Searching for Factors Influencing the Severity of the Symptoms of Long COVID
Abstract
:1. Introduction
2. Materials and Methods
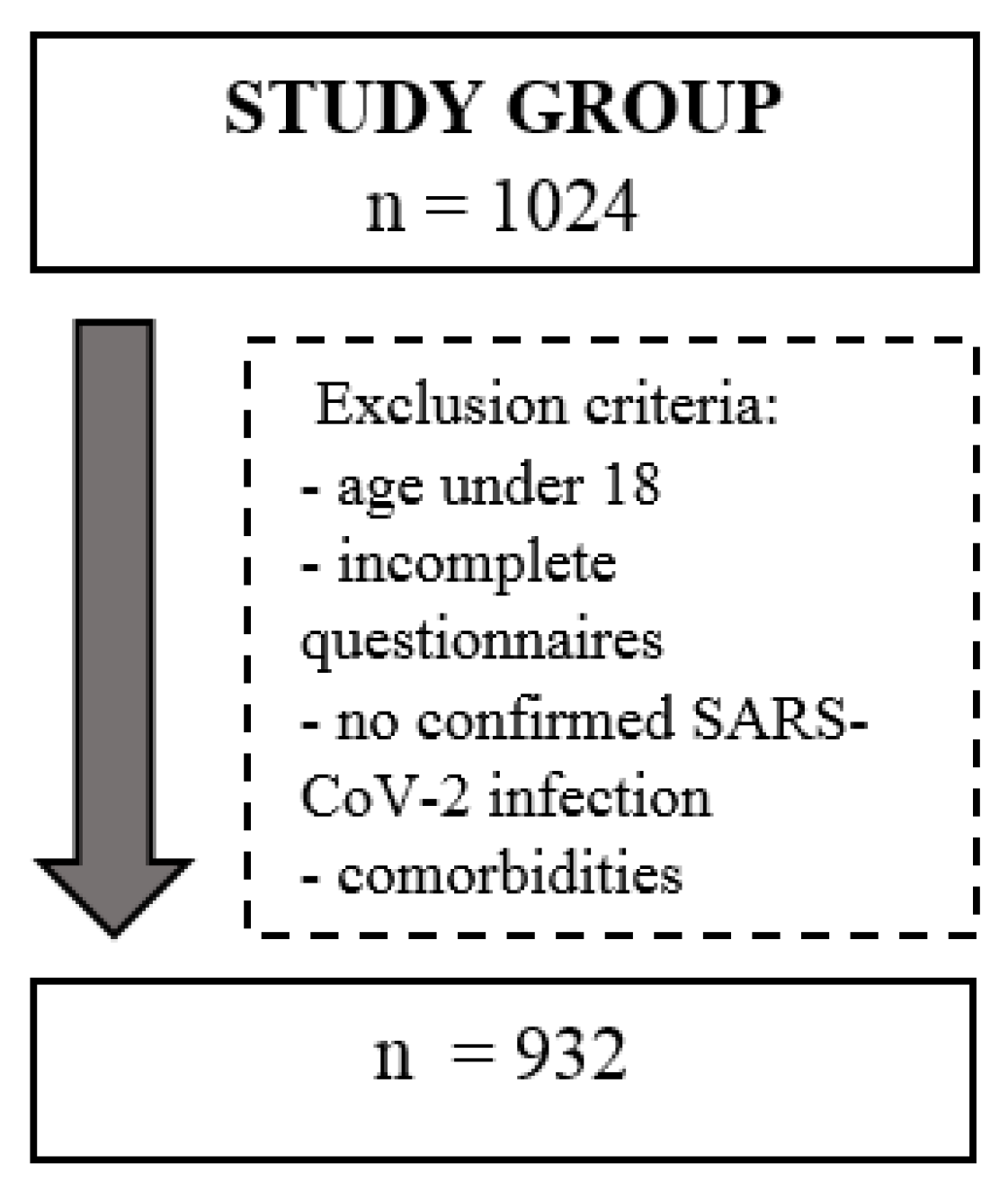
2.1. Survey Sample
2.2. Questionnaires
2.3. Statistical Analysis
3. Results
3.1. Group Characteristics
3.2. Correlations between Complications after COVID-19 and Individual Variables
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020, 16, 1446. [Google Scholar] [CrossRef]
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Ladny, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Hunt, R.H.; East, J.E.; Lanas, A.; Malfertheiner, P.; Satsangi, J.; Scarpignato, C.; Webb, G.J. COVID-19 and Gastrointestinal Disease: Implications for the Gastroenterologist. Dig. Dis. 2021, 39, 119–139. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [Green Version]
- Beom, J.; Jung, J.; Hwang, I.C.; Cho, Y.J.; Kim, E.S.; Kim, H.B.; Lim, J.Y.; Song, K.H. Early rehabilitation in a critically ill inpatient with COVID-19. Eur. J. Phys. Rehabil. Med. 2020, 56, 858–861. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, W.Q.; Li, Y.R.; He, J.X.; Guan, W.J. The Short- and Long-Term Clinical, Radiological and Functional Consequences of COVID-19. Arch. Bronconeumol. 2022, 58, 32–38. [Google Scholar] [CrossRef]
- De Biase, S.; Cook, L.; Skelton, D.A.; Witham, M.; Ten Hove, R. The COVID-19 rehabilitation pandemic. Age Ageing 2020, 49, 696–700. [Google Scholar] [CrossRef]
- Tan, S.C.; Haines, K.; Zhang, N. Beyond the ventilator: Rehabilitation for critically ill patients with coronavirus disease 2019. Aust. Crit. Care 2020, 33, 485–487. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef]
- Wong, A.W.; Shah, A.S.; Johnston, J.C.; Carlsten, C.; Ryerson, C.J. Patient-reported outcome measures after COVID-19: A prospective cohort study. Eur. Respir. J. 2020, 56, 2003276. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Akbarialiabad, H.; Taghrir, M.H.; Abdollahi, A.; Ghahramani, N.; Kumar, M.; Paydar, S.; Razani, B.; Mwangi, J.; Asadi-Pooya, A.A.; Malekmakan, L.; et al. Long COVID, a comprehensive systematic scoping review. Infection 2021, 49, 1163–1186. [Google Scholar] [CrossRef]
- Taribagil, P.; Creer, D.; Tahir, H. ‘Long COVID’ syndrome. BMJ Case Rep. 2021, 14, e241485. [Google Scholar] [CrossRef]
- Wijeratne, T.; Crewther, S. Post-COVID 19 neurological syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2020, 419, 117179. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ARC Study Group. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; Spruit, M.A.; Vijlbrief, H.; Janssen, D.J.A.; Hajian, B.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542–02020. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. TLC Study Group. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- Raj, S.R.; Arnold, A.C.; Barboi, A.; Claydon, V.E.; Limberg, J.K.; Lucci, V.M.; Numan, M.; Peltier, A.; Snapper, H.; Vernino, S. American Autonomic Society. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 2021, 31, 365–368. [Google Scholar] [CrossRef]
- Humphreys, H.; Kilby, L.; Kudiersky, N.; Copeland, R. Long COVID and the role of physical activity: A qualitative study. BMJ Open. 2021, 11, e047632. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Misra, A. Post COVID-19 Syndrome (“Long COVID”) and Diabetes: Challenges in Diagnosis and Management. Diabetes Metab. Syndr. 2021, 15, 102235. [Google Scholar] [CrossRef]
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 2021, 75, e14357. [Google Scholar] [CrossRef]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- The Lancet. Understanding long COVID: A modern medical challenge. Lancet 2021, 398, 725. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Fazavana, J.; Mallon, P.W.; Rehill, A.M.; Baker, R.I.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”-A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 2022, 10, e00911. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Naik, S.; Haldar, S.N.; Soneja, M.; Mundadan, N.G.; Garg, P.; Mittal, A.; Desai, D.; Trilangi, P.K.; Chakraborty, S.; Begam, N.N.; et al. Post COVID-19 sequelae: A prospective observational study from Northern India. Drug Discov. Ther. 2021, 15, 254–260. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Guijarro, C.; Plaza-Canteli, S.; Hernández-Barrera, V.; Torres-Macho, J. Prevalence of Post-COVID-19 Cough One Year After SARS-CoV-2 Infection: A Multicenter Study. Lung 2021, 199, 249–253. [Google Scholar] [CrossRef]
- Walia, N.; Lat, J.O.; Tariq, R.; Tyagi, S.; Qazi, A.M.; Salari, S.W.; Jafar, A.; Kousar, T.; Bieniek, S. Post-acute sequelae of COVID-19 and the mental health implications. Discoveries 2021, 9, e140. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V.; Canning, B.J. Is There (Will There Be) a Post-COVID-19 Chronic Cough? Lung 2020, 198, 863–865. [Google Scholar] [CrossRef]
- D’Cruz, R.F.; Waller, M.D.; Perrin, F.; Periselneris, J.; Norton, S.; Smith, L.J.; Jolley, C.J.; Walder, D.; Heitmann, A.; Madula, R.; et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021, 7, 00655–02020. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Barratt, S.L.; Hyams, C.; Bibby, A.; Moran, E.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gherlone, E.F.; Polizzi, E.; Tetè, G.; De Lorenzo, R.; Magnaghi, C.; Rovere Querini, P.; Ciceri, F. Frequent and Persistent Salivary Gland Ectasia and Oral Disease After COVID-19. J. Dent. Res. 2021, 100, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Kofler, M.; Lindner, A.; Mahlknecht, P.; Helbok, R.; Weiss, G.; Tancevski, I.; Seppi, K.; et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur. J. Neurol. 2021, 28, 3348–3359. [Google Scholar] [CrossRef]
- Parente-Arias, P.; Barreira-Fernandez, P.; Quintana-Sanjuas, A.; Patiño-Castiñeira, B. Recovery rate and factors associated with smell and taste disruption in patients with coronavirus disease 2019. Am. J. Otolaryngol. 2021, 42, 102648. [Google Scholar] [CrossRef]
- Poyraz, B.Ç.; Poyraz, C.A.; Olgun, Y.; Gürel, Ö.; Alkan, S.; Özdemir, Y.E.; Karaali, R.; Balkan, İ.İ. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2021, 295, 113604. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Steves, C.J.; Menni, C.; Fall, T.; Ganesh, S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Cao, B.; Tu, S.; Zhou, X.; Huang, Z.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Albashir, A.A.D. The potential impacts of obesity on COVID-19. Clin. Med. 2020, 20, e109–e113. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef] [Green Version]
- Hendren, N.S.; de Lemos, J.A.; Ayers, C.; Das, S.R.; Rao, A.; Carter, S.; Rosenblatt, A.; Walchok, J.; Omar, W.; Khera, R.; et al. Association of Body Mass Index and Age With Morbidity and Mortality in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation 2021, 143, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N. Long COVID: How to define it and how to manage it. BMJ 2020, 370, m3489. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Iqbal, K.; Arshad Ali, S.; Azim, D.; Farid, E.; Baig, M.D.; Bin Arif, T.; Raza, M. The COVID-19 Sequelae: A Cross-Sectional Evaluation of Post-recovery Symptoms and the Need for Rehabilitation of COVID-19 Survivors. Cureus 2021, 13, e13080. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.G.; Gourna Paleoudis, E.; Lesky-Di Bari, D.; Nyirenda, T.; Friedman, T.; Gupta, A.; Aschner, J.L.; Rasouli, L.; Zetkulic, M.; Balani, B.; et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE 2020, 15, e0243882. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Tafuri, S.; Cavone, D.; Sponselli, S.; Pipoli, A.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Loosen, S.H.; Jensen, B.O.; Tanislav, C.; Luedde, T.; Roderburg, C.; Kostev, K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: A cross-sectional study from 50,402 COVID-19 patients. Infection 2022, 30, 1–6. [Google Scholar] [CrossRef]
- Clark, S.; Morris, M.; Lomax, N.; Birkin, M. Can a data driven obesity classification system identify those at risk of severe COVID-19 in the UK Biobank cohort study? Int. J. Obes. 2021, 45, 2281–2285. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Fabio, C.; Patrizia, R.-Q.; Francesco, B.; The COVID-19 BioB Outpatient Clinic Study group. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef] [PubMed]
| Variable | n | % | |
|---|---|---|---|
| Gender | female | 786 | 84.33 |
| male | 146 | 15.67 | |
| Age | 18–25 years | 86 | 9.23 |
| 26–35 years | 244 | 26.18 | |
| 36–45 years | 325 | 34.87 | |
| 46–55 years | 169 | 18.13 | |
| 56–65 years | 83 | 8.91 | |
| over 60 years | 25 | 2.68 | |
| Place of residence | village | 196 | 21.03 |
| city up to 10 thousand | 37 | 3.97 | |
| city from 10 to 50 thousand | 159 | 17.06 | |
| city with 50 to 100 thousand | 91 | 9.76 | |
| city with over 100 thousand | 449 | 48.18 | |
| Hospitalization | yes | 85 | 9.12 |
| no | 847 | 90.88 | |
| Nutritional status (BMI) | 17.0–18.49 (underweight) | 108 | 11.59 |
| 18.5–24.99 (norm) | 351 | 37.66 | |
| 25.0–29.9 (overweight) | 269 | 28.86 | |
| 30.0–34.99 (1st degree obesity) | 136 | 14.59 | |
| 35.0–39.99 (2nd degree obesity) | 50 | 5.36 | |
| Time from SARS-CoV-2 infection | up to 3 months | 399 | 42.81 |
| from 3 to 6 months | 125 | 13.41 | |
| over 6 months | 408 | 43.78 | |
| Pair of Variables | R | p | |
|---|---|---|---|
| Time since SARS-CoV-2 infection | Shortness of breath at rest | −0.050792 | 0.121 |
| Shortness of breath during exercise | −0.112570 | <0.001 * | |
| Coughing/loud breathing | −0.028147 | 0.505 | |
| Problems with mobility | −0.083910 | 0.010 * | |
| Fatigue | −0.007824 | 0.831 | |
| Muscle/joint pain | −0.071215 | 0.029 * | |
| Eating problems | −0.075123 | 0.101 | |
| Problems with everyday activities | −0.081740 | 0.012 * | |
| Disturbances in mental comfort | −0.023006 | 0.482 | |
| Overall health perception | 0.064012 | 0.050 * | |
| Age | Shortness of breath at rest | 0.026036 | 0.427 |
| Shortness of breath during exercise | −0.001883 | 0.954 | |
| Coughing/loud breathing | 0.041950 | 0.320 | |
| Problems with mobility | 0.172705 | <0.001 * | |
| Fatigue | 0.029857 | 0.416 | |
| Muscle/joint pain | −0.013400 | 0.683 | |
| Eating problems | −0.082353 | 0.073 | |
| Problems with everyday activities | 0.080511 | 0.014 * | |
| Disturbances in mental comfort | −0.091730 | 0.005 * | |
| Overall health perception | −0.004706 | 0.886 | |
| BMI | Shortness of breath at rest | 0.111565 | <0.001 * |
| Shortness of breath during exercise | 0.150855 | <0.001 * | |
| Coughing/loud breathing | 0.023634 | 0.584 | |
| Problems with mobility | 0.134040 | <0.001 * | |
| Fatigue | 0.085030 | 0.023 * | |
| Muscle/joint pain | 0.023721 | 0.478 | |
| Eating problems | −0.066431 | 0.158 | |
| Problems with everyday activities | 0.103615 | 0.002 * | |
| Disturbances in mental comfort | −0.001368 | 0.967 | |
| Overall health perception | −0.104278 | 0.002 * | |
| Variable | Underweight | Normal Weight | Overweight | 1st Degree Obesity | 2nd Degree Obesity | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Min–Max | SD | M | Min–Max | SD | M | Min–Max | SD | M | Min–Max | SD | M | Min–Max | SD | ||
| Shortness of breath at rest | 1.48 | 0.0–10.0 | 2.49 | 1.42 | 0.0–10.0 | 2.16 | 1.68 | 0.0–10.0 | 2.50 | 2.13 | 0.0–10.0 | 2.64 | 2.00 | 0.0–9.0 | 2.62 | 0.042 * |
| Shortness of breath during exercise | 3.04 | 0.0–10.0 | 2.86 | 3.29 | 0.0–10.0 | 2.91 | 3.59 | 0.0–10.0 | 2.97 | 4.23 | 0.0–10.0 | 3.11 | 4.19 | 0.0–9.0 | 2.97 | <0.001 * |
| Coughing/loud breathing | 4.49 | 0.0–10.0 | 3.08 | 4.05 | 0.0–10.0 | 3.06 | 4.20 | 0.0–10.0 | 3.07 | 4.21 | 0.0–10.0 | 3.26 | 4.90 | 1.0–10.0 | 2.69 | 0.574 |
| Problems with mobility | 1.41 | 0.0–9.0 | 2.20 | 1.36 | 0.0–10.0 | 2.27 | 1.70 | 0.0–10.0 | 2.32 | 2.07 | 0.0–10.0 | 2.60 | 1.64 | 0.0–8.0 | 2.13 | <0.001 * |
| Fatigue | 3.86 | 0.0–10.0 | 3.05 | 3.74 | 0.0–10.0 | 2.88 | 4.01 | 0.0–10.0 | 2.82 | 4.40 | 0.0–10.0 | 3.24 | 4.41 | 0.0–10.0 | 2.49 | 0.321 |
| Muscle/joint pain | 2.27 | 0.0–10.0 | 2.98 | 1.98 | 0.0–9.0 | 2.35 | 2.11 | 0.0–10.0 | 2.59 | 2.33 | 0.0–10.0 | 2.74 | 1.89 | 0.0–8.0 | 2.41 | 0.930 |
| Eating problems | 4.13 | 0.0–10.0 | 3.28 | 4.23 | 0.0–10.0 | 3.23 | 3.89 | 0.0–10.0 | 3.06 | 4.14 | 0.0–10.0 | 3.46 | 2.62 | 0.0–9.0 | 3.01 | 0.371 |
| Problems with everyday activities | 2.29 | 0.0–10.0 | 2.87 | 2.31 | 0.0–10.0 | 2.49 | 2.41 | 0.0–10.0 | 2.65 | 2.73 | 0.0–10.0 | 2.73 | 2.19 | 0.0–8.0 | 2.45 | 0.454 |
| Disturbances in mental comfort | 3.83 | 0.0–10.0 | 3.40 | 3.21 | 0.0–10.0 | 3.02 | 2.96 | 0.0–10.0 | 3.03 | 3.80 | 0.0–10.0 | 3.36 | 3.42 | 0.0–10.0 | 3.33 | 0.099 |
| Overall health perception | 5.32 | 0.0–10.0 | 3.09 | 4.74 | 0.0–10.0 | 2.65 | 4.61 | 0.0–10.0 | 2.69 | 4.38 | 0.0–10.0 | 2.67 | 4.22 | 0.0–10.0 | 2.45 | 0.137 |
| Variable | <3 Months | 3–6 Months | >6 Months | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | Min–Max | SD | M | Min-Max | SD | M | Min–Max | SD | ||
| Shortness of breath at rest | 1.76 | 0.0–10.0 | 2.47 | 1.90 | 0.0–10.0 | 2.66 | 1.51 | 0.0–10.0 | 2.34 | 0.245 |
| Shortness of breath during exercise | 1.71 | 0.0–10.0 | 2.18 | 1.76 | 0.0–9.0 | 2.40 | 1.34 | 0.0–10.0 | 2.12 | 0.003 * |
| Coughing/loud breathing | 4.29 | 0.0–10.0 | 3.04 | 4.49 | 0.0–10.0 | 3.28 | 4.08 | 0.0–10.0 | 3.08 | 0.579 |
| Problems with mobility | 1.70 | 0.0–10.0 | 2.31 | 2.10 | 0.0–10.0 | 2.82 | 1.39 | 0.0–10.0 | 2.22 | 0.015 * |
| Fatigue | 4.01 | 0.0–10.0 | 2.98 | 4.10 | 0.0–10.0 | 3.05 | 3.93 | 0.0–10.0 | 2.87 | 0.920 |
| Muscle/joint pain | 2.22 | 0.0–10.0 | 2.59 | 2.40 | 0.0–9.0 | 2.94 | 1.88 | 0.0–10.0 | 2.41 | 0.078 |
| Eating problems | 2.40 | 0.0–10.0 | 3.18 | 3.15 | 0.0–10.0 | 3.29 | 2.19 | 0.0–10.0 | 3.25 | 0.221 |
| Problems with everyday activities | 2.58 | 0.0–10.0 | 2.67 | 0.95 | 0.0–10.0 | 2.04 | 0.64 | 0.0–10.0 | 1.61 | <0.001 * |
| Disturbances in mental comfort | 2.32 | 0.0–10.0 | 2.77 | 2.93 | 0.0–10.0 | 3.02 | 2.48 | 0.0–10.0 | 2.98 | 0.175 |
| Overall health perception | 4.48 | 0.0–10.0 | 2.69 | 4.90 | 0.0–10.0 | 2.64 | 4.86 | 0.0–10.0 | 2.77 | 0.099 |
| Variable | Female | Male | p | ||||
|---|---|---|---|---|---|---|---|
| M | Min–Max | SD | M | Min–Max | SD | ||
| Shortness of breath at rest | 1.69 | 0.0–10.0 | 2.45 | 1.50 | 0.0–9.0 | 2.34 | 0.248 |
| Shortness of breath during exercise | 3.60 | 0.0–10.0 | 2.98 | 3.13 | 0.0–10.0 | 2.98 | 0.029 * |
| Coughing/loud breathing | 4.39 | 0.0–10.0 | 3.06 | 3.31 | 0.0–10.0 | 3.06 | 0.002 * |
| Problems with mobility | 1.62 | 0.0–10.0 | 2.35 | 1.57 | 0.0–10.0 | 2.31 | 0.015 * |
| Fatigue | 4.00 | 0.0–10.0 | 2.91 | 3.85 | 0.0–10.0 | 3.08 | 0.507 |
| Muscle/joint pain | 2.16 | 0.0–10.0 | 2.57 | 1.70 | 0.0–9.0 | 2.49 | 0.012 * |
| Eating problems | 4.12 | 0.0–10.0 | 3.18 | 3.38 | 0.0–10.0 | 3.48 | 0.081 |
| Problems with everyday activities | 0.89 | 0.0–10.0 | 1.89 | 0.76 | 0.0–7.0 | 1.64 | 0.571 |
| Disturbances in mental comfort | 3.48 | 0.0–10.0 | 3.18 | 2.47 | 0.0–10.0 | 2.96 | <0.001 * |
| Overall health perception | 4.73 | 0.0–10.0 | 2.66 | 4.55 | 0.0–10.0 | 2.99 | 0.383 |
| Pair of Variables | p | |
|---|---|---|
| Difference between before and after COVID-19 | Shortness of breath at rest | <0.001 * |
| Shortness of breath during exercise | <0.001 * | |
| Coughing/loud breathing | <0.001 * | |
| Problems with mobility | <0.001 * | |
| Fatigue | <0.001 * | |
| Problems with everyday activities | <0.001 * | |
| Disturbances in mental comfort | <0.001 * | |
| Overall health perception | 0.534 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mińko, A.; Turoń-Skrzypińska, A.; Rył, A.; Tomska, N.; Bereda, Z.; Rotter, I. Searching for Factors Influencing the Severity of the Symptoms of Long COVID. Int. J. Environ. Res. Public Health 2022, 19, 8013. https://doi.org/10.3390/ijerph19138013
Mińko A, Turoń-Skrzypińska A, Rył A, Tomska N, Bereda Z, Rotter I. Searching for Factors Influencing the Severity of the Symptoms of Long COVID. International Journal of Environmental Research and Public Health. 2022; 19(13):8013. https://doi.org/10.3390/ijerph19138013
Chicago/Turabian StyleMińko, Alicja, Agnieszka Turoń-Skrzypińska, Aleksandra Rył, Natalia Tomska, Zuzanna Bereda, and Iwona Rotter. 2022. "Searching for Factors Influencing the Severity of the Symptoms of Long COVID" International Journal of Environmental Research and Public Health 19, no. 13: 8013. https://doi.org/10.3390/ijerph19138013
APA StyleMińko, A., Turoń-Skrzypińska, A., Rył, A., Tomska, N., Bereda, Z., & Rotter, I. (2022). Searching for Factors Influencing the Severity of the Symptoms of Long COVID. International Journal of Environmental Research and Public Health, 19(13), 8013. https://doi.org/10.3390/ijerph19138013






