Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy
Abstract
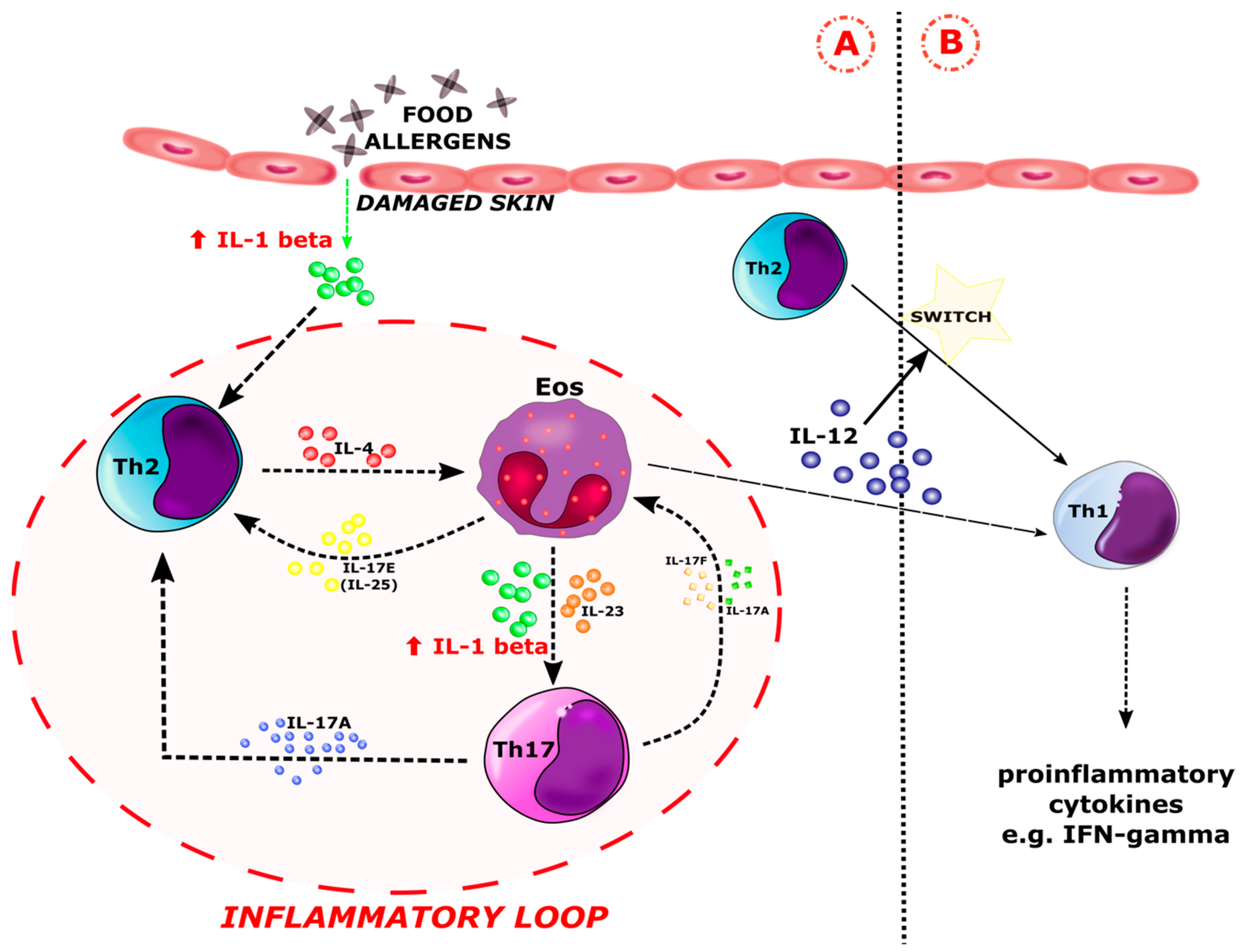
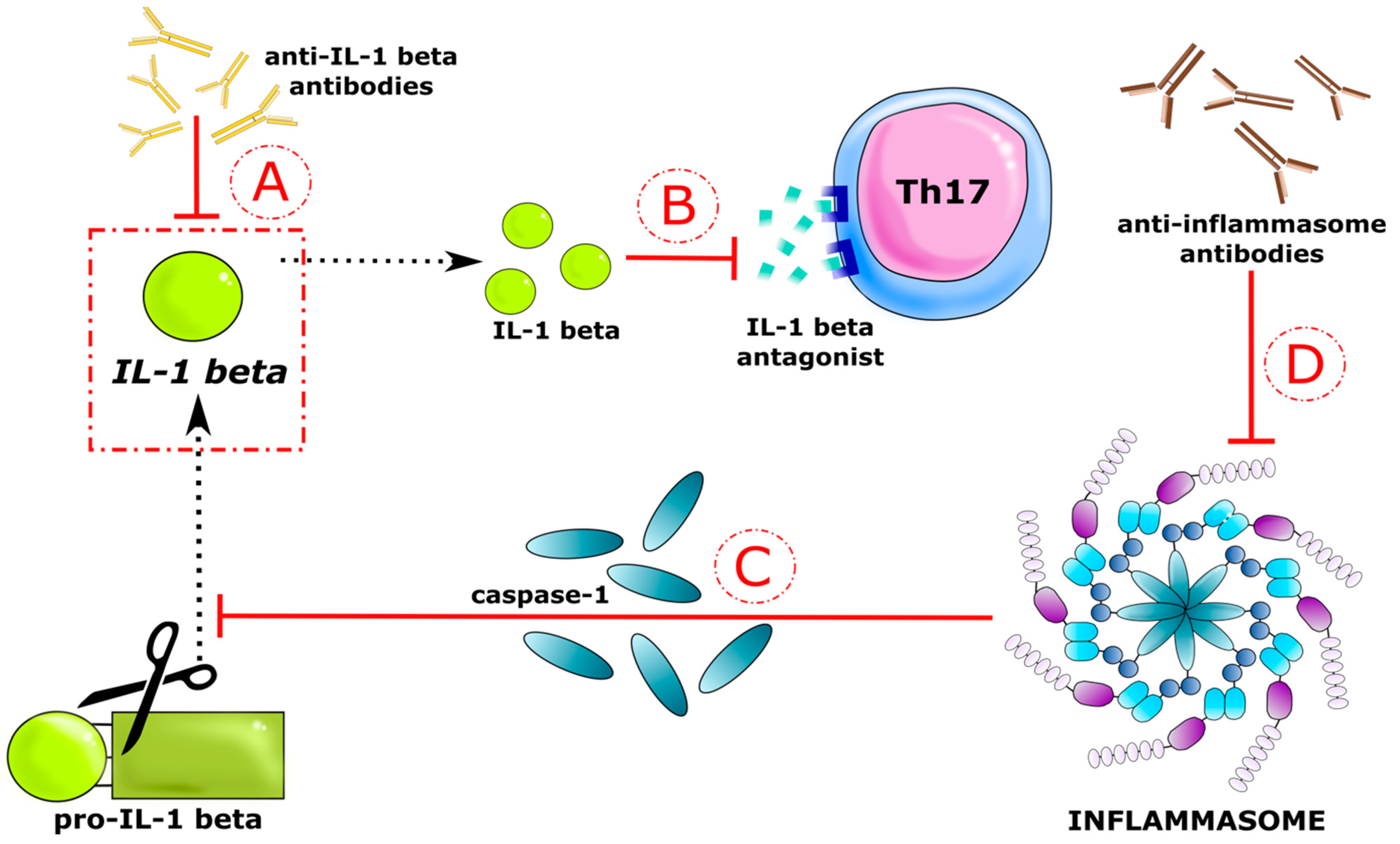
:1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Diagnosis of IgE-Mediated Food Allergy
2.3. Diagnosis of Delayed-Type Food Allergy
2.4. Measurement of Th17 Cytokine Panel
2.5. Data Analysis
3. Results
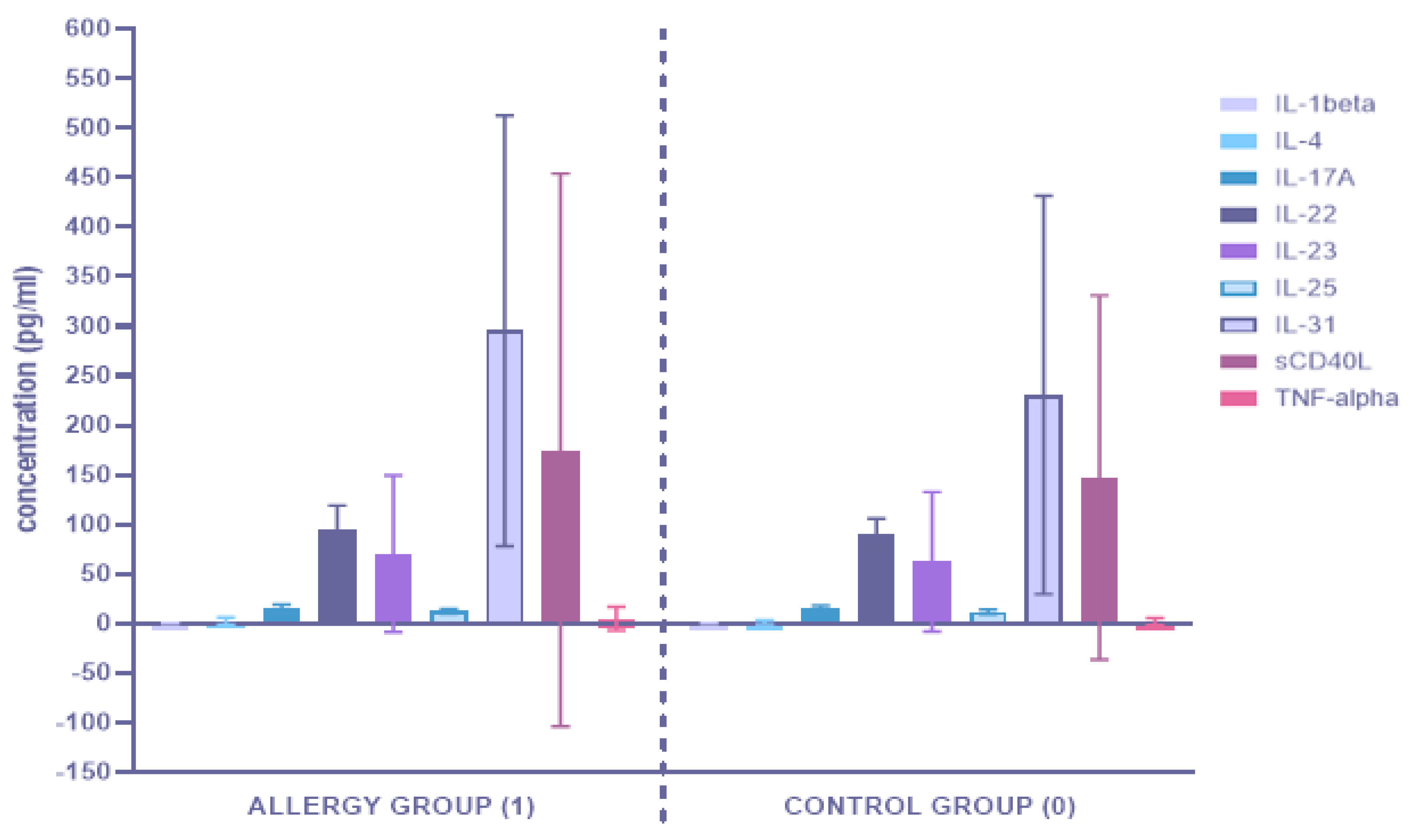
3.1. Alterations in Serum Concentration of the Th17-Related Cytokines
3.1.1. Comparison of Serum Cytokine Concentrations between the Allergy Group (1) and the Control Group (0)
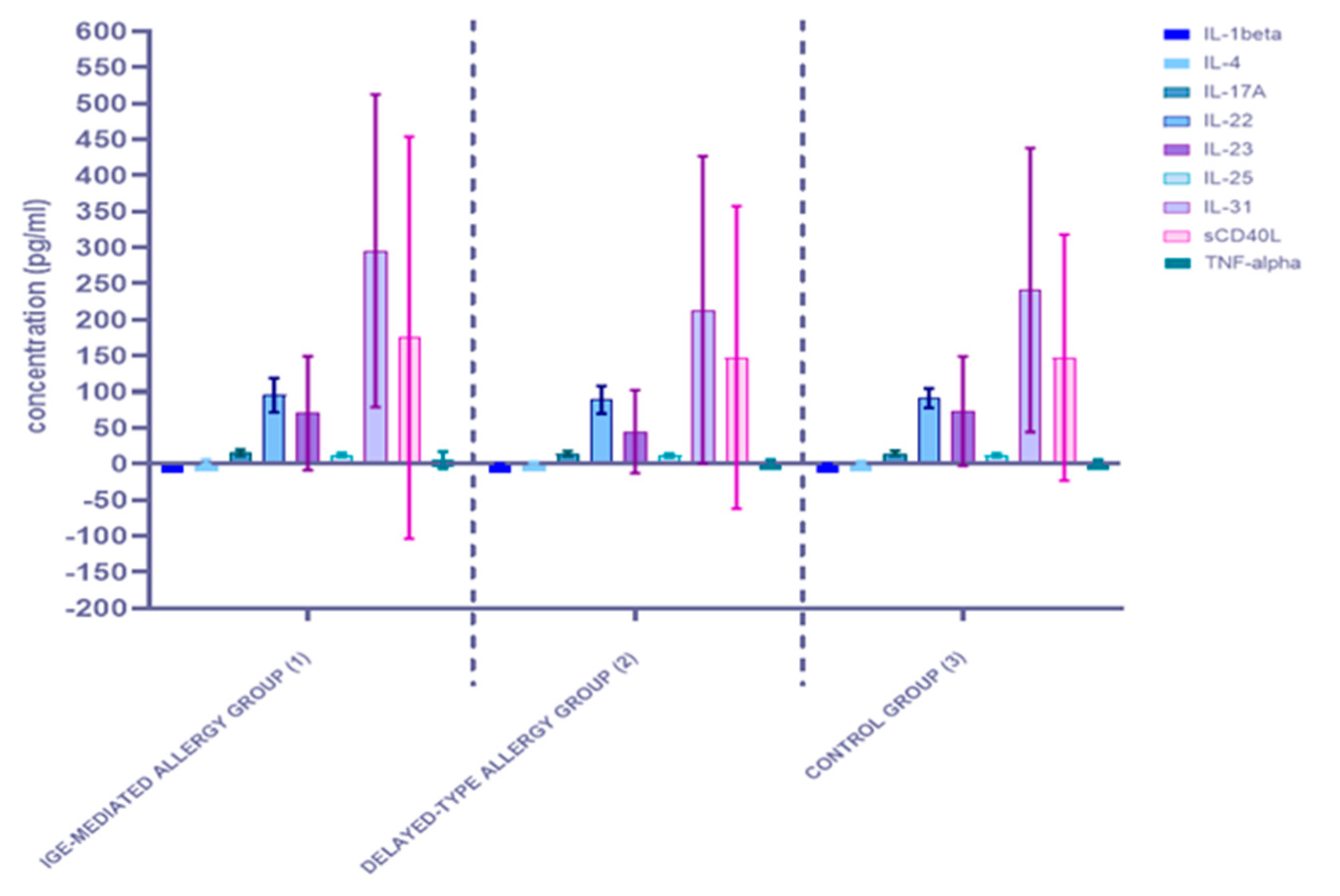
3.1.2. Comparison of Serum Cytokine Concentrations between the Three Studied Groups: The IgE-Mediated Allergy Group (1), the Delayed-Type Allergy Group (2), and the Control Group (3)
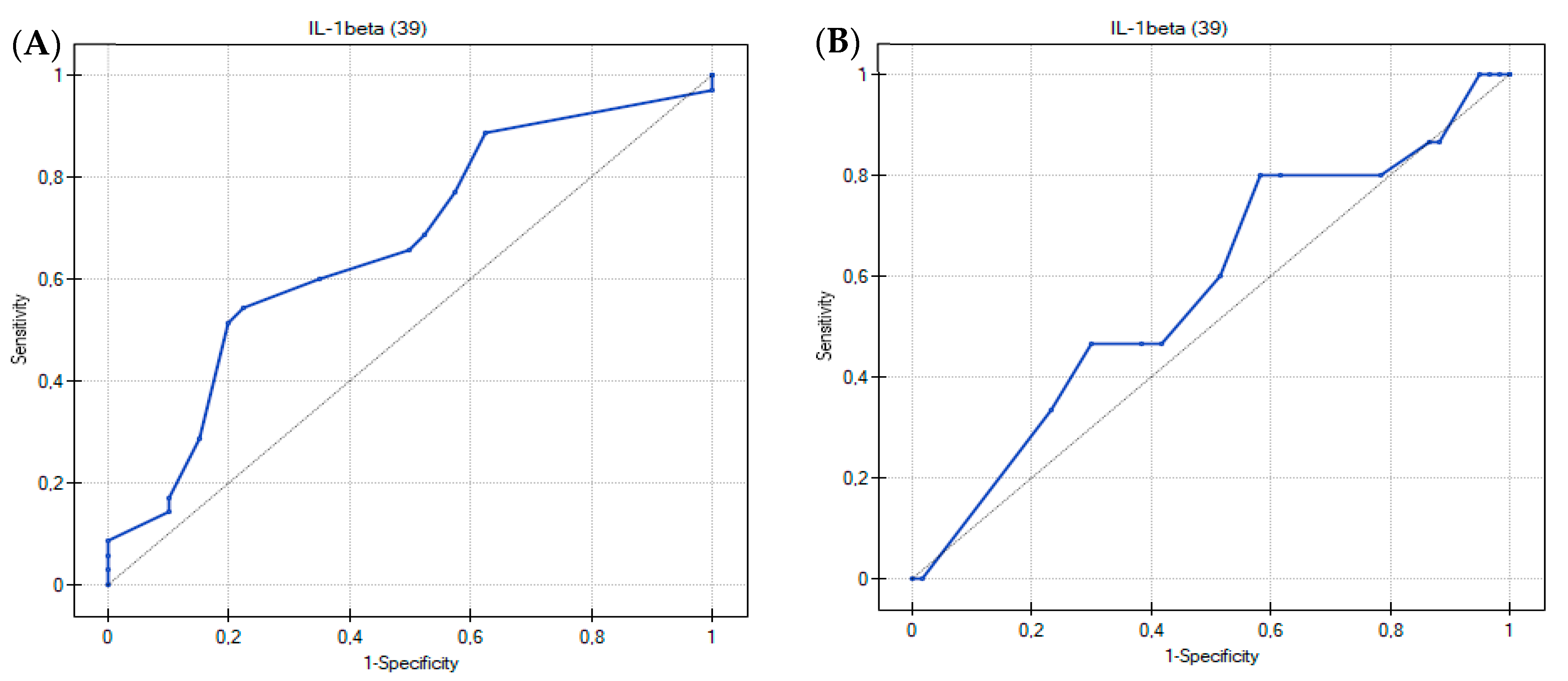
3.2. Usefulness of the Th17-Related Cytokines in the Differentiation of Food Hypersensitivities in Children with Atopic Dermatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- JCI—Precision Medicine and Phenotypes, Endotypes, Genotypes, Regiotypes, and Theratypes of Allergic Diseases. Available online: https://www.jci.org/articles/view/124611 (accessed on 6 June 2022).
- Urashima, M.; Mezawa, H.; Okuyama, M.; Urashima, T.; Hirano, D.; Gocho, N.; Tachimoto, H. Primary Prevention of Cow’s Milk Sensitization and Food Allergy by Avoiding Supplementation with Cow’s Milk Formula at Birth: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Burney, P.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Clausen, M.; Dubakiene, R.; Fernandez-Perez, C.; Fritsche, P.; Jedrzejczak-Czechowicz, M.; et al. Food Allergy in Adults: Substantial Variation in Prevalence and Causative Foods Across Europe. J. Allergy Clin. Immunol. Pract. 2019, 7, 1920–1928.e11. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.A.; Dharmage, S.; Allen, K.; Koplin, J.; Garcia-Larsen, V.; Boyle, R.; Waidyatillake, N.; Lodge, C.J. Age at introduction to complementary solid food and food allergy and sensitization: A systematic review and meta-analysis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019, 49, 754–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Borro, M.; Alonzi, S.; Sindher, S.; Nadeau, K.; Chinthrajah, R.S. Chinthrajah, Improvement in Health-Related Quality of Life in Food-Allergic Patients: A Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2021, 9, 3705–3714. [Google Scholar] [CrossRef]
- Stróżek, J.; Samoliński, B.K.; Kłak, A.; Drużba, E.G.; Izdebski, R.; Fałta, E.K.; Raciborski, F. The indirect costs of allergic diseases. Int. J. Occup. Med. Environ. Health 2019, 32, 281–290. [Google Scholar] [CrossRef]
- Umasunthar, T.; Leonardi-Bee, J.; Turner, P.J.; Hodes, M.; Gore, C.; Warner, J.O.; Boyle, R.J. Incidence of food anaphylaxis in people with food allergy: A systematic review and meta-analysis. Clin. Exp. Allergy 2014, 45, 1621–1636. [Google Scholar] [CrossRef] [Green Version]
- Caubet, J.-C.; Sampson, H.A. Beyond Skin Testing: State of the Art and New Horizons in Food Allergy Diagnostic Testing. Immunol. Allergy Clin. 2012, 32, 97–109. [Google Scholar] [CrossRef]
- Manea, I.; Ailenei, E.; Deleanu, D. Overview of food allergy diagnosis. Clujul Med. 2016, 89, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Sampson, H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J. Allergy Clin. Immunol. 2001, 107, 891–896. [Google Scholar] [CrossRef]
- Szeinbach, S.L.; Barnes, J.H.; Sullivan, T.J.; Williams, P.B. Precision and accuracy of commercial laboratories’ ability to classify positive and/or negative allergen-specific IgE results. Ann. Allergy. Asthma. Immunol. 2001, 86, 373–381. [Google Scholar] [CrossRef]
- Meng, S.; Tan, Y.; Chang, S.; Li, J.; Maleki, S.; Puppala, N. Peanut allergen reduction and functional property improvement by means of enzymatic hydrolysis and transglutaminase crosslinking. Food Chem. 2020, 302, 125186. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Martelli, A.; Calvani, M.; Foiadelli, T.; Tosca, M.; Pingitore, G.; Licari, A.; Marseglia, A.; Ciprandi, G.; Caffarelli, C. Component resolved diagnosis and risk assessment in food allergy. Acta Bio-Med. Atenei Parm. 2021, 92, e2021528. [Google Scholar] [CrossRef]
- Lidholm, J.; Ballmer-Weber, B.K.; Mari, A.; Vieths, S. Component-resolved diagnostics in food allergy. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 234–240. [Google Scholar] [CrossRef]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025.e43. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; The EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.R.; Nachshon, L.; Sinai, T.; Epstein-Rigbi, N.; Oren, Y.; Eisenberg, E.; Katz, Y.; Elizur, A. Risk factors for reduced bone mineral density measurements in milk-allergic patients. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2018, 29, 850–856. [Google Scholar] [CrossRef]
- Dilley, M.A.; Rettiganti, M.; Christie, L.; O’Brien, E.; Patterson, M.; Weeks, C.; Aronson, J.; Scurlock, A.M.; Perry, T.T.; Pesek, R.D.; et al. Impact of food allergy on food insecurity and health literacy in a tertiary care pediatric allergy population. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2019, 30, 363–369. [Google Scholar] [CrossRef]
- Grimshaw, K.E.; Roberts, G.; Selby, A.; Reich, A.; Butiene, I.; Clausen, M.; Dubakiene, R.; Fiandor, A.; Fiocchi, A.; Grabenhenrich, L.; et al. Risk Factors for Hen’s Egg Allergy in Europe: EuroPrevall Birth Cohort. J. Allergy Clin. Immunol. Pract. 2019, 8, 1341–1348.e5. [Google Scholar] [CrossRef]
- Martin, P.E.; Eckert, J.K.; Koplin, J.; Lowe, A.; Gurrin, L.; Dharmage, S.; Vuillermin, P.; Tang, M.L.K.; Ponsonby, A.-L.; Matheson, M.; et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014, 45, 255–264. [Google Scholar] [CrossRef]
- Cartledge, N.; Chan, S. Atopic Dermatitis and Food Allergy: A Paediatric Approach. Curr. Pediatr. Rev. 2018, 14, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Mavroudi, A.; Karagiannidou, A.; Xinias, I.; Cassimos, D.; Karantaglis, N.; Farmaki, E.; Imvrios, G.; Fotoulaki, M.; Eboriadou, M.; Tsanakas, J. Assessment of IgE-mediated food allergies in children with atopic dermatitis. Allergol. Immunopathol. Madr. 2017, 45, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, P.A.; Sicherer, S.H.; Borkowski, T.A.; Cohen, B.A.; Sampson, H.A. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998, 101, e8. [Google Scholar] [CrossRef] [Green Version]
- Maggi, E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998, 3, 233–244. [Google Scholar] [CrossRef]
- Mu, Z.; Zhao, Y.; Liu, X.; Chang, C.; Zhang, J. Molecular Biology of Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2014, 47, 193–218. [Google Scholar] [CrossRef]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. A Role for Th17 Cells in the Immunopathogenesis of Atopic Dermatitis? J. Investig. Dermatol. 2008, 128, 2569–2571. [Google Scholar] [CrossRef] [Green Version]
- Helm, R.M.; Burks, A.W. Mechanisms of food allergy. Curr. Opin. Immunol. 2000, 12, 647–653. [Google Scholar] [CrossRef]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. In Management of Atopic Dermatitis: Methods and Challenges; Fortson, E.A., Feldman, S.R., Strowd, L.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–37. [Google Scholar] [CrossRef]
- Ebiedermann, T.; Eskabytska, Y.; Ekaesler, S.; Evolz, T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015, 6, 353. Available online: https://www.frontiersin.org/article/10.3389/fimmu.2015.00353 (accessed on 7 June 2022).
- McAleer, M.; Jakasa, I.; Hurault, G.; Sarvari, P.; McLean, W.; Tanaka, R.; Kezic, S.; Irvine, A. Systemic and stratum corneum biomarkers of severity in infant atopic dermatitis include markers of innate and T helper cell-related immunity and angiogenesis. Br. J. Dermatol. 2018, 180, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Oyoshi, M.K.; He, R.; Kumar, L.; Yoon, J.; Geha, R.S. Chapter 3 Cellular and Molecular Mechanisms in Atopic Dermatitis. In Advances in Immunology 102; Academic Press: Cambridge, MA, USA, 2009; pp. 135–226. [Google Scholar] [CrossRef]
- Bohle, B. T lymphocytes and food allergy. Mol. Nutr. Food Res. 2004, 48, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Veres, G.; Westerholm-Ormio, M.; Kokkonen, J.; Arato, A.; Savilahti, E. Cytokines and Adhesion Molecules in Duodenal Mucosa of Children with Delayed-Type Food Allergy. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adkinson, N.F., Jr.; Bochner, B.S.; Burks, A.W.; Busse, W.W.; Holgate, S.T.; Lemanske, R.F.; O’Hehir, R.E. Middleton’s Allergy E-Book: Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Fouser, L.A.; Wright, J.F.; Dunussi-Joannopoulos, K.; Collins, M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol. Rev. 2008, 226, 87–102. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-065X.2008.00712.x (accessed on 7 June 2022). [CrossRef]
- Hu, Y.; Shen, F.; Crellin, N.K.; Ouyang, W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann. N. Y. Acad. Sci. 2010, 1217, 60–76. Available online: https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.2010.05825.x (accessed on 7 June 2022). [CrossRef]
- Tesmer, L.A.; Lundy, S.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible Pathogenic Role of Th17 Cells for Atopic Dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [Green Version]
- TSLP Directly Interacts with Skin-Homing Th2 Cells Highly Expressing its Receptor to Enhance IL-4 Production in Atopic Dermatitis—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0022202X1560166X (accessed on 7 June 2022).
- Guenova, E.; Skabytska, Y.; Hoetzenecker, W.; Weindl, G.; Sauer, K.; Tham, M.; Kim, K.W.; Park, J.H.; Seo, J.H.; Ignatova, D.; et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2015, 112, 2163–2168. Available online: https://www.pnas.org/doi/abs/10.1073/pnas.1416922112 (accessed on 7 June 2022). [CrossRef] [Green Version]
- Guttman-Yassky, E.; Lowes, M.A.; Fuentes-Duculan, J.; Zaba, L.C.; Cardinale, I.; Nograles, K.E.; Khatcherian, A.; Novitskaya, I.; Carucci, J.A.; Bergman, R.; et al. Low Expression of the IL-23/Th17 Pathway in Atopic Dermatitis Compared to Psoriasis. J. Immunol. 2008, 181, 7420–7427. [Google Scholar] [CrossRef]
- Toda, M.; Leung, D.Y.; Molet, S.; Boguniewicz, M.; Taha, R.; Christodoulopoulos, P.; Fukuda, T.; Elias, J.A.; Hamid, Q.A. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 2003, 111, 875–881. [Google Scholar] [CrossRef]
- Wang, B.; Hu, J.; Liu, Y.; Liu, Q.; Li, D. Food allergy promotes a Th2/Th17 response that drives house dust mite-induced allergic airway inflammation in humanized mice. Clin. Exp. Immunol. 2020, 202, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bin Dhuban, K.; D’Hennezel, E.; Ben-Shoshan, M.; McCusker, C.; Clarke, A.; Fiset, P.; Mazer, B.; Piccirillo, C.A. Altered T helper 17 responses in children with food allergy. Int. Arch. Allergy Immunol. 2013, 162, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Packi, K.; Matysiak, J.; Matuszewska, E.; Bręborowicz, A.; Kycler, Z.; Matysiak, J. New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors. Int. J. Environ. Res. Public Health 2021, 18, 4011. Available online: https://www.mdpi.com/1660-4601/18/8/4011 (accessed on 7 June 2022). [CrossRef]
- Nakajima, H.; Takatsu, K. Role of Cytokines in Allergic Airway Inflammation. Int. Arch. Allergy Immunol. 2007, 142, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Saggini, A.; Maccauro, G.; Tripodi, D.; De Lutiis, M.; Conti, F.; Felaco, P.; Fulcheri, M.; Galzio, R.; Caraffa, A.; Antinolfi, P.; et al. Allergic Inflammation: Role of Cytokines with Special Emphasis on IL-4. Int. J. Immunopathol. Pharmacol. 2011, 24, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhajj, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK555922/ (accessed on 4 April 2022).
- Calvani, M.; Bianchi, A.; Reginelli, C.; Peresso, M.; Testa, A. Oral Food Challenge. Medicina 2019, 55, 651. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Urisu, A. Diagnosis of Food Allergy Based on Oral Food Challenge Test. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2009, 58, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Houser, B. Bio-Rad’s Bio-Plex® suspension array system, xMAP technology overview. Arch. Physiol. Biochem. 2012, 118, 192–196. [Google Scholar] [CrossRef] [Green Version]
- The Genesis and Evolution of Bead-Based Multiplexing—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1046202318301737 (accessed on 10 June 2022).
- Lu, B.; Liu, M.; Wang, J.; Fan, H.; Yang, D.; Zhang, L.; Gu, X.; Nie, J.; Chen, Z.; Corbett, A.J.; et al. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 2020, 13, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Bouadma, L.; Wiedemann, A.; Patrier, J.; Surénaud, M.; Wicky, P.-H.; Foucat, E.; Diehl, J.-L.; Hejblum, B.P.; Sinnah, F.; de Montmollin, E.; et al. Immune Alterations in a Patient with SARS-CoV-2-Related Acute Respiratory Distress Syndrome. J. Clin. Immunol. 2020, 40, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Gery, I.; Gershon, R.K.; Waksman, B.H. Potentiation of the T-lymphocyte response to mitogens. J. Exp. Med. 1972, 136, 128–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gery, I.; Waksman, B.H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J. Exp. Med. 1972, 136, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A.; Renfer, L.; Wolff, S.M. Human leukocytic pyrogen: Purification and development of a radioimmunoassay. Proc. Natl. Acad. Sci. USA 1977, 74, 4624–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Ross, M.; Meisenberg, B.; O’Reilly, R.; Lilleby, K.; Buckner, C.D.; Appelbaum, F.R.; Buhles, W.; Singer, J.; Peters, W.P. Phase I study of recombinant human interleukin-1 beta (rhIL-1 beta) in patients with bone marrow failure. Bone Marrow Transplant. 1994, 14, 583–588. [Google Scholar]
- Vadhan-Raj, S.; Kudelka, A.P.; Garrison, L.; Gano, J.; Edwards, C.L.; Freedman, R.S.; Kavanagh, J.J. Effects of interleukin-1 alpha on carboplatin-induced thrombocytopenia in patients with recurrent ovarian cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994, 12, 707–714. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, H.R.; Sundy, J.S.; Terkeltaub, R.; Knapp, H.R.; Mellis, S.J.; Stahl, N.; Yancopoulos, G.D.; Soo, Y.; King-Davis, S.; Weinstein, S.P.; et al. Rilonacept (interleukin-1 trap) in the prevention of acute gout flares during initiation of urate-lowering therapy: Results of a phase II randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012, 64, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Liu, W.; Luo, Y.; Tanaka, A.; Cai, X.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J. Biol. Chem. 2010, 285, 6477–6488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Kambe, N.; Saito, M.; Nishikomori, R.; Kim, Y.-G.; Murakami, M.; Núñez, G.; Matsue, H. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J. Exp. Med. 2009, 206, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Gaide, O.; Petrilli, V.; Martinon, F.; Contassot, E.; Roques, S.; Kummer, J.A.; Tschopp, J.; French, L.E. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J. Investig. Dermatol. 2007, 127, 1956–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Sayama, K.; Tohyama, M.; Shirakata, Y.; Hanakawa, Y.; Tokumaru, S.; Yang, L.; Hirakawa, S.; Hashimoto, K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J. Allergy Clin. Immunol. 2011, 127, 806–814.e4. [Google Scholar] [CrossRef]
- Willart, M.; Hammad, H. Lung dendritic cell-epithelial cell crosstalk in Th2 responses to allergens. Curr. Opin. Immunol. 2011, 23, 772–777. [Google Scholar] [CrossRef]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, H.; Cambier, S.; Somanath, S.; Barker, T.; Minagawa, S.; Markovics, J.; Goodsell, A.; Publicover, J.; Reichardt, L.; Jablons, D.; et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Investig. 2011, 121, 2863–2875. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.C. Anti-cytokines and cytokines in the treatment of rheumatoid arthritis. Curr. Pharm. Des. 2003, 9, 1095–1126. [Google Scholar] [CrossRef]
- Nutan, F.; Kanwar, A.; Parsad, D. The effect of topically applied corticosteroids on interleukin 1β levels in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. JEADV 2012, 26, 1020–1022. [Google Scholar] [CrossRef]
- Sutton, C.; Brereton, C.; Keogh, B.; Mills, K.H.; Lavelle, E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1685–1691. [Google Scholar] [CrossRef]
- Benedé, S.; Blázquez, A.B.; Chiang, D.; Tordesillas, L.; Berin, M.C. The rise of food allergy: Environmental factors and emerging treatments. eBioMedicine 2016, 7, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahiner, U.M.; Layhadi, J.A.; Golebski, K.; Komlósi, Z.I.; Peng, Y.; Sekerel, B.; Durham, S.R.; Brough, H.; Morita, H.; Akdis, M.; et al. Innate lymphoid cells: The missing part of a puzzle in food allergy. Allergy 2021, 76, 2002–2016. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.F.Y.; Wong, C.K.; Lam, C.W.K. Molecular Mechanisms of Cytokine and Chemokine Release from Eosinophils Activated by IL-17A, IL-17F, and IL-23: Implication for Th17 Lymphocytes-Mediated Allergic Inflammation. J. Immunol. 2008, 180, 5625–5635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esnault, S.; Kelly, E.A.B.; Nettenstrom, L.M.; Cook, E.B.; Seroogy, C.M.; Jarjour, N.N. Human Eosinophils Release IL-1β and Increase Expression of IL-17A in Activated CD4+ T Lymphocytes. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2012, 42, 1756–1764. [Google Scholar] [CrossRef] [Green Version]
- Simon, H.-U.; Yousefi, S.; Dibbert, B.; Hebestreit, H.; Weber, M.; Branch, D.R.; Blaser, K.; Levi-Schaffer, F.; Anderson, G.P. Role for tyrosine phosphorylation and Lyn tyrosine kinase in fas receptor-mediated apoptosis in eosinophils. Blood 1998, 92, 547–557. [Google Scholar] [CrossRef]
- Hebestreit, H.; Dibbert, B.; Balatti, I.; Braun, D.; Schapowal, A.; Blaser, K.; Simon, H.-U. Disruption of fas receptor signaling by nitric oxide in eosinophils. J. Exp. Med. 1998, 187, 415–425. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kouzaki, H.; Kita, H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J. Immunol. 2010, 184, 6350–6358. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.; Cheung, P.F.Y.; Ip, W.K.; Lam, C.W.K. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am. J. Respir. Cell Mol. Biol. 2007, 37, 85–96. [Google Scholar] [CrossRef]
- Simon, D.; Braathen, L.R.; Simon, H.-U. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Johnson, S.H.; DeLain, L.P.; Haedt, M.J.; Noll, A.L.; Sandbo, N.; Jarjour, N.N. Matrix Metalloproteinase-9-Dependent Release of IL-1β by Human Eosinophils. Mediat. Inflamm. 2019, 2019, e7479107. [Google Scholar] [CrossRef] [Green Version]
- Sutton, C.E.; Lalor, S.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.; Mills, K.H. Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewe, M.; Czech, W.; Morita, K.; Werfel, T.; Klammer, M.; Kapp, A.; Ruzicka, T.; Schöpf, E.; Krutmann, J. Human Eosinophils Produce Biologically Active IL-12: Implications for Control of T Cell Responses. J. Immunol. 1998, 161, 415–420. [Google Scholar] [PubMed]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Krause, K.; Metz, M.; Makris, M.; Zuberbier, T.; Maurer, M. The role of interleukin-1 in allergy-related disorders. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 477–484. [Google Scholar] [CrossRef]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev. Clin. Immunol. 2021, 17, 835–852. [Google Scholar] [CrossRef]
- Tazawa, T.; Sugiura, H.; Sugiura, Y.; Uehara, M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch. Dermatol. Res. 2004, 295, 459–464. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.-C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Żbikowska-Gotz, M.; Pałgan, K.; Gawrońska-Ukleja, E.; Kuźmiński, A.; Przybyszewski, M.; Socha, E.; Bartuzi, Z. Expression of IL-17A concentration and effector functions of peripheral blood neutrophils in food allergy hypersensitivity patients. Int. J. Immunopathol. Pharmacol. 2016, 29, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, M.A.; Fluhr, J.W.; Ruwwe-Glösenkamp, C.; Stevanovic, K.; Bergmann, K.; Zuberbier, T. Role of IL-17 in atopy—A systematic review. Clin. Transl. Allergy 2021, 11, e12047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Angkasekwinai, P.; Lu, N.; Voo, K.S.; Arima, K.; Hanabuchi, S.; Hippe, A.; Corrigan, C.; Dong, C.; Homey, B.; et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007, 204, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeşilova, Y.; Çalka, Ö.; Akdeniz, N.; Berktaş, M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann. Dermatol. 2012, 24, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.-H.; Kang, E.-K.; Quan, S.-H.; Rhee, C.-S.; Lee, C.H.; Kim, D.-Y. Anti-tumor necrosis factor-alpha treatment reduces allergic responses in an allergic rhinitis mouse model. Allergy 2011, 66, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Beser, O.; Konukoglu, D.; Cokugras, H.C.; Erkan, T.; Kutlu, T.; Cokugras, F. The utility of TNF-α, IL-6 and IL-10 in the diagnosis and/or follow-up food allergy. Allergol. Immunopathol. 2020, 48, 48–55. [Google Scholar] [CrossRef]
- Amarasekera, M. Immunoglobulin E in health and disease. Asia Pac. Allergy 2011, 1, 12–15. [Google Scholar] [CrossRef] [Green Version]
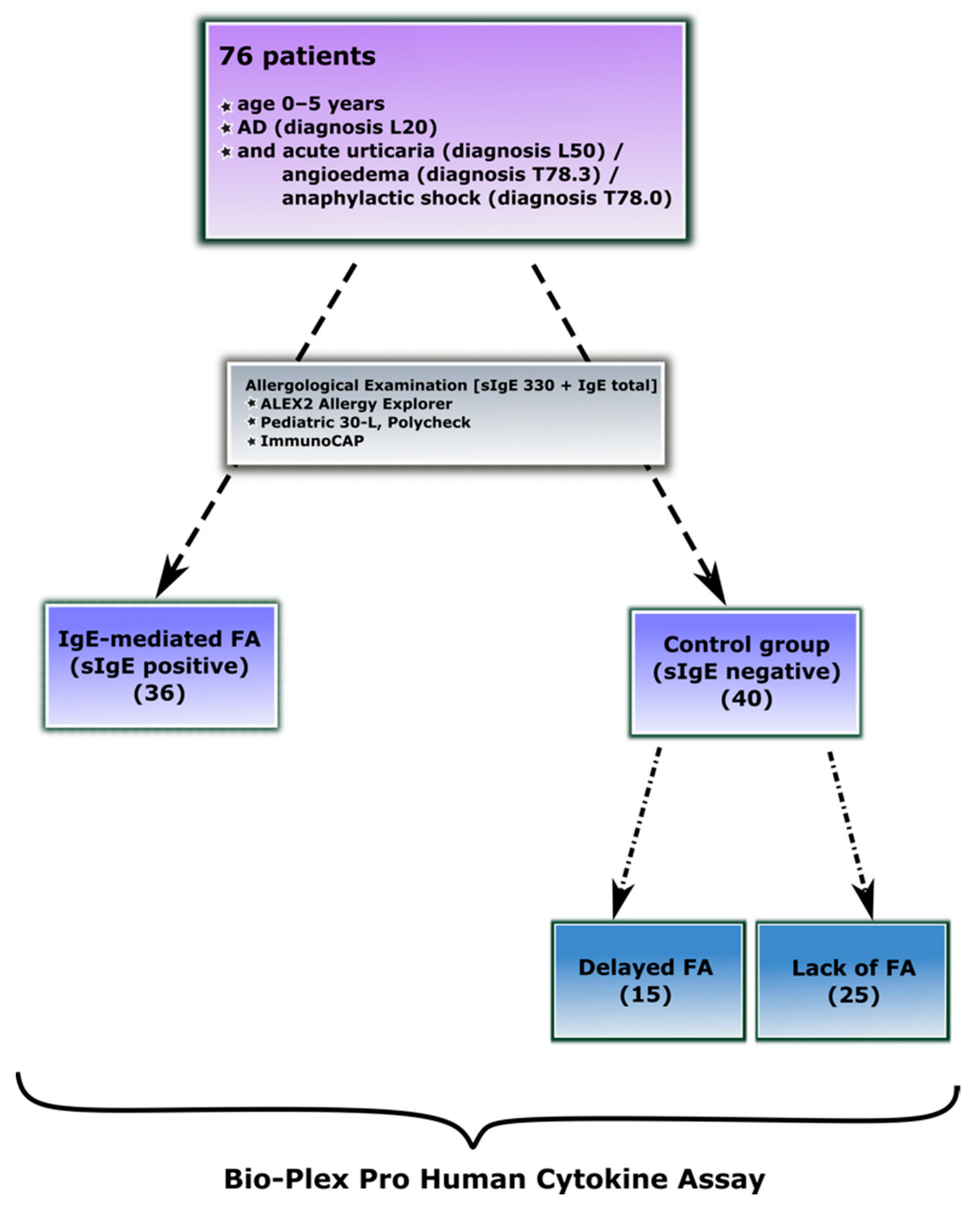

| Characteristics of the Participants | IgE-Mediated Food Allergy (1) | Control Group Lack of IgE-Mediated Food Allergy (0) | |
|---|---|---|---|
| Delayed-Type Food Allergy (2) | Control Group Lack of Food Allergy (3) | ||
| No. of subjects | 36 | 15 | 25 |
| Sex | |||
| Male | 19 (52.8%) | 11 (73.3%) | 14 (56%) |
| Female | 17 (47.2%) | 4 (26.7%) | 11 (44%) |
| Age [months] | |||
| Median | 23.5 | 12 | 20.5 |
| Mean | 25.81 | 13.2 | 26.6 |
| Range | 6–60 | 6–36 | 2–60 |
| Eczema [for the last 6 weeks or more] | 36 | 15 | 25 |
| Patient age on the onset of eczema [months] | |||
| Median | 3 | 3 | 4 |
| Mean | 4.4 | 3.73 | 8.9 |
| Range | 1–24 | 1–10 | 2–36 |
| Atopic dermatitis (L20) | 36 | 15 | 25 |
| Association with foods: | |||
| Milk | 27 | 11 | 13 |
| Egg | 9 | 5 | 2 |
| Egg white | 1 | 1 | 0 |
| Egg yolk | 1 | 0 | 0 |
| Cocoa | 5 | 0 | 3 |
| Chocolate | 2 | 0 | 0 |
| Oatmeal | 0 | 1 | 0 |
| Flour | 0 | 1 | 0 |
| Wheat | 1 | 1 | 3 |
| Gluten | 3 | 1 | 0 |
| Rye bread | 0 | 1 | 0 |
| Ketchup | 1 | 0 | 0 |
| Sweets | 1 | 0 | 0 |
| Nuts | 6 | 0 | 1 |
| Coconut | 2 | 0 | 0 |
| Fruits | 10 | 3 | 2 |
| Banana | 0 | 1 | 1 |
| Strawberry | 4 | 1 | 0 |
| Apple | 2 | 1 | 0 |
| Peach | 1 | 0 | 0 |
| Citrus | 2 | 0 | 1 |
| Juice | 0 | 0 | 2 |
| Carrot | 2 | 0 | 0 |
| Tomato | 1 | 0 | 0 |
| Potato | 1 | 0 | 0 |
| Soy | 0 | 1 | 0 |
| Chickpeas | 0 | 0 | 1 |
| Silage | 1 | 0 | 0 |
| Allergic Urticaria (L50) | 8 | 0 | 2 |
| Angiodema (T78.3) | 5 | 0 | 0 |
| The causative food: | |||
| Hazelnut | 1 | 0 | 0 |
| Milk | 2 | 0 | 0 |
| Egg | 3 | 0 | 0 |
| Egg white | 1 | 0 | 0 |
| Peanut | 2 | 0 | 0 |
| White fish | 1 | 0 | 0 |
| Raisins | 1 | 0 | 0 |
| Gluten | 1 | 0 | 0 |
| Cauliflower | 0 | 0 | 1 |
| Anaphylactic shock (T78.0) | 5 | 0 | 0 |
| Symptoms of a generalized reaction: | |||
| Dyspnoea | 5 | 0 | 0 |
| Vascular edema | 2 | 0 | 0 |
| Hives | 1 | 0 | 0 |
| Adrenaline | 0 | 0 | 0 |
| The causative food: | |||
| Hazelnut | 1 | 0 | 0 |
| Milk | 1 | 0 | 0 |
| Peanut | 1 | 0 | 0 |
| Egg | 1 | 0 | 0 |
| Fish (cod) | 1 | 0 | 0 |
| Chronic symptoms of the digestive system: | |||
| Colic | 0 | 6 | 0 |
| Abdominal pain | 2 | 3 | 0 |
| Abdominal gas | 1 | 2 | 0 |
| Vomiting, Downpouring | 0 | 3 | 0 |
| Diarrhea | 3 | 1 | 2 |
| Constipation | 2 | 1 | 0 |
| Mucus in the stool | 1 | 1 | 0 |
| Blood in the stool | 0 | 0 | 0 |
| Chronic diseases: | |||
| Early childhood asthma | 3 | 2 | 1 |
| Recurrent bronchitis | 0 | 0 | 0 |
| Allergic rhinitis | 7 | 4 | 3 |
| Recurrent upper respiratory tract infections | 1 | 0 | 0 |
| Neurogenic bladder | |||
| 1 | 0 | 0 | |
| Main Criteria for Inclusion | Justification for the Selection of the Test Group |
|---|---|
|
|
|
|
|
|
| Cytokine | p Value | ||
|---|---|---|---|
| Shapiro-Wilk Test | Mann-Whitney U Test | ||
| Allergy Group (1) | Control Group (0) | ||
| IL-1beta | 0.079521 | 0.000159 | 0.014583 |
| IL-4 | <0.0001 | <0.0001 | 0.087304 |
| IL-17A | 0.003265 | 0.008829 | 0.395553 |
| IL-22 | 0.000282 | 0.012779 | 0.121022 |
| IL-23 | 0.000128 | <0.0001 | 0.659412 |
| IL-25 | 0.281176 | 0.005543 | 0.274022 |
| IL-31 | 0.016542 | 0.000028 | 0.260290 |
| sCD40L | <0.0001 | <0.0001 | 0.923855 |
| TNF-alpha | <0.0001 | <0.0001 | 0.815267 |
| Cytokine | p Value | ||||
|---|---|---|---|---|---|
| Shapiro-Wilk Test/K-S | ANOVA Kruskal-Wallis Test | ANOVA Test | |||
| IgE-Mediated Allergy Group (1) | Delayed-Type Allergy Group (2) | Control Group (3) | |||
| IL-1beta | 0.079521 | 0.024564 | 0.003197 | 0.0448 | |
| IL-4 | <0.0001 | 0.021308 | 0.000147 | 0.2189 | |
| IL-17A | 0.003265 | 0.042197 | 0.151014 | 0.6334 | |
| IL-22 | 0.000282 | 0.014216 | 0.657141 | 0.2055 | |
| IL-23 | 0.000128 | 0.002405 | 0.001771 | 0.5333 | |
| IL-25 | 0.281176 | 0.061647 | 0.067417 | 0.3687 | |
| IL-31 | 0.016542 | 0.001127 | 0.003738 | 0.4952 | |
| sCD40L | <0.0001 | 0.000361 | 0.000706 | 0.9312 | |
| TNF-alpha | <0.0001 | <0.0001 | <0.0001 | 0.7305 | |
| ROC Curve Analysis | Cytokines | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IL-1beta | IL-4 | IL-17A | IL-22 | IL-23 | IL-25 | IL-31 | sCD40L | TNF-Alpha | |
| AUC | 0.67 | 0.62 | 0.56 | 0.61 | 0.53 | 0.57 | 0.58 | 0.51 | 0.52 |
| SE(AUC) | 0.063 | 0.065 | 0.067 | 0.066 | 0.066 | 0.067 | 0.066 | 0.066 | 0.060 |
| −95% CI | 0.540589 | 0.488565 | 0.425263 | 0.475555 | 0.401245 | 0.443203 | 0.446278 | 0.377998 | 0.399014 |
| +95% CI | 0.788697 | 0.742149 | 0.689737 | 0.733731 | 0.658755 | 0.704655 | 0.705864 | 0.635574 | 0.633129 |
| p value | 0.0144 | 0.0863 | 0.3926 | 0.1198 | 0.6556 | 0.2717 | 0.2580 | 0.9196 | 0.8111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Packi, K.; Matysiak, J.; Klimczak, S.; Matuszewska, E.; Bręborowicz, A.; Pietkiewicz, D.; Matysiak, J. Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy. Int. J. Environ. Res. Public Health 2022, 19, 7877. https://doi.org/10.3390/ijerph19137877
Packi K, Matysiak J, Klimczak S, Matuszewska E, Bręborowicz A, Pietkiewicz D, Matysiak J. Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy. International Journal of Environmental Research and Public Health. 2022; 19(13):7877. https://doi.org/10.3390/ijerph19137877
Chicago/Turabian StylePacki, Kacper, Joanna Matysiak, Sylwia Klimczak, Eliza Matuszewska, Anna Bręborowicz, Dagmara Pietkiewicz, and Jan Matysiak. 2022. "Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy" International Journal of Environmental Research and Public Health 19, no. 13: 7877. https://doi.org/10.3390/ijerph19137877
APA StylePacki, K., Matysiak, J., Klimczak, S., Matuszewska, E., Bręborowicz, A., Pietkiewicz, D., & Matysiak, J. (2022). Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy. International Journal of Environmental Research and Public Health, 19(13), 7877. https://doi.org/10.3390/ijerph19137877





