Erectile Dysfunction in Relation to Metabolic Disorders and the Concentration of Sex Hormones in Aging Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Determination of Sex Hormones
2.3. Determination of the Concentrations of Biochemical Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braun, M.; Wassmer, G.; Klotz, T.; Reifenrath, B.; Mathers, M.; Engelmann, U. Epidemiology of Erectile Dysfunction: Results of the “Cologne Male Survey”. Int. J. Impot. Res. 2000, 12, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.B.; Araujo, A.B.; Feldman, H.A.; Derby, C.A.; Kleinman, K.P.; McKinlay, J.B. Incidence of Erectile Dysfunction in Men 40 to 69 Years Old: Longitudinal Results from the Massachusetts Male Aging Study. J. Urol. 2000, 163, 460–463. [Google Scholar] [CrossRef]
- Schouten, B.W.V.; Bosch, J.L.H.R.; Bernsen, R.M.D.; Blanker, M.H.; Thomas, S.; Bohnen, A.M. Incidence Rates of Erectile Dysfunction in the Dutch General Population. Effects of Definition, Clinical Relevance and Duration of Follow-up in the Krimpen Study. Int. J. Impot. Res. 2005, 17, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.T.; Kim, N.N.; Paick, J.S.; Simonsen, U.U.; Ückert, S.; Wespes, E.; Andersson, K.E.; et al. Anatomy, Physiology, and Pathophysiology of Erectile Dysfunction. J. Sex. Med. 2010, 7, 445–475. [Google Scholar] [CrossRef]
- McCabe, M.P.; Sharlip, I.D.; Atalla, E.; Balon, R.; Fisher, A.D.; Laumann, E.; Lee, S.W.; Lewis, R.; Segraves, R.T. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J. Sex. Med. 2016, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Serefoglu, E.C.; Mcmahon, C.G.; Waldinger, M.D.; Althof, S.E.; Shindel, A.; Adaikan, G.; Becher, E.F.; Dean, J.; Giuliano, F.; Hellstrom, W.J.G.; et al. An Evidence-Based Unified Definition of Lifelong and Acquired Premature Ejaculation: Report of the Second International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation. Sex. Med. 2014, 2, 41–59. [Google Scholar] [CrossRef]
- Coskuner, E.R.; Ozkan, B. Premature ejaculation and endocrine disorders: A literature review. World J. Mens Health 2022, 40, 38–51. [Google Scholar] [CrossRef]
- Soni, K.K.; Jeong, H.-S.; Jang, S. Neurons for Ejaculation and Factors Affecting Ejaculation. Biology 2022, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Cappelleri, J.C.; Gendrano, N. The International Index of Erectile Function (IIEF): A State-of-the-Science Review. Int. J. Impot. Res. 2002, 14, 226–244. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, Y.H.; Qin, L.Q. Erectile Dysfunction and Risk of Cardiovascular Disease: Meta-Analysis of Prospective Cohort Studies. J. Am. Coll. Cardiol. 2011, 58, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Briganti, A.; Jackson, G.; Kloner, R.A.; Montorsi, F.; Montorsi, P.; Vlachopoulos, C. A Systematic Review of the Association between Erectile Dysfunction and Cardiovascular Disease. Eur. Urol. 2014, 65, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The International Index of Erectile Function (IIEF): A Multidimensional Scale for Assessment of Erectile Dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A Critical Evaluation of Simple Methods for the Estimation of Free Testosterone in Serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.D.; Malkin, C.J.; Channer, K.S.; Jones, T.H. A Mathematical Comparison of Techniques to Predict Biologically Available Testosterone in a Cohort of 1072 Men. Eur. J. Endocrinol. 2004, 151, 241–249. [Google Scholar] [CrossRef][Green Version]
- Kahn, H.S. The “Lipid Accumulation Product” Performs Better than the Body Mass Index for Recognizing Cardiovascular Risk: A Population-Based Comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. Obesity, the Metabolic Syndrome, and Sexual Dysfunction. Int. J. Impot. Res. 2005, 17, 391–398. [Google Scholar] [CrossRef]
- Loves, S.; Ruinemans-Koerts, J.; de Boer, H. Letrozole Once a Week Normalizes Serum Testosterone in Obesity-Related Male Hypogonadism. Eur. J. Endocrinol. 2008, 158, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Rajfer, J. Relationship between testosterone and erectile dysfunction. Rev. Urol. 2000, 2, 122–128. [Google Scholar]
- Barone, B.; Napolitano, L.; Abate, M.; Cirillo, L.; Reccia, P.; Passaro, F.; Turco, C.; Morra, S.; Mastrangelo, F.; Scarpato, A.; et al. The Role of Testosterone in the Elderly: What Do We Know? Int. J. Mol. Sci. 2022, 23, 3535. [Google Scholar] [CrossRef]
- Saad, F.; Caliber, M.; Doros, G.; Haider, K.S.; Haider, A. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male 2020, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, L.; Liu, Z.; Wu, S.; Xu, L. Leptin Level and Oxidative Stress Contribute to Obesity-Induced Low Testosterone in Murine Testicular Tissue. Oxidative Med. Cell. Longev. 2014, 2014, 190945. [Google Scholar] [CrossRef]
- Haider, K.S.; Haider, A.; Doros, G.; Traish, A. Long-Term Testosterone Therapy Improves Urinary and Sexual Function, and Quality of Life in Men with Hypogonadism: Results from a Propensity Matched Subgroup of a Controlled Registry Study. J. Urol. 2018, 199, 257–265. [Google Scholar] [CrossRef]
- Ahn, H.S.; Park, C.M.; Lee, S.W. The Clinical Relevance of Sex Hormone Levels and Sexual Activity in the Ageing Male. BJU Int. 2002, 89, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G.R.; Stephens-Shields, A.J.; Rosen, R.C.; Wang, C.; Bhasin, S.; Matsumoto, A.M.; Parsons, J.K.; Gill, T.M.; Molitch, M.E.; Farrar, J.T.; et al. Testosterone Treatment and Sexual Function in Older Men With Low Testosterone Levels. J. Clin. Endocrinol. Metab. 2016, 101, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Andersen, I.; Heitman, B.L.; Wagner, G. Obesity and Sexual Dysfunction in Younger Danish Men. J. Sex. Med. 2008, 5, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.A.; Nettleship, J.E.; Salman, M.; Almehmadi, Y. Waist Circumference Is Superior to Weight and BMI in Predicting Sexual Symptoms, Voiding Symptoms and Psychosomatic Symptoms in Men with Hypogonadism and Erectile Dysfunction. Andrologia 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.J.; King, W.C.; White, G.E.; Subak, L.L.; Mitchell, J.E.; Courcoulas, A.P.; Flum, D.R.; Strain, G.; Sarwer, D.B.; Kolotkin, R.L.; et al. Sexual Functioning of Men and Women with Severe Obesity before Bariatric Surgery. Surg. Obes. Relat. Dis. 2017, 13, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Dursun, M.; Besiroglu, H.; Cakir, S.S.; Otunctemur, A.; Ozbek, E. Increased Visceral Adiposity Index Associated with Sexual Dysfunction in Men. Aging Male 2018, 21, 187–192. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Patients with Erectile Dysfunction n = 72 | Patients without Erectile Dysfunction n = 34 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X | SD | Min | Max | X | SD | Min | Max | ||
| Age (years) | 65.48 | 4.28 | 60.00 | 75.00 | 64.68 | 4.52 | 60.00 | 74.00 | 0.382 |
| Body weight (kg) | 90.88 | 14.91 | 57.00 | 125.00 | 90.92 | 12.34 | 70.00 | 114.00 | 0.900 |
| WC (cm) | 105.60 | 11.50 | 73.00 | 126.00 | 103.10 | 9.54 | 80.00 | 118.00 | 0.352 |
| BMI (kg/m2) | 29.54 | 4.44 | 14.99 | 42.97 | 29.52 | 3.73 | 21.91 | 37.89 | 0.927 |
| WHR | 1.03 | 0.06 | 0.89 | 1.26 | 1.00 | 0.06 | 0.94 | 1.16 | 0.044 * |
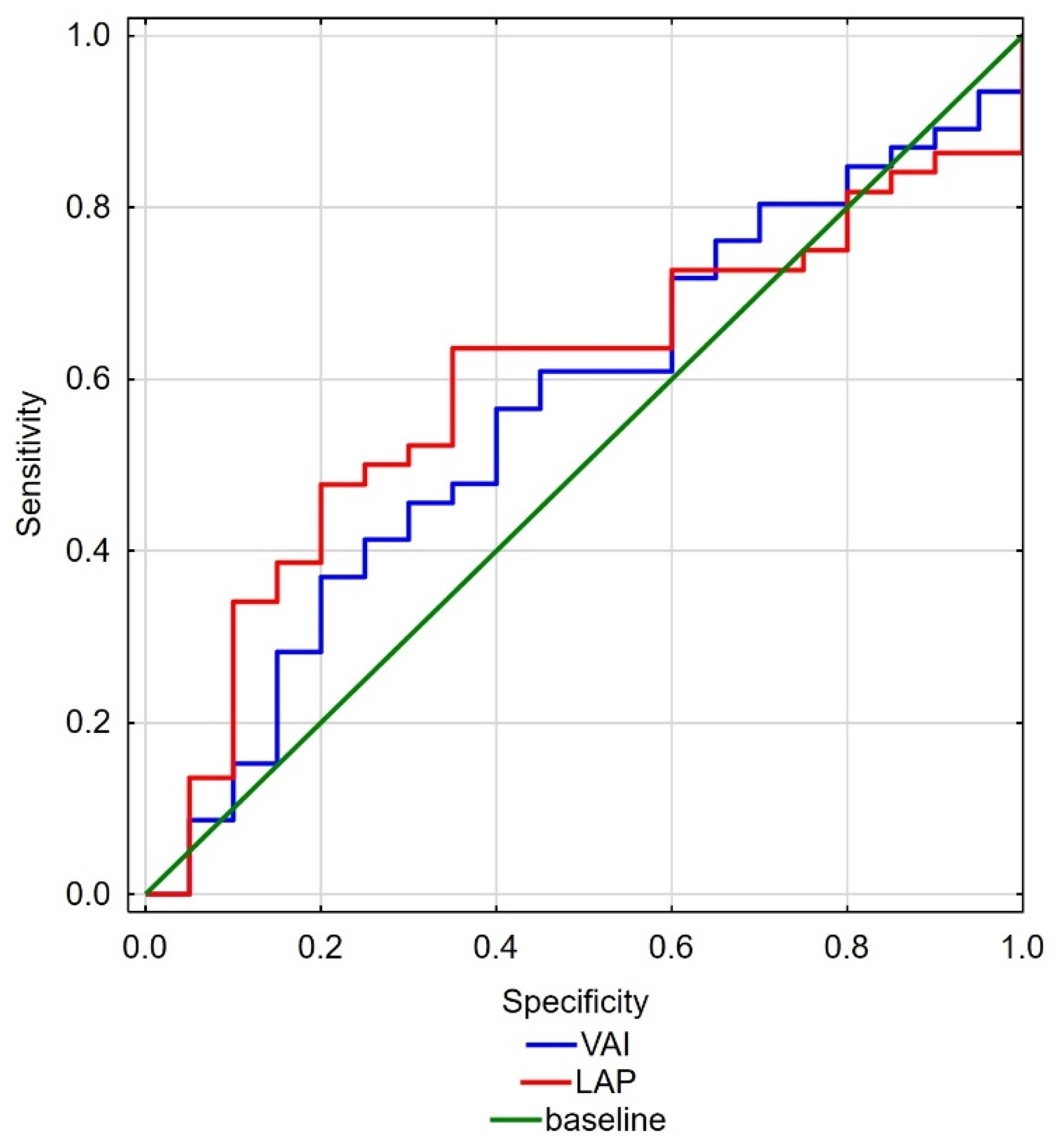
| VAI | 3.07 | 1.69 | 0.88 | 8.30 | 2.32 | 1.22 | 0.84 | 5.55 | 0.048 * |
| LAP | 84.03 | 43.18 | 8.39 | 192.36 | 63.96 | 25.59 | 34.48 | 133.44 | 0.050 * |
| HOMA-IR | 3.76 | 7.36 | 0.58 | 52.86 | 2.79 | 3.59 | 0.64 | 19.11 | 0.969 |
| Parameters | Patients with Erectile Dysfunction n = 72 | Patients without Erectile Dysfunction n = 34 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X | SD | Min | Max | X | SD | Min | Max | ||
| TT (ng/mL) | 4.79 | 2.12 | 1.38 | 10.54 | 5.75 | 2.29 | 1.69 | 12.90 | 0.018 * |
| FT (ng/mL) | 0.09 | 0.04 | 0.02 | 0.23 | 0.08 | 0.04 | 0.02 | 0.16 | 0.501 |
| Bioavailable T (ng/dL) | 2.00 | 0.90 | 0.46 | 5.06 | 1.92 | 0.95 | 0.42 | 3.87 | 0.735 |
| E2 (pg/mL) | 82.52 | 35.45 | 28.26 | 180.53 | 89.77 | 38.39 | 20.92 | 170.83 | 0.442 |
| SHBG (nmol/L) | 45.95 | 29.87 | 5.68 | 143.55 | 75.42 | 57.66 | 12.65 | 215.49 | 0.026 * |
| DHEAS (µg/mL) | 0.88 | 0.65 | 0.11 | 3.28 | 0.98 | 0.77 | 0.09 | 2.75 | 0.773 |
| Parameters | Patients with Erectile Dysfunction n = 72 | Patients without Erectile Dysfunction n = 34 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X | SD | Min | Max | X | SD | Min | Max | ||
| ChT | 180.54 | 49.61 | 105.3 | 308.7 | 179.85 | 47.01 | 97.9 | 264.6 | 0.871 |
| HDL | 39.42 | 9.75 | 18.2 | 63.1 | 41.83 | 9.87 | 22.6 | 56.6 | 0.201 |
| TG | 196.02 | 157.52 | 11.4 | 1033 | 176 | 105.65 | 77.6 | 583.7 | 0.201 |
| LDL | 113.2 | 82.14 | 36.46 | 612.68 | 103.57 | 45.16 | 12.28 | 189.9 | 0.779 |
| FPG | 86.78 | 29.26 | 59.1 | 134.3 | 80.66 | 18.2 | 58.7 | 128.8 | 0.957 |
| Albumins (g/dL) | 4.12 | 0.31 | 3.2 | 4.9 | 4.31 | 0.31 | 3.6 | 5.1 | 0.029 * |
| Parameters | p | OR | Confidence OR−95% | Confidence OR 95% |
|---|---|---|---|---|
| VAI | 0.072 | 1.536 | 0.962 | 2.453 |
| LAP | 0.050 * | 1.017 | 1.000 | 1.034 |
| BMI | 0.604 | 1.035 | 0.908 | 1.181 |
| TT | 0.046 * | 1.216 | 0.967 | 1.530 |
| SHBG | 0.007 * | 1.020 | 1.005 | 1.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandra, R.; Aleksandra, S.; Iwona, R. Erectile Dysfunction in Relation to Metabolic Disorders and the Concentration of Sex Hormones in Aging Men. Int. J. Environ. Res. Public Health 2022, 19, 7576. https://doi.org/10.3390/ijerph19137576
Aleksandra R, Aleksandra S, Iwona R. Erectile Dysfunction in Relation to Metabolic Disorders and the Concentration of Sex Hormones in Aging Men. International Journal of Environmental Research and Public Health. 2022; 19(13):7576. https://doi.org/10.3390/ijerph19137576
Chicago/Turabian StyleAleksandra, Rył, Szylińska Aleksandra, and Rotter Iwona. 2022. "Erectile Dysfunction in Relation to Metabolic Disorders and the Concentration of Sex Hormones in Aging Men" International Journal of Environmental Research and Public Health 19, no. 13: 7576. https://doi.org/10.3390/ijerph19137576
APA StyleAleksandra, R., Aleksandra, S., & Iwona, R. (2022). Erectile Dysfunction in Relation to Metabolic Disorders and the Concentration of Sex Hormones in Aging Men. International Journal of Environmental Research and Public Health, 19(13), 7576. https://doi.org/10.3390/ijerph19137576






