Association of Sleep Duration with Hyperuricemia in Chinese Adults: A Prospective Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Sleep Duration and Hyperuricemia
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
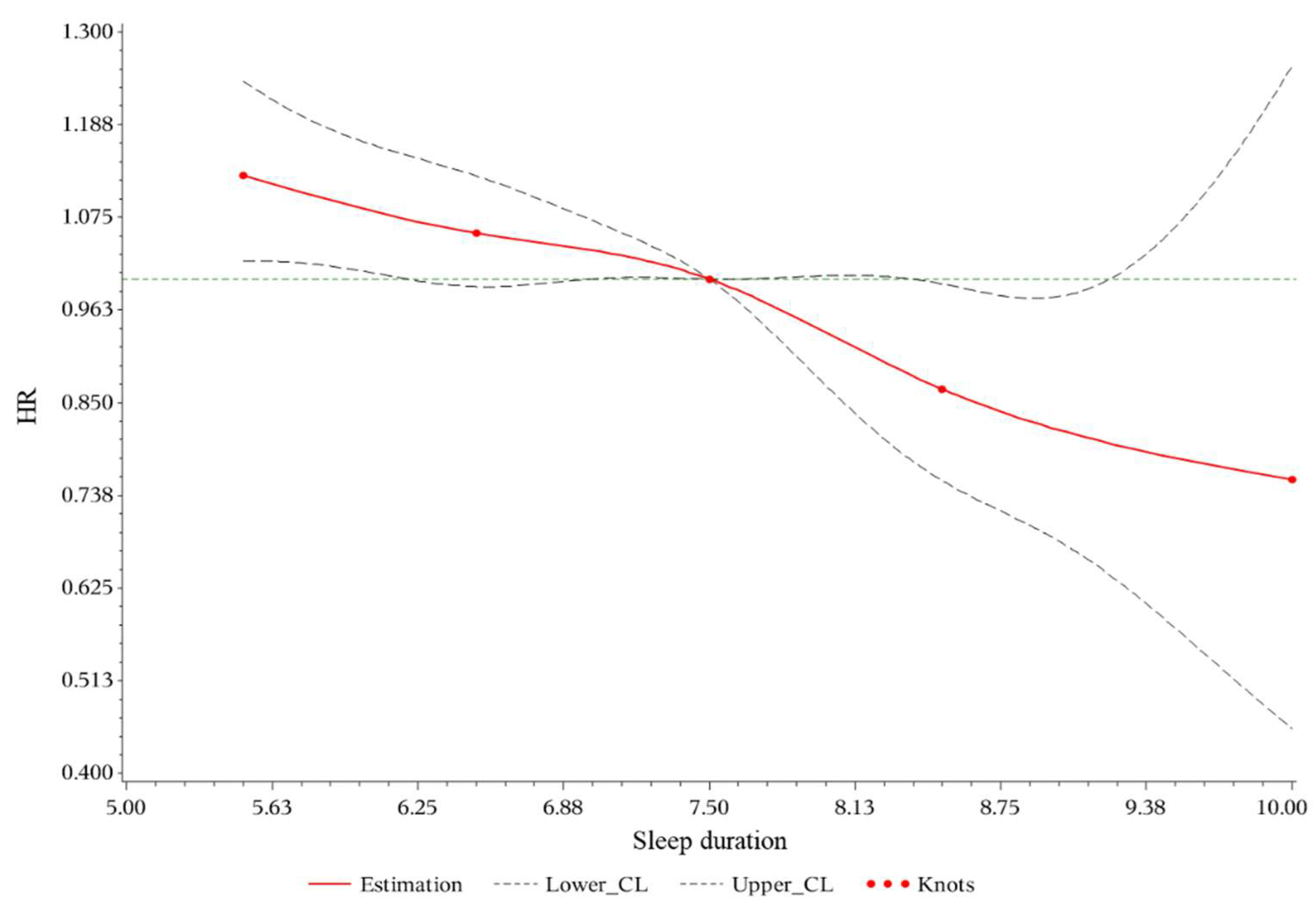
3.2. Association between Sleep Duration and Hyperuricemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Models | HR (95%CI) | ||
|---|---|---|---|
| <7 h | 7–8 h | ≥8 h | |
| Events | 1598 | 837 | 126 |
| Person year | 37,809.5 | 22,176.08 | 4411.167 |
| Incidence Rate (/1000 py) | 42.27 | 37.74 | 28.56 |
| Model 1 | 1.055 (0.970, 1.148) | ref | 0.804 (0.667, 0.970) |
| Model 2 | 1.055 (0.969, 1.148) | ref | 0.798 (0.662, 0.963) |
| Model 3 | 1.018 (0.935, 1.108) | ref | 0.788 (0.653, 0.950) |
| Model 4 | 1.014 (0.931, 1.104) | ref | 0.785 (0.651, 0.947) |
| Models | HR (95%CI) | p for Trend | ||||
|---|---|---|---|---|---|---|
| <6 h | 6–7 h | 7–8 h | 8–9 h | ≥9 h | ||
| Events | 571 | 2382 | 1668 | 232 | 15 | |
| Person year | 12,943.17 | 56,837.42 | 44,966.25 | 7913.58 | 598.33 | |
| Incidence Rate (/1000 py) | 44.12 | 41.91 | 37.09 | 29.32 | 25.07 | |
| Model 1 | 1.200 (1.089, 1.322) | 1.090 (1.023, 1.160) | ref | 0.865 (0.754, 0.992) | 0.836 (0.503, 1.389) | <0.001 |
| Model 2 | 1.176 (1.067, 1.296) | 1.084 (1.017, 1.155) | ref | 0.855 (0.745, 0.981) | 0.795 (0.478, 1.322) | <0.001 |
| Model 3 | 1.128 (1.023, 1.243) | 1.058 (0.993, 1.128) | ref | 0.854 (0.744, 0.980) | 0.752 (0.452, 1.251) | <0.001 |
| Model 4 | 1.116 (1.012, 1.230) | 1.055 (0.990, 1.125) | ref | 0.852 (0.743, 0.978) | 0.734 (0.441, 1.221) | <0.001 |
References
- Li, X.; Meng, X.; Timofeeva, M.; Tzoulaki, I.; Tsilidis, K.K.; Ioannidis, J.P.; Campbell, H.; Theodoratou, E. Serum uric acid levels and multiple health outcomes: Umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ 2017, 357, j2376. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Hara, S.; Roncal-Jimenez, C.A.; Bjornstad, P.; Nakagawa, T.; Andres-Hernando, A.; Sato, Y.; Jensen, T.; et al. Uric Acid Is a Strong Risk Marker for Developing Hypertension From Prehypertension: A 5-Year Japanese Cohort Study. Hypertension 2018, 71, 78–86. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Hisatome, I.; Nakagawa, T.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Bjornstad, P.; Jensen, T.; Sato, Y.; Milagres, T.; et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension 2017, 69, 1036–1044. [Google Scholar] [CrossRef]
- Han, T.; Meng, X.; Shan, R.; Zi, T.; Li, Y.; Ma, H.; Zhao, Y.; Shi, D.; Qu, R.; Guo, X.; et al. Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. Int. J. Obes. 2018, 42, 1336–1344. [Google Scholar] [CrossRef]
- Jayachandran, M.; Qu, S. Harnessing hyperuricemia to atherosclerosis and understanding its mechanistic dependence. Med. Res. Rev. 2021, 41, 616–629. [Google Scholar] [CrossRef]
- Ponticelli, C.; Podestà, M.A.; Moroni, G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020, 98, 1149–1159. [Google Scholar] [CrossRef]
- Borghi, C.; Palazzuoli, A.; Landolfo, M.; Cosentino, E. Hyperuricemia: A novel old disorder-relationship and potential mechanisms in heart failure. Heart Fail. Rev. 2020, 25, 43–51. [Google Scholar] [CrossRef]
- Chaudhary, N.S.; Bridges, S.L., Jr.; Saag, K.G.; Rahn, E.J.; Curtis, J.R.; Gaffo, A.; Limdi, N.A.; Levitan, E.B.; Singh, J.A.; Colantonio, L.D.; et al. Severity of Hypertension Mediates the Association of Hyperuricemia With Stroke in the REGARDS Case Cohort Study. Hypertension 2020, 75, 246–256. [Google Scholar] [CrossRef]
- Cabău, G.; Crișan, T.O.; Klück, V.; Popp, R.A.; Joosten, L.A.B. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Joosten, L.A.B.; Crişan, T.O.; Bjornstad, P.; Johnson, R.J. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat. Rev. Rheumatol. 2020, 16, 75–86. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Song, P.; Wang, H.; Xia, W.; Chang, X.; Wang, M.; An, L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci. Rep. 2018, 8, 4314. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, K.; Yang, L.; Wang, H.; Xiao, Y.; Qiu, G.; Liu, X.; Yuan, Y.; Bai, Y.; Li, X.; et al. Sleep duration, midday napping, and sleep quality and incident stroke: The Dongfeng-Tongji cohort. Neurology 2020, 94, e345–e356. [Google Scholar] [CrossRef]
- Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. [Google Scholar] [CrossRef] [Green Version]
- Prather, A.A.; Janicki-Deverts, D.; Hall, M.H.; Cohen, S. Behaviorally Assessed Sleep and Susceptibility to the Common Cold. Sleep 2015, 38, 1353–1359. [Google Scholar] [CrossRef]
- Wang, C.; Bangdiwala, S.I.; Rangarajan, S.; Lear, S.A.; AlHabib, K.F.; Mohan, V.; Teo, K.; Poirier, P.; Tse, L.A.; Liu, Z.; et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: A study of 116,632 people from 21 countries. Eur. Heart J. 2019, 40, 1620–1629. [Google Scholar] [CrossRef]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. Morb. Mortal Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Bakour, C.; Schwartz, S.; O’Rourke, K.; Wang, W.; Sappenfield, W.; Couluris, M.; Chen, H. Sleep Duration Trajectories and Systemic Inflammation in Young Adults: Results From the National Longitudinal Study of Adolescent to Adult Health (Add Health). Sleep 2017, 40, zsx156. [Google Scholar] [CrossRef]
- Holingue, C.; Owusu, J.T.; Feder, K.A.; Spira, A.P. Sleep duration and C-reactive protein: Associations among pregnant and non-pregnant women. J. Reprod. Immunol. 2018, 128, 9–15. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.; Son, D.H.; Kwon, Y.J. U-Shaped Association between Sleep Duration, C-Reactive Protein, and Uric Acid in Korean Women. Int. J. Environ. Res. Public Health 2020, 17, 2657. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Babio, N.; Díaz-López, A.; Martínez-González, M.; Becerra-Tomas, N.; Corella, D.; Schröder, H.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Sleep Duration is Inversely Associated with Serum Uric Acid Concentrations and Uric Acid to Creatinine Ratio in an Elderly Mediterranean Population at High Cardiovascular Risk. Nutrients 2019, 11, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MJ Health Care. Available online: https://www.mjlife.com.cn/ (accessed on 17 May 2022).
- Lin, K.C.; Lin, H.Y.; Chou, P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J. Rheumatol. 2000, 27, 1045–1050. [Google Scholar]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Chen, C.; Lu, F.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. BES 2004, 17, 1–36. [Google Scholar]
- Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 390–419. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012, 35 (Suppl. S1), S4–S10. [CrossRef] [Green Version]
- Chou, Y.T.; Li, C.H.; Shen, W.C.; Yang, Y.C.; Lu, F.H.; Wu, J.S.; Chang, C.J. Association of sleep quality and sleep duration with serum uric acid levels in adults. PLoS ONE 2020, 15, e0239185. [Google Scholar] [CrossRef]
- van Wamelen, D.J.; Taddei, R.N.; Calvano, A.; Titova, N.; Leta, V.; Shtuchniy, I.; Jenner, P.; Martinez-Martin, P.; Katunina, E.; Chaudhuri, K.R. Serum Uric Acid Levels and Non-Motor Symptoms in Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S. Sleep Apnea and Cardiovascular Disease: An Enigmatic Risk Factor. Circ. Res. 2018, 122, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Z.; McEvoy, R.D.; Anderson, C.S.; Rodgers, A.; Perkovic, V.; Neal, B. Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2017, 318, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Koene, R.J.; Johnson, A.R.; Lin, G.M.; Ferguson, J.D. Sleep, sleep apnea and atrial fibrillation: Questions and answers. Sleep Med. Rev. 2018, 39, 134–142. [Google Scholar] [CrossRef]
- Du, N.; Xu, D.; Hou, X.; Song, X.; Liu, C.; Chen, Y.; Wang, Y.; Li, X. Inverse Association Between Serum Uric Acid Levels and Alzheimer’s Disease Risk. Mol. Neurobiol. 2016, 53, 2594–2599. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Hayat, H.; Regev, N.; Matosevich, N.; Sales, A.; Paredes-Rodriguez, E.; Krom, A.J.; Bergman, L.; Li, Y.; Lavigne, M.; Kremer, E.J.; et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020, 6, eaaz4232. [Google Scholar] [CrossRef] [Green Version]
- Sumi, T.; Umeda, Y. Adrenergic regulation of the plasma levels of purine metabolites in the rat. Eur. J. Pharmacol. 1977, 46, 243–247. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [Green Version]
- Tobaldini, E.; Fiorelli, E.M.; Solbiati, M.; Costantino, G.; Nobili, L.; Montano, N. Short sleep duration and cardiometabolic risk: From pathophysiology to clinical evidence. Nat. Rev. Cardiol. 2019, 16, 213–224. [Google Scholar] [CrossRef]
- Boudjeltia, K.Z.; Faraut, B.; Stenuit, P.; Esposito, M.J.; Dyzma, M.; Brohée, D.; Ducobu, J.; Vanhaeverbeek, M.; Kerkhofs, M. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: A pilot study. Vasc. Health Risk Manag. 2008, 4, 1467–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; D’Addato, S.; Borghi, C. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension: Data from the Brisighella Heart Study. J. Hypertens. 2019, 37, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Kuwabara, R.; Niwa, K.; Hisatome, I.; Smits, G.; Roncal-Jimenez, C.A.; MacLean, P.S.; Yracheta, J.M.; Ohno, M.; Lanaspa, M.A.; et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients 2018, 10, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Y.; Cappuccio, F.P.; Wainwright, N.W.; Surtees, P.G.; Luben, R.; Brayne, C.; Khaw, K.T. Sleep duration and risk of fatal and nonfatal stroke: A prospective study and meta-analysis. Neurology 2015, 84, 1072–1079. [Google Scholar] [CrossRef]

| Characters | Missing | Habitual Sleep Duration | Overall | ||
|---|---|---|---|---|---|
| <7 h | 7–8 h | ≥8 h | |||
| Women (N, %) | 0 | 10,087 (51.94) | 6924 (56.27) | 1491 (64.80) | 18,502 (54.38) |
| Age (years, mean ± SD) | - | 39.60 ± 9.83 | 36.59 ± 8.98 | 36.58 ± 8.97 | 38.31 ± 9.59 |
| Married (n, %) | 1588 | 14,870 (76.57) | 9234 (75.05) | 1754 (76.23) | 25,858 (76.00) |
| School (n, %) | 10,833 | ||||
| Less than Master’s degree | 1462 (7.53) | 609 (4.95) | 193 (8.39) | 2264 (6.65) | |
| Master’s degree | 3594 (18.51) | 1752 (14.24) | 374 (16.25) | 5720 (16.81) | |
| Doctoral degree | 8715 (44.88) | 5482 (44.55) | 1011 (43.94) | 15,208 (44.70) | |
| Annual income (n, %) | 3466 | ||||
| CNY < 100,000 | 9183 (47.29) | 5644 (45.87) | 1165 (50.63) | 15,992 (47.00) | |
| CNY 100,000–200,000 | 5126 (26.40) | 3547 (28.83) | 568 (24.68) | 9241 (27.16) | |
| CNY ≥ 200,000 | 3099 (15.96) | 1891 (15.37) | 336 (14.60) | 5326 (15.65) | |
| Smoke (n, %) | 1010 | 6369 (32.80) | 3053 (24.81) | 584 (25.38) | 10,006 (29.41) |
| Drink (n, %) | 1161 | 4540 (23.38) | 2191 (17.81) | 395 (17.17) | 7126 (20.94) |
| Habitual sports (n, %) | 0 | 1042 (5.37) | 184 (1.50) | 0 (0.00) | 1226 (3.60) |
| Overweight or obesity | - | 4186 (34.02) | 8223 (42.34) | 720 (31.29) | 13,129 (38.59) |
| Abdominal obesity (n, %) | 50 | 4069 (20.95) | 1784 (14.50) | 325 (14.12) | 6178 (18.16) |
| Hypertension (n, %) | 18 | 2159 (11.12) | 920 (7.48) | 180 (7.82) | 3259 (9.58) |
| Diabetes (n, %) | 0 | 1015 (5.23) | 398 (3.23) | 80 (3.48) | 1493 (4.39) |
| High level WBC | 0 | 4405 (22.68) | 2467 (20.05) | 512 (22.25) | 7384 (21.70) |
| Low HDL-C (n, %) | 2798 | 2579 (13.28) | 1460 (11.87) | 233 (10.13) | 4272 (12.56) |
| High TG (n, %) | 26 | 3519 (18.12) | 1740 (14.14) | 353 (15.34) | 5612 (16.49) |
| Models | HR (95%CI) | p for Trend | ||
|---|---|---|---|---|
| <7 h | 7–8 h | ≥8 h | ||
| Events | 2953 | 1668 | 247 | |
| Person year | 69,780.58 | 44,966.25 | 8511.92 | |
| Incidence Rate (/1000 py) | 42.32 | 37.09 | 29.02 | |
| Model 1 | 1.108 (1.043–1.178) | ref | 0.863 (0.755–0.986) | <0.001 |
| Model 2 | 1.100 (1.034–1.169) | ref | 0.851 (0.744–0.973) | <0.001 |
| Model 3 | 1.070 (1.006–1.138) | ref | 0.846 (0.740–0.968) | <0.001 |
| Model 4 | 1.066 (1.002–1.133) | ref | 0.844 (0.738–0.965) | <0.001 |
| Subgroup | HR (95%CI) by Habitual Sleep Duration | p for Interaction | ||
|---|---|---|---|---|
| <7 h | 7–8 h | ≥8 h | ||
| Sex | ||||
| women | 1.055 (0.941, 1.184) | ref | 0.905 (0.729, 1.123) | 0.220 |
| men | 1.067 (0.992, 1.147) | ref | 0.824 (0.694, 0.978) | |
| Age | ||||
| ≥50 | 0.930 (0.779, 1.113) | ref | 0.688 (0.444, 1.065) | 0.405 |
| <50 | 1.084 (1.015, 1.157) | ref | 0.874 (0.759, 1.006) | |
| Education | ||||
| Less than Master’s degree | 0.947 (0.746, 1.202) | ref | 0.652 (0.403, 1.054) | 0.193 |
| Master’s degree | 0.950 (0.815, 1.106) | ref | 0.767 (0.556, 1.059) | |
| Doctoral degree | 1.062 (0.971, 1.163) | ref | 0.772 (0.626, 0.952) | |
| Marital status | ||||
| Unmarried | 1.005 (0.873, 1.157) | ref | 1.119 (0.837, 1.495) | 0.152 |
| Married | 1.100 (1.025, 1.181) | ref | 0.796 (0.681, 0.932) | |
| Annual income | ||||
| CNY < 100,000 | 1.017 (0.929, 1.112) | ref | 0.882 (0.734, 1.060) | 0.440 |
| CNY 100,000–200,000 | 1.114 (0.990, 1.254) | ref | 0.975 (0.750, 1.267) | |
| CNY ≥ 200,000 | 1.148 (0.987, 1.336) | ref | 0.653 (0.447, 0.954) | |
| Smoking | ||||
| Never | 1.087 (1.003, 1.178) | ref | 0.845 (0.711, 1.005) | 0.709 |
| Current and ever | 1.038 (0.941, 1.146) | ref | 0.866 (0.696, 1.077) | |
| Drinking | ||||
| Never | 1.080 (1.003, 1.164) | ref | 0.904 (0.773, 1.058) | 0.522 |
| Current and ever | 1.034 (0.923, 1.158) | ref | 0.708 (0.540, 0.930) | |
| BMI | ||||
| ≥24 | 1.005 (0.928, 1.089) | ref | 0.801 (0.666, 0.963) | 0.035 |
| <24 | 1.163 (1.056, 1.280) | ref | 0.923 (0.759, 1.122) | |
| Abdominal obesity | ||||
| No | 1.070 (0.993, 1.151) | ref | 0.881 (0.754, 1.029) | 0.624 |
| Yes | 1.061 (0.950, 1.187) | ref | 0.764 (0.585, 0.996) | |
| Hypertension | ||||
| No | 1.102 (1.031, 1.177) | ref | 0.878 (0.760, 1.013) | 0.274 |
| Yes | 0.878 (0.744, 1.036) | ref | 0.664 (0.457, 0.965) | |
| Low HDL-C | ||||
| No | 1.006 (0.935, 1.083) | ref | 0.824 (0.703, 0.967) | 0.156 |
| Yes | 1.216 (1.061, 1.394) | ref | 0.815 (0.588, 1.130) | |
| High TG | ||||
| No | 0.996 (0.927, 1.070) | ref | 0.813 (0.693, 0.955) | 0.053 |
| Yes | 1.271 (1.129, 1.431) | ref | 0.964 (0.755, 1.231) | |
| Diabetes | ||||
| No | 1.071 (1.006, 1.141) | ref | 0.859 (0.749, 0.985) | 0.753 |
| Yes | 1.076 (0.811, 1.426) | ref | 0.747 (0.394, 1.418) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Shi, K.; Yang, H.; Sun, D.; Lv, J.; Ma, Y.; Man, S.; Yin, J.; Wang, B.; Yu, C.; et al. Association of Sleep Duration with Hyperuricemia in Chinese Adults: A Prospective Longitudinal Study. Int. J. Environ. Res. Public Health 2022, 19, 8105. https://doi.org/10.3390/ijerph19138105
Yu H, Shi K, Yang H, Sun D, Lv J, Ma Y, Man S, Yin J, Wang B, Yu C, et al. Association of Sleep Duration with Hyperuricemia in Chinese Adults: A Prospective Longitudinal Study. International Journal of Environmental Research and Public Health. 2022; 19(13):8105. https://doi.org/10.3390/ijerph19138105
Chicago/Turabian StyleYu, Huan, Kexiang Shi, Haiming Yang, Dianjianyi Sun, Jun Lv, Yuan Ma, Sailimai Man, Jianchun Yin, Bo Wang, Canqing Yu, and et al. 2022. "Association of Sleep Duration with Hyperuricemia in Chinese Adults: A Prospective Longitudinal Study" International Journal of Environmental Research and Public Health 19, no. 13: 8105. https://doi.org/10.3390/ijerph19138105
APA StyleYu, H., Shi, K., Yang, H., Sun, D., Lv, J., Ma, Y., Man, S., Yin, J., Wang, B., Yu, C., & Li, L. (2022). Association of Sleep Duration with Hyperuricemia in Chinese Adults: A Prospective Longitudinal Study. International Journal of Environmental Research and Public Health, 19(13), 8105. https://doi.org/10.3390/ijerph19138105






