Participation in Low Back Pain Management: It Is Time for the To-Be Scenarios in Digital Public Health
Abstract
:1. Introduction
2. Materials and Methods
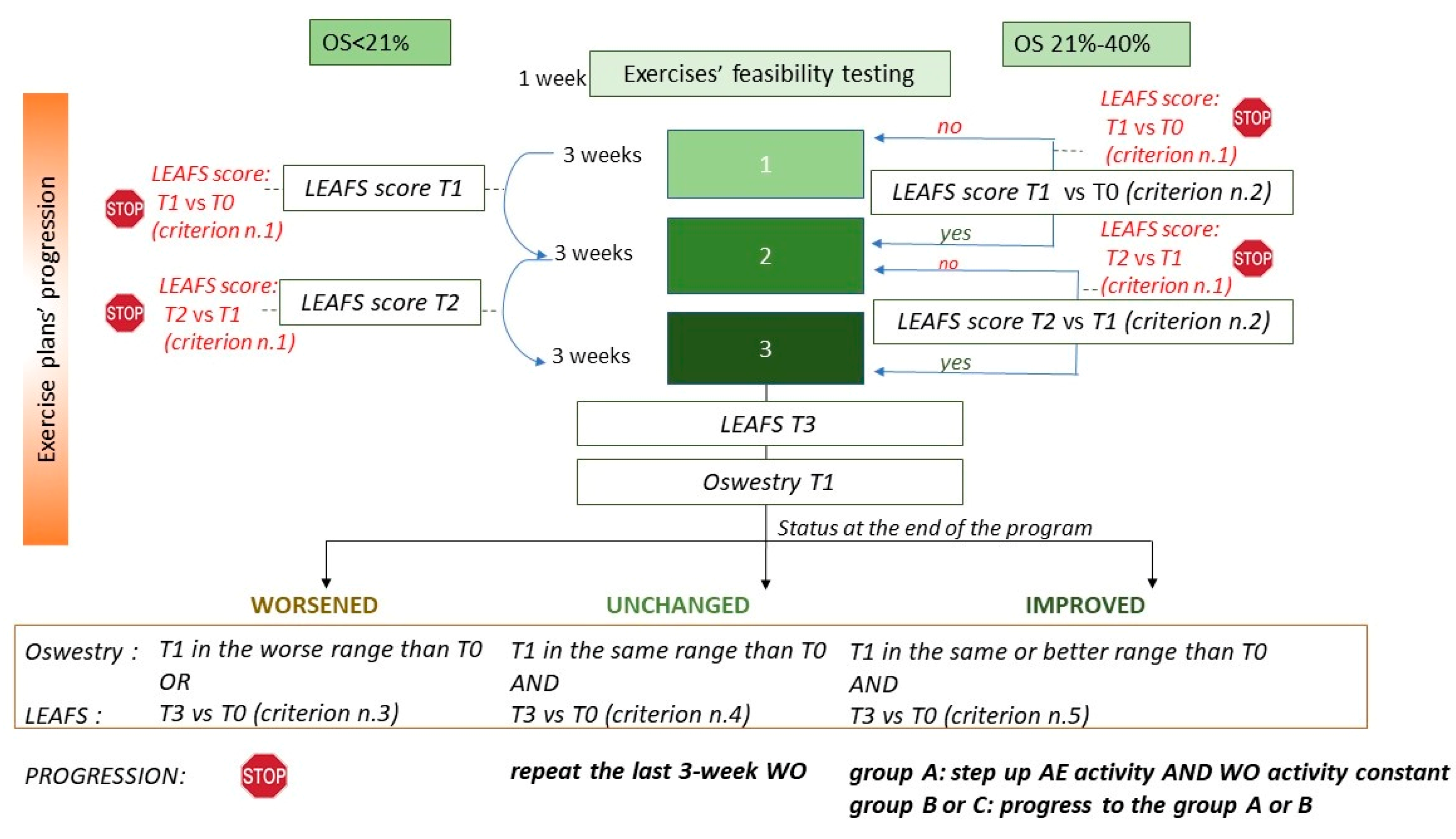
2.1. The Dress-KINESIS Tool
- The WO section (lasting from 45 min to one hour) includes a warm-up phase and some daily exercises aimed at strengthening the core muscles and the deep abdominal muscles, promoting respiratory control and increasing the range of motion of the spine and joints as well as overall body flexibility. These movements focus on improving balance, developing the muscular rate of force and reducing the neuromuscular fatigability.
- The AE section (lasting about 30 min) includes some specific aerobic activities (running, walking and climbing steps) and stretching movements. The AE section aims at preventing/limiting bone loss and metabolic syndrome. The AE sections are tailored based on some abilities of the tool users, identified through the answers provided to specific items of the LEAFS scale.
- Aim (improving balance, strengthening the core muscles, hip mobilization, spine flexibility, promoting respiratory control, etc.);
- Starting position (lying prone or supine, standing, kneeling, etc.);
- Type of movement;
- Target muscles;
- Effort intensity, based on the work metabolic rate of each type of exercise (MET, metabolic equivalent) [29] and the number of repetitions planned for each user.
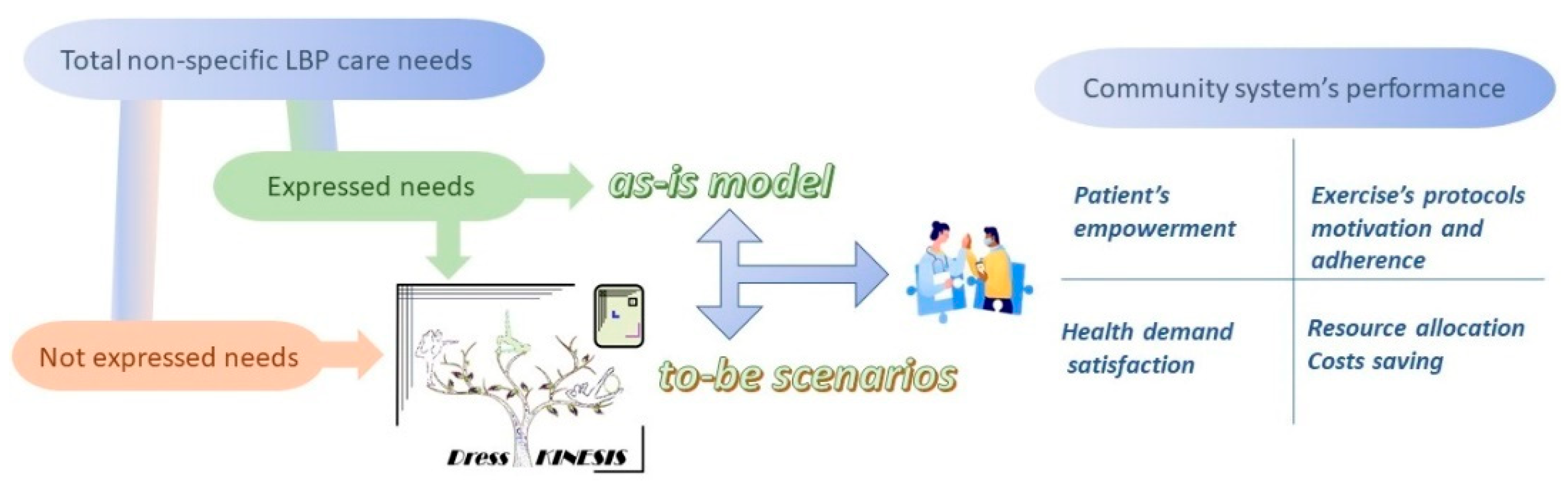
2.2. The Scenarios Approach
- The Ministry of Health surveyed 1141 rehabilitation institutes in Italy. During 2019, each institute counted an average of 9.91 outpatient accesses per day (95% CI: 2.17–17.65%) in the area of motor rehabilitation (expressed needs) [33].
- About 90% of all patients show non-specific LBP [34]. To manage uncomplicated acute LBP, international guidelines recommend the provision of advice, education, reassurance and simple analgesics, but 33% of patients who follow first-line care experience a recurrence in the next 12 months, and 20% to 30% develop chronic pain [35].
- Early engagement of patients reduces healthcare costs through the avoidance of unnecessary investigations and treatments [36]. Childs et al. estimated that in the 2-year follow-up period after a new episode of LBP, patients involved in early physical therapy protocols (within 0–14 days following pain onset) save EUR 1106 of healthcare costs (prescription of drugs and hospital costs) compared to patients undergoing delayed physical therapy [37].
- Between 58% and 75% of LBP patients (medium and high risk of poor prognosis) require further physiotherapy treatments after having performed the first activity protocol [40].
- LBP therapy strategies mainly depend on pain classification in terms of intensity and duration [41]. Many clinical trials have been conducted to evaluate the effects of particular physical interventions, but heterogeneity among LBP patients included in the same intervention tends to dilute the treatment results [42].
- Subgroup-matched treatment approaches have been proposed to improve evidence-based guidelines for supporting clinical decision-making. In the field of conservative management of LBP, the Treatment-Based Classification (TBC) system was first described by Delitto and colleagues in 1995 and has been further updated based on emerging evidence [27].
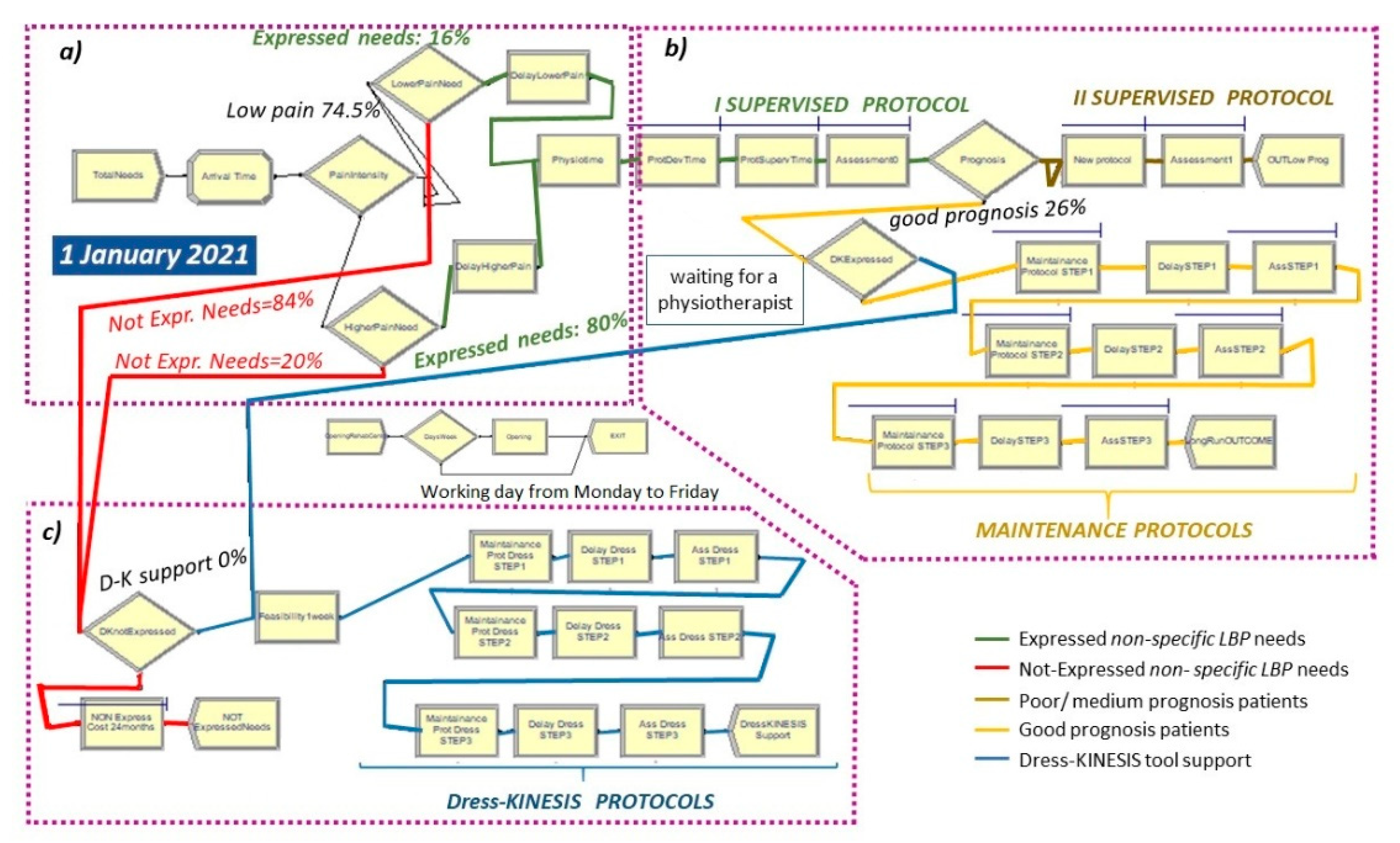
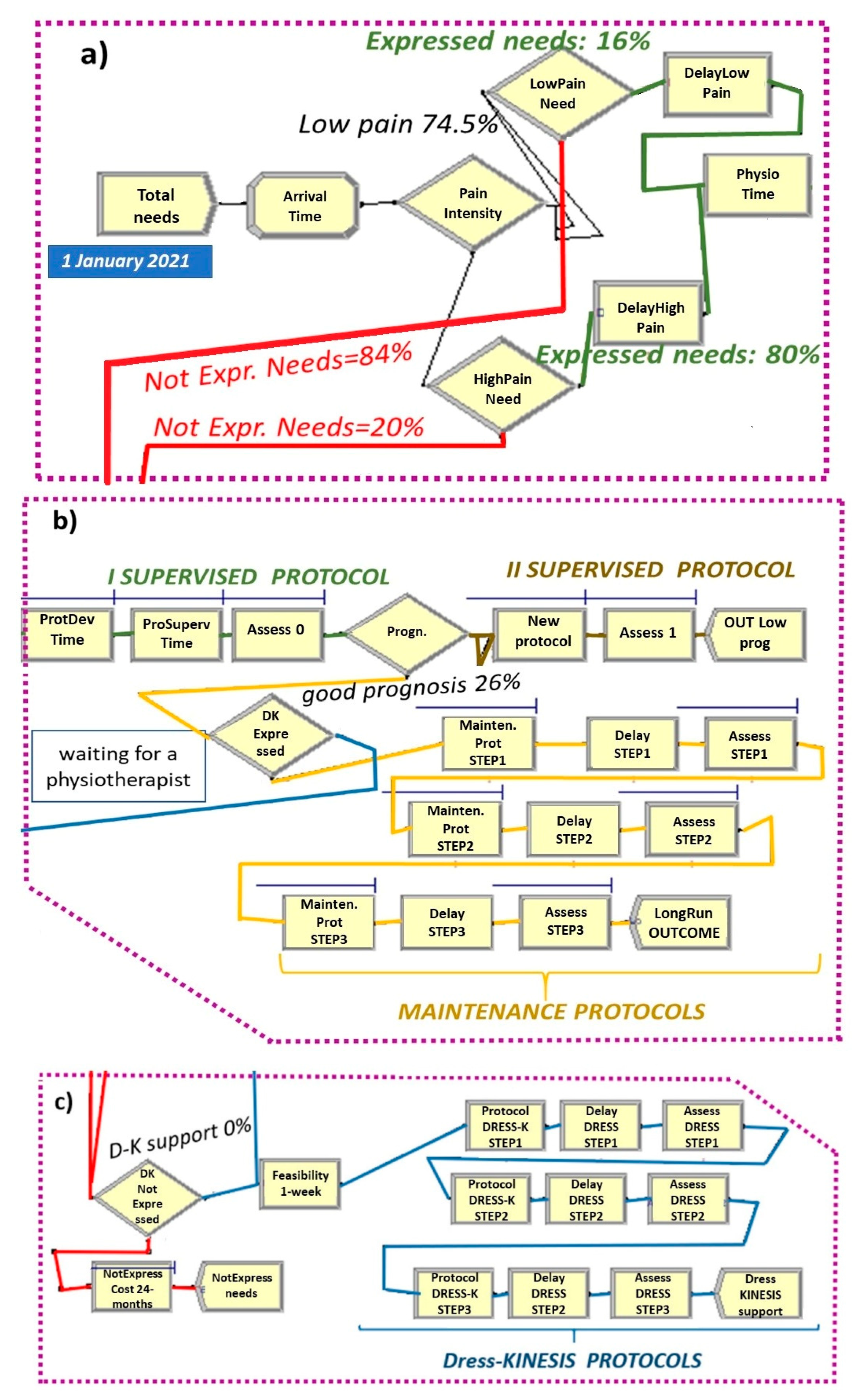
2.3. The As-Is Scenario
- Entities, the dynamic objects in the simulation, representing non-specific LBP patients;
- Resources including (a) four physiotherapists with their work cost (EUR 12.00/h) and (b) drug prescription and inpatient costs during the 24 months after LBP onset (EUR 1106.00). Healthcare costs regard patients who did not express their care needs only.
- Total Needs create module (Figure 5 and Figure 6a), set by considering: (a) total demand of 5.8 patients/day per rehabilitation institute, (b) time between arrivals of patients with expressed and non-expressed needs, following a “random(expo)” distribution with an average interarrival time of 0.98 h (λ) and an arrival rate of 1.02 patients/hour (1/λ). These parameters are estimated from an LBP point prevalence of 7.5% [31].
- Attribute module: TNOW. This attribute is set to the system time (TNOW) when a new patient arrives. It records the simulation clock time as the model progresses.
- PhysioTime hold and signal module (Figure 5): used for restricting the physiotherapists’ working days to Monday-to-Friday intervals.
- ProtSupervTime seize–delay–release module (Figure 5 and Figure 6b): used for modeling the time spent by a physiotherapist on the supervision of each patient during protocol execution. Under the hypothesis that a physiotherapist spends three working hours per week on each patient and follows a group of three patients at a time, the physiotherapy daily effort is set to 2.5% of his total time. Additionally, the ProtSupervTime follows a triangular distribution of 20, 30, 40 days.
- New Protocol seize–delay–release module (Figure 5 and Figure 6b): used for modeling the time spent by a physiotherapist on the supervision of each patient with a poor/medium prognosis during the adjunctive protocol execution. This module has the same parameters of the ProtSupervTime seize–delay–release module.
- (1)
- (2)
2.4. The To-Be Scenarios
2.5. Systems Replication Parameters
- NUMBER OF REPLICATIONS (of each model): 100;
- STARTING DATE AND TIME of replication: Friday 2021/01/01 9:00;
- LENGTH OF EACH REPLICATION: 30 days;
- HOURS PER DAY of replication: 8.
2.6. Output Measures and Statistical Analysis
3. Results
4. Discussion
- One-size-fits-all examination and long waiting times could lead patients to feel that they are not being treated as individuals, thus promoting distrust in healthcare services and hesitation to take health assessments [50].
- Consumers’ spending attitudes are influenced by the cost level of preventive medical services, in particular the out-of-pocket cost, as well as the expected health profit from a medical intervention. This makes health information/education a central point in consumers’ decision-making processes [50].
- In the real world, up to 70% of patients do not engage in prescribed home exercise, and the lack of time and/or motivation are the main reasons pointed out by people to justify this [51]. This reduces both the adherence to the maintenance activity among patients who have performed a first cycle of supervised exercise and the propensity to perform any structured physical activity among those who do not seek care, in particular when the perceived intensity of pain is low.
- The most effective treatments for LBP consist of tailored designed exercise programs which are delivered in a supervised format (e.g., home exercise with regular therapist follow-up) [52].
Strength and Limitations:
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchini, M.; Molinaro, S.; Caiolfa, M.; Salvatori, M.; Pieroni, S. Facing the National Recovery and Resilience Plan: Sources of Data, Indicators, and Participatory Strategies in Healthcare and Social Fields. Int. J. Environ. Res. Public Health 2021, 18, 10457. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, H.C.; Ostermann, T.; Redaèlli, M. Using the scenario method in the context of health and health care—A scoping review. BMC Med Res. Methodol. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmar, H.C.; Goluchowicz, K.; Beckert, B.; Dönitz, E.; Bartholomeyczik, S.; Ostermann, T.; Boustani, M.; Buscher, I. Health care for people with dementia in 2030—Results of a multidisciplinary scenario process. Health Policy 2014, 114, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.R. City scenarios for the future of personalized medicine: A participatory scenario process in Germany. Int. J. Interdiscip. Soc. Community Stud. 2013, 7, 1–16. [Google Scholar] [CrossRef]
- Richards, T.; Montori, V.; Godlee, F.; Lapsley, P.; Paul, D. Let the patient revolution begin. BMJ 2013, 346, f2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, V. The Participatory Zeitgeist in Health Care: It is Time for a Science of Participation. J. Particip. Med. 2020, 12, e15101. [Google Scholar] [CrossRef]
- Sampalli, T.; Dickson, R.; Hayden, J.; Edwards, L.; Salunkhe, A. Meeting the needs of a complex population: A functional health- and patient-centered approach to managing multimorbidity. J. Comorb. 2016, 6, 76–84. [Google Scholar] [CrossRef]
- Vuong, Q.-H.; Le, T.-T.; La, V.-P.; Nguyen, H.T.T.; Ho, M.-T.; Van Khuc, Q.; Nguyen, M.-H. Covid-19 vaccines production and societal immunization under the serendipity-mindsponge-3D knowledge management theory and conceptual framework. Humanit. Soc. Sci. Commun. 2022, 9, 22. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberg, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement. Altern. Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- Kongsted, A.; Ris, I.; Kjaer, P.; Hartvigsen, J. Self-management at the core of back pain care: 10 key points for clinicians. Braz. J. Phys. Ther. 2021, 25, 396–406. [Google Scholar] [CrossRef]
- Ministero delle Finanze. The National Recovery and Resilience Plan (NRRP). Available online: https://italiadomani.gov.it/en/home.htmlh (accessed on 21 April 2022).
- Odone, A.; Buttigieg, S.; Ricciardi, W.; Azzopardi-Muscat, N.; Staines, A. Public health digitalization in Europe. Eur. J. Public Health 2019, 29 (Suppl. 3), 28–35, Erratum in Eur. J. Public Health 2021, 31, e1. [Google Scholar] [CrossRef] [PubMed]
- Iyamu, I.; Xu, A.X.T.; Gómez-Ramírez, O.; Ablona, A.; Chang, H.-J.; Mckee, G.; Gilbert, M. Defining Digital Public Health and the Role of Digitization, Digitalization, and Digital Transformation: Scoping Review. JMIR Public Health Surveill. 2021, 7, e30399. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, W.; Pita Barros, P.; Bourek, A.; Brouwer, W.; Kelsey, T.; Lehtonen, L. Expert Panel on Effective Ways of Investing in Health (EXPH). How to govern the digital transformation of health services. Eur. J. Public Health 2019, 29 (Suppl. 3), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Cummins, N.; Schuller, B.W. Five Crucial Challenges in Digital Health. Front. Digit. Health 2020, 2, 536203. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, T.A.; White, G.O., III. The World Economic Forum and Nike: Emerging ‘Shared Responsibility’ and Institutional Control Models for Achieving a Socially Responsible Global Supply Chain? Bus. Hum. Rights J. 2016, 1, 307–313. [Google Scholar] [CrossRef]
- Khalil, A.-A.; Meyliana; Hidayanto, A.N.; Prabowo, H. Identification of Factor Affecting Continuance Usage Intention of mHealth Application: A Systematic Literature Review. In Proceedings of the 4th International Conference on Informatics and Computational Sciences (ICICoS), Semarang, Indonesia,, 10–11 November 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Alpay, L.; Van Der Boog, P.; Dumaij, A. An empowerment-based approach to developing innovative e-health tools for self-management. Health Inform. J. 2011, 17, 247–255. [Google Scholar] [CrossRef]
- Dutmer, A.L.; Preuper, H.R.S.; Soer, R.; Brouwer, S.; Bültmann, U.; Dijkstra, P.U.; Coppes, M.H.; Stegeman, P.; Buskens, E.; van Asselt, A.D.; et al. Personal and Societal Impact of Low Back Pain: The Groningen Spine Cohort. Spine 2019, 44, E1443–E1451. [Google Scholar] [CrossRef]
- Garattini, L.; Koleva, D.; Motterlini, N.; Cornago, D. Medical Costs of Chronic Musculoskeletal Pain in Italy. Clin. Drug Investig. 2007, 27, 139–148. [Google Scholar] [CrossRef]
- Traeger, A.C.; Buchbinder, R.; Elshaug, A.G.; Croft, P.R.; Maher, C. Care for low back pain: Can health systems deliver? Bull. World Health Organ. 2019, 97, 423–433. [Google Scholar] [CrossRef]
- Chenot, J.-F.; Scherer, M.; Becker, A.; Donner-Banzhoff, N.; Baum, E.; Leonhardt, C.; Keller, S.; Pfingsten, M.; Hildebrandt, J.; Basler, H.-D.; et al. Acceptance and perceived barriers of implementing a guideline for managing low back in general practice. Implement Sci. 2008, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Bishop, F.; Dima, A.L.; Ngui, J.; Little, P.; Moss-Morris, R.; Foster, N.E.; Lewith, G.T. “Lovely Pie in the Sky Plans”: A Qualitative Study of Clinicians’ Perspectives on Guidelines for Managing Low Back Pain in Primary Care in England. Spine 2015, 40, 1842–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchini, M.; Pieroni, S.; Martini, N.; Ripoli, A.; Chiappino, D.; Denoth, F.; Liebman, M.N.; Molinaro, S.; Della Latta, D. Shifting the Paradigm: The Dress-COV Telegram Bot as a Tool for Participatory Medicine. Int. J. Environ. Res. Public Health 2020, 17, 8786. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Baiardi, P.; Ferrari, S.; Foti, C.; Mugnai, R.; Pillastrini, P.; Vanti, C.; Zanoli, G. Development of the Italian version of the Oswestry Disability Index (ODI-I): A cross-cultural adaptation, reliability, and validity study. Spine 2009, 34, 2090–2095. [Google Scholar] [CrossRef]
- Cacchio, A.; De Blasis, E.; Necozione, S.; Rosa, F.; Riddle, D.L.; di Orio, F.; De Blasis, D.; Santilli, V. The Italian version of the lower extremity functional scale was reliable, valid, and responsive. J. Clin. Epidemiol. 2010, 63, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Delitto, A.; E Erhard, R.; Bowling, R.W. A treatment-based classification approach to low back syndrome: Identifying and staging patients for conservative treatment. Phys. Ther. 1995, 75, 470–485; discussion 485-9. [Google Scholar] [CrossRef]
- Costa, L.D.C.M.; Maher, C.G.; Hancock, M.J.; McAuley, J.H.; Herbert, R.D.; Costa, L.O. The prognosis of acute and persistent low-back pain: A meta-analysis. Can. Med. Assoc. J. 2012, 184, E613–E624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, M.D.A.; da Silva, I.C.M.; Ramires, V.; Reichert, F.; Martins, R.; Ferreira, R.; Tomasi, E. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PLoS ONE 2018, 13, e0200701. [Google Scholar] [CrossRef] [Green Version]
- Glenn, J.C. The Futures Group International. Scenarios. In Futures Research Methodology Version 3.0. The Millenium Project; Glenn, J.C., Gordon, T.J., Eds.; Milennium Project: Washington, DC, USA, 2011; p. 52. ISBN -10:0981894119. [Google Scholar]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Walker, B.F. The prevalence of low back pain: A systematic review of the literature from 1966 to 1998. J. Spinal Disord. 2000, 13, 205–217. [Google Scholar] [CrossRef]
- Ministero della Salute. Annuario Statistico del Servizio Sanitario Nazionale, Anno 2019. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3073_allegato.pdf (accessed on 21 April 2022).
- Koes, B.W.; van Tulder, M.; Thomas, S. Diagnosis and treatment of low back pain. BMJ 2006, 332, 1430–1434. [Google Scholar] [CrossRef] [Green Version]
- da Silva, T.; Mills, K.; Brown, B.T.; Herbert, R.D.; Maher, C.G.; Hancock, M.J. Risk of Recurrence of Low Back Pain: A Systematic Review. J. Orthop. Sports Phys. Ther. 2017, 47, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Dentzer, S. Rx for the ‘blockbuster drug’ of patient engagement. Health Aff. 2013, 32, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, J.D.; Fritz, J.M.; Wu, S.S.; Flynn, T.W.; Wainner, R.S.; Robertson, E.K.; Kim, F.S.; George, S.Z. Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv. Res. 2015, 15, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, S.A.; Merryweather, A.; Thiese, M.S.; Hegmann, K.T.; Lu, M.-L.; Kapellusch, J.M.; Marras, W.S. Prevalence of low back pain, seeking medical care, and lost time due to low back pain among manual material handling workers in the United States. BMC Musculoskelet. Disord. 2019, 20, 243. [Google Scholar] [CrossRef] [Green Version]
- Freburger, J.K.; Holmes, G.M.; Agans, R.P.; Jackman, A.M.; Darter, J.D.; Wallace, A.S.; Castel, L.D.; Kalsbeek, W.D.; Carey, T.S. The rising prevalence of chronic low back Pain. Arch. Intern. Med. 2009, 169, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.C.; Whitehurst, D.G.; Lewis, M.; Bryan, S.; Dunn, K.M.; E Foster, N.; Konstantinou, K.; Main, C.J.; Mason, E.; Somerville, S.; et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): A randomised controlled trial. Lancet 2011, 378, 1560–1571. [Google Scholar] [CrossRef] [Green Version]
- Webb, R.; Brammah, T.; Lunt, M.; Urwin, M.; Allison, T.; Symmons, D. Prevalence and predictors of intense, chronic, and disabling neck and back pain in the UK general Population. Spine 2003, 28, 1195–1202. [Google Scholar] [CrossRef]
- Alrwaily, M.; Timko, M.; Schneider, M.; Stevans, J.; Bise, C.; Hariharan, K.; Delitto, A. Treatment-Based Classification System for Low Back Pain: Revision and Update. Phys. Ther. 2016, 96, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Kelton, W.D.; Sadowski, R.P.; Zupick, N.B. Simulation with Arena, 6th ed.; McGraw Hill: New York, NY, USA, 2014; ISBN -10:0073401315. [Google Scholar]
- Sawilowsky, S.S. Fermat, Schubert, Einstein, and Behrens-Fisher: The probable difference between two means with different variance. J. Mod. Appl. Stat. Methods 2002, 1, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Zigenfus, G.C.; Yin, J.; Giang, G.M.; Fogarty, W.T. Effectiveness of early physical therapy in the treatment of acute low back musculoskeletal Disorders. J. Occup. Environ. Med. 2000, 42, 35–39. [Google Scholar] [CrossRef]
- Flannery, D.; Jarrin, R. Building A Regulatory and Payment Framework Flexible Enough to Withstand Technological Progress. Health Aff. 2018, 37, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Ekman, B. Cost Analysis of a Digital Health Care Model in Sweden. PharmacoEconomics Open 2018, 2, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dullet, N.W.; Geraghty, E.M.; Kaufman, T.; Kissee, J.L.; King, J.; Dharmar, M.; Smith, A.C.; Marcin, J.P. Impact of a University-Based Outpatient Telemedicine Program on Time Savings, Travel Costs, and Environmental Pollutants. Value Health 2017, 20, 542–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.Z.; Childs, J.D.; Teyhen, D.S.; Wu, S.S.; Wright, A.C.; Dugan, J.L.; E Robinson, M. Rationale, design, and protocol for the prevention of low back pain in the military (POLM) trial (NCT00373009). BMC Musculoskelet. Disord. 2007, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.-H.; Ho, T.-M.; Nguyen, H.-K.; Vuong, T.-T. Healthcare consumers’ sensitivity to costs: A reflection on behavioural economics from an emerging market. Palgrave Commun. 2018, 4, 70. [Google Scholar] [CrossRef]
- Rodrigues, F.; Bento, T.; Cid, L.; Neiva, H.; Teixeira, D.; Moutão, J.; Marinho, D.A.; Monteiro, D. Can Interpersonal Behavior Influence the Persistence and Adherence to Physical Exercise Practice in Adults? A Systematic Review. Front. Psychol. 2018, 9, 2141. [Google Scholar] [CrossRef]
- Beinart, N.A.; Goodchild, C.E.; Weinman, J.A.; Ayis, S.; Godfrey, E. Individual and intervention-related factors associated with adherence to home exercise in chronic low back pain: A systematic review. Spine J. 2013, 13, 1940–1950. [Google Scholar] [CrossRef]
- Sinsky, C.A.; Bavafa, H.; Roberts, R.G.; Beasley, J.W. Standardization vs. Customization: Finding the Right Balance. Ann. Fam. Med. 2021, 19, 171–177. [Google Scholar] [CrossRef]
- Seron, P.; Oliveros, M.-J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef]
- Albahrouh, S.I.; Buabbas, A.J. Physiotherapists’ perceptions of and willingness to use telerehabilitation in Kuwait during the COVID-19 pandemic. BMC Med. Inform. Decis. Mak. 2021, 21, 122. [Google Scholar] [CrossRef]
- D’Souza, A.F.; Rebello, S.R. Perceptions and Willingness of Physiotherapists in India to Use Telerehabilitation During the COVID-19 Pandemic. Int. J. Telerehabilitation 2021, 13, e6425. [Google Scholar] [CrossRef] [PubMed]
- Cranen, K.; Groothuis-Oudshoorn, C.G.; Vollenbroek-Hutten, M.M.; IJzerman, M.J. Toward Patient-Centered Telerehabilitation Design: Understanding Chronic Pain Patients’ Preferences for Web-Based Exercise Telerehabilitation Using a Discrete Choice Experiment. J. Med. Internet Res. 2017, 19, e26. [Google Scholar] [CrossRef] [PubMed]
- Fioratti, I.; Fernandes, L.G.; Reis, F.J.; Saragiotto, B.T. Strategies for a safe and assertive telerehabilitation practice. Braz. J. Phys. Ther. 2021, 25, 113–116. [Google Scholar] [CrossRef]
- Ashwood, J.S.; Mehrotra, A.; Cowling, D. Uscher-Pines L. Direct-To-Consumer Telehealth May Increase Access To Care But Does Not Decrease Spending. Health Aff. 2017, 36, 485–491. [Google Scholar] [CrossRef] [PubMed]
- EU Missions & Citizen Engagement Activities. Available online: https://ec.europa.eu/info/research-and-innovation/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe/eu-missions-horizon-europe/eu-missions-citizen-engagement-activities_en (accessed on 16 June 2022).


| Physiotherapists | Healthcare Costs Next 2 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | % +D-K Support | Pat. IN 30 Days (95% CI Half Width) | Av. WIP (95% CI Half Width) | %Δ WIP (p) | Av. Number physio. Use over Time (95% CI Half Width) | Av. Number Patients Waiting for a physio. (95% CI half width) | Average Waiting Days for a Physio. (95% CI Half Width) | Av. Cost (€) (95% CI Half Width) | %Δ Cost (p) | Av. Costs (€) (95% CI Half Width) | %Δ Costs (p) |
| AS IS | E:0; NE:0 | 245.52 (±3.09) | 45.43 (±0.86) | - | 0.85 (±0.02) | 1.97 (±0.05) | 0.84 (±0.02) | 439.10 (±13.47) | - | 183,662.36 (±2914.46) | - |
| TO BE Expr | E:50 | 245.59 (±3.08) | 45.43 (±0.87) | 0.0% (>0.05) | 0.85 (±0.02) | 1.97 (±0.05) | 0.84 (±0.02) | 438.65 (±13.39) | −0.10% (>0.05) | 183,695.54 (±2914.33) | 0.02% (>0.05) |
| E:60 | 245.63 (±3.05) | 45.43 (±0.87) | 0.0% (>0.05) | 0.85 (±0.02) | 1.97 (±0.05) | 0.84 (±0.02) | 438.52 (±13.38) | −0.18% (>0.05) | 183,717.66 (±2910.66) | 0.04% (>0.05) | |
| E:70 | 245.66 (±3.05) | 45.43 (±0.87) | 0.0% (>0.05) | 0.85 (±0.02) | 1.97 (±0.05) | 0.84 (±0.02) | 438.31 (±13.38) | −0.18% (>0.05) | 183,739.78 (±2910.66) | 0.04% (>0.05) | |
| E:80 | 245.63 (±3.02) | 45.43 (±0.86) | 0.0% (>0.05) | 0.85 (±0.02) | 1.97 (±0.05) | 0.84 (±0.02) | 438.19 (±13.35) | −0.21% (>0.05) | 183,784.02 (±2888.98) | 0.07% (>0.05) | |
| E:100 | 245.80 (±3.04) | 45.43 (±0.86) | 0.0% (>0.05) | 0.85 (±0.02) | 1.97 (±0.05) | 0.84 (±0.02) | 437.96 (±13.36) | −0.26% (>0.05) | 183,994.16 (±2933.87) | 0.18% (>0.05) | |
| TO BE Not-Expr | NE:10 | 246.50 (±3.44) | 53.27 (±1.02) | 17.26% (<0.05) | 0.85 (±0.02) | 2.03 (±0.06) | 0.87 (±0.02) | 439.36 (±13.73) | −0.16% (>0.05) | 167,028.12 (±3068.02) | −9.04% (<0.05) |
| NE:15 | 247.32 (±3.26) | 57.45 (±1.10) | 26.46% (<0.05) | 0.86 (±0.02) | 2.06 (±0.06) | 0.87 (±0.02) | 447.36 (±13.71) | 1.88% (>0.05) | 157,394.86 (±2700.72) | −14.30% (<0.05) | |
| NE:20 | 247.11 (±3.64) | 60.89 (±1.16) | 34.03% (<0.05) | 0.85 (±0.02) | 2.06 (±0.06) | 0.87 (±0.02) | 441.66 (±13.90) | 0.58% (>0.05) | 149,210.46 (±2666.38) | −18.76% (<0.05) | |
| TO BE Mixed | E:80; NE:10 | 246.50 (±3.48) | 53.27 (±1.02) | 17.26% (<0.05) | 0.85 (±0.02) | 2.03 (±0.06) | 0.87 (±0.02) | 438.41 (±14.52) | −0.16% (>0.05) | 167,050.24 (±3062.47) | −9.04% (<0.05) |
| E:80; NE:15 | 247.23 (±3.24) | 57.44 (±1.10) | 26.44% (<0.05) | 0.86 (±0.02) | 2.05 (±0.06) | 0.87 (±0.02) | 445.85 (±13.53) | 1.54% (>0.05) | 157,350.62 (±2714.94) | −14.33% (<0.05) | |
| E:80; NE:20 | 247.02 (±3.59) | 60.89 (±1.16) | 34.03% (<0.05) | 0.85 (±0.02) | 2.06 (±0.06) | 0.87 (±0.02) | 440.48 (±13.69) | 0.31% (>0.05) | 149,011.38 (±2617.97) | −18.87% (<0.05) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchini, M.; Salvatori, M.; Denoth, F.; Molinaro, S.; Pieroni, S. Participation in Low Back Pain Management: It Is Time for the To-Be Scenarios in Digital Public Health. Int. J. Environ. Res. Public Health 2022, 19, 7805. https://doi.org/10.3390/ijerph19137805
Franchini M, Salvatori M, Denoth F, Molinaro S, Pieroni S. Participation in Low Back Pain Management: It Is Time for the To-Be Scenarios in Digital Public Health. International Journal of Environmental Research and Public Health. 2022; 19(13):7805. https://doi.org/10.3390/ijerph19137805
Chicago/Turabian StyleFranchini, Michela, Massimiliano Salvatori, Francesca Denoth, Sabrina Molinaro, and Stefania Pieroni. 2022. "Participation in Low Back Pain Management: It Is Time for the To-Be Scenarios in Digital Public Health" International Journal of Environmental Research and Public Health 19, no. 13: 7805. https://doi.org/10.3390/ijerph19137805
APA StyleFranchini, M., Salvatori, M., Denoth, F., Molinaro, S., & Pieroni, S. (2022). Participation in Low Back Pain Management: It Is Time for the To-Be Scenarios in Digital Public Health. International Journal of Environmental Research and Public Health, 19(13), 7805. https://doi.org/10.3390/ijerph19137805






