Spot in Response to HIV: Reaching Men and Boys; UNAIDS: Geneva, Switzerland, 2017. [Google Scholar]
- Conroy, A.; Leddy, A.; Johnson, M.; Ngubane, T.; Van Rooyen, H.; Darbes, L. “I told her this is your life”: Relationship Dynamics, Partner Support, and Adherence to Antiretroviral Therapy among South African Couples. Cult. Health Sex. 2017, 19, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Zungu, N.P.; Simbayi, L.C.; Mabaso, M.; Evans, M.; Zuma, K.; Ncitakalo, N.; Sifunda, S. HIV risk perception and behavior among medically and traditionally circumcised males in South Africa. BMC Public Health 2016, 16, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akullian, A.; Bershteyn, A.; Klein, D.; Vandormael, A.; Bärnighausen, T.; Tanser, F. Sexual partnership age pairings and risk of HIV acquisition in rural South Africa. AIDS 2017, 31, 1755–1764. [Google Scholar] [CrossRef]
- Gouws, E.; Williams, B.G. Age-mixing and the incidence of HIV among young women. Lancet HIV 2016, 4, e6–e8. [Google Scholar] [CrossRef]
- De Oliveira, T.; Kharsany, A.B.; Gräf, T.; Cawood, C.; Khanyile, D.; Grobler, A.; Puren, A.; Madurai, S.; Baxter, C.; Karim, Q.A.; et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: A community-wide phylogenetic study. Lancet HIV 2017, 4, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Onoya, D.; Zuma, K.; Zungu, N.; Shisana, O.; Mehlomakhulu, V. Determinants of multiple sexual partnerships in South Africa. J. Public Health 2015, 37, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Nyirenda, M.; Wand, H.; Ramjee, G. Cohort effects on sexual behavior and risk of acquisition of sexually transmitted infections and HIV in a South African HIV prevention trial. HIV AIDS Rev. 2018, 17, 181–188. [Google Scholar] [CrossRef]
- Evans, M.; Maughan-Brown, B.; Zungu, N.; George, G. HIV Prevalence and ART Use Among Men in Partnerships with 15–29 Year Old Women in South Africa: HIV Risk Implications for Young Women in Age-Disparate Partnerships. AIDS Behav. 2017, 21, 2533–2542. [Google Scholar] [CrossRef]
- Dellar, R.C.; Dlamini, S.; Karim, Q.A. Adolescent girls and young women: Key populations for HIV epidemic control. J. Int. AIDS Soc. 2015, 18, 19408. [Google Scholar] [CrossRef]
- Auvert, B.; Taljaard, D.; Lagarde, E.; Sobngwi-Tambekou, J.; Sitta, R.; Puren, A. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLOS Med. 2005, 2, e298. [Google Scholar] [CrossRef] [Green Version]
- Gray, R.H.; Kigozi, G.; Serwadda, D.; Makumbi, F.; Watya, S.; Nalugoda, F.; Kiwanuka, N.; Moulton, L.H.; Chaudhary, M.A.; Chen., M.Z.; et al. Male circumcision for HIV prevention in men in Rakai, Uganda: A randomised trial. Lancet 2007, 369, 657–666. [Google Scholar] [CrossRef]
- Bailey, R.C.; Moses, S.; Parker, C.B.; Agot, K.; Maclean, I.; Krieger, J.N.; Williams, C.F.; Campbell, R.T.; Ndinya-Achola, J.O. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet 2007, 369, 643–656. [Google Scholar] [CrossRef]
- UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic; UNAIDS: Geneva, Switzerland, 2014. [Google Scholar]
- Makusha, T.; Mabaso, M.; Richter, L.; Desmond, C.; Jooste, S.; Simbayi, L. Trends in HIV testing and associated factors among men in South Africa: Evidence from 2005, 2008 and 2012 national population-based household surveys. Public Health 2017, 143, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Statistics South Africa. Spatial Metadata; Stats SA: Pretoria, South Africa, 2011.
- Statistics South Africa. Mid-Year Population Estimates 2017; Statistics South Africa: Pretoria, South Africa, 2017.
- Kassanjee, R.; Pilcher, C.D.; Busch, M.P.; Murphy, G.; Facente, S.N.; Keating, S.; McKinney, E.; Marson, K.; Price, M.; Martin, J.N.; et al. Viral load criteria and threshold optimization to improve HIV incidence assay characteristics. AIDS 2016, 30, 2361–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.F.; Dorrington, R.E.; Moolla, H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. S. Afr. J. HIV Med. 2017, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Grebe, E.; Welte, A.; Johnson, L.F.; van Cutsem, G.; Puren, A.; Ellman, T.; Etard, J.-F.; Huerga, H.; the Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA). Population-level HIV incidence estimates using a combination of synthetic cohort and recency biomarker approaches in KwaZulu-Natal, South Africa. PLoS ONE 2018, 13, e0203638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, S.S.A.; Baxter, C. HIV incidence trends in Africa: Young women at highest risk. Lancet HIV 2021, 8, e389–e390. [Google Scholar] [CrossRef]
- Birdthistle, I.; Tanton, C.; Tomita, A.; de Graaf, K.; Schaffnit, S.; Tanser, F.; Slaymaker, E. Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e1521–e1540. [Google Scholar] [CrossRef]
- Frank, T.D.; Carter, A.; Jahagirdar, D.; Biehl, M.H.; Douwes-Schultz, D.; Larson, S.L. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019, 6, e831–e859. [Google Scholar] [CrossRef] [Green Version]
- Moyo, S.; Young, P.W.; Gouws, E.; Naidoo, I.; Wamicwe, J.; Mukui, I.; Marsh, K.; Igumbor, E.; Kim, A.A.; Rehle, T. Equity of antiretroviral treatment use in high HIV burden countries: Analyses of data from nationally-representative surveys in Kenya and South Africa. PLoS ONE 2018, 13, e0201899. [Google Scholar] [CrossRef]
- Tang, H.; Mao, Y.; Tang, W.; Han, J.; Xu, J.; Li, J. “Late for testing, early for antiretroviral therapy, less likely to die”: Results from a large HIV cohort study in China, 2006–2014. BMC Infect. Dis. 2018, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, S.; Hertzmark, E.; Muya, A.; Sando, D.; Ulenga, N.; Machumi, L.; Spiegelman, D.; Fawzi, W.W. Equity of child and adolescent treatment, continuity of care and mortality, according to age and gender among enrollees in a large HIV programme in Tanzania. J. Int. AIDS Soc. 2018, 21, e25070. [Google Scholar] [CrossRef]
- Earnshaw, V.A.; Reisner, S.L.; Menino, D.; Poteat, V.P.; Bogart, L.M.; Barnes, T.N.; Schuster, M.A. Stigma-based bullying interventions: A systematic review. Dev. Rev. 2018, 48, 178–200. [Google Scholar] [CrossRef]
- Croome, N.; Ahluwalia, M.; Hughes, L.; Abas, M. Patient-reported barriers and facilitators to antiretroviral adherence in sub-Saharan Africa. AIDS 2017, 31, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan-Brown, B.; Venkataramani, A.S. Accuracy and determinants of perceived HIV risk among young women in South Africa. BMC Public Health 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Treves-Kagan, S.; Steward, W.T.; Ntswane, L.; Haller, R.; Gilvydis, J.M.; Gulati, H.; Barnhart, S.; Lippman, S.A. Why increasing availability of ART is not enough: A rapid, community-based study on how HIV-related stigma impacts engagement to care in rural South Africa. BMC Public Health 2015, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.J.; Cyrus, E.; Bhatt, C.; Lyons, N.; LaVia, L.-O.; Boyce, G. Viral suppression among persons living with HIV in Trinidad & Tobago: Implications for targeted prevention programmes. Glob. Public Health 2019, 14, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Zuma, K.; Mzolo, T.; Makonko, E. Determinants of age at sexual debut and associated risks among South African youths. Afr. J. AIDS Res. 2011, 10, 189–194. [Google Scholar] [CrossRef]
- Mukanyangezi, M.F.; Manzi, O.; Tobin, G.; Rulisa, S.; Bienvenu, E.; Giglio, D. Sexual risk behaviour in a cohort of HIV-negative and HIV-positive Rwandan women. Epidemiol. Infect. 2018, 147, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.M.T.; Boggiano, V.L.; Tran, B.X.; Nguyen, L.H.; Tran, T.T.; Latkin, C.A.; Ho, C.S.H.; Ho, R.C.M. Sexual Risk Behaviors of Patients with HIV/AIDS over the Course of Antiretroviral Treatment in Northern Vietnam. Int. J. Environ. Res. Public Health 2018, 15, 1106. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Pagano, M. Role of survey response rates on valid inference: An application to HIV prevalence estimates. Emerg. Themes Epidemiol. 2018, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Harling, G.; Moyo, S.; McGovern, M.E.; Mabaso, M.; Marra, G.; Bärnighausen, T.; Rehle, T. National South African HIV prevalence estimates robust despite substantial test non-participation. South Afr. Med. J. 2017, 107, 590–594. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuma, K.; Simbayi, L.; Zungu, N.; Moyo, S.; Marinda, E.; Jooste, S.; North, A.; Nadol, P.; Aynalem, G.; Igumbor, E.; et al. The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey. Int. J. Environ. Res. Public Health 2022, 19, 8125. https://doi.org/10.3390/ijerph19138125
Zuma K, Simbayi L, Zungu N, Moyo S, Marinda E, Jooste S, North A, Nadol P, Aynalem G, Igumbor E, et al. The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey. International Journal of Environmental Research and Public Health. 2022; 19(13):8125. https://doi.org/10.3390/ijerph19138125
Chicago/Turabian StyleZuma, Khangelani, Leickness Simbayi, Nompumelelo Zungu, Sizulu Moyo, Edmore Marinda, Sean Jooste, Alicia North, Patrick Nadol, Getahun Aynalem, Ehimario Igumbor, and et al. 2022. "The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey" International Journal of Environmental Research and Public Health 19, no. 13: 8125. https://doi.org/10.3390/ijerph19138125
APA StyleZuma, K., Simbayi, L., Zungu, N., Moyo, S., Marinda, E., Jooste, S., North, A., Nadol, P., Aynalem, G., Igumbor, E., Dietrich, C., Sigida, S., Chibi, B., Makola, L., Kondlo, L., Porter, S., Ramlagan, S., & on behalf of the SABSSM V Study Group Contributors. (2022). The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey. International Journal of Environmental Research and Public Health, 19(13), 8125. https://doi.org/10.3390/ijerph19138125




