Effects of Tongue-Strengthening Exercise on Tongue Strength Reserve and Detraining Effects among Healthy Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Training Protocol
2.3. Detraining
2.4. Measurement
2.5. Validity and Reliability
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
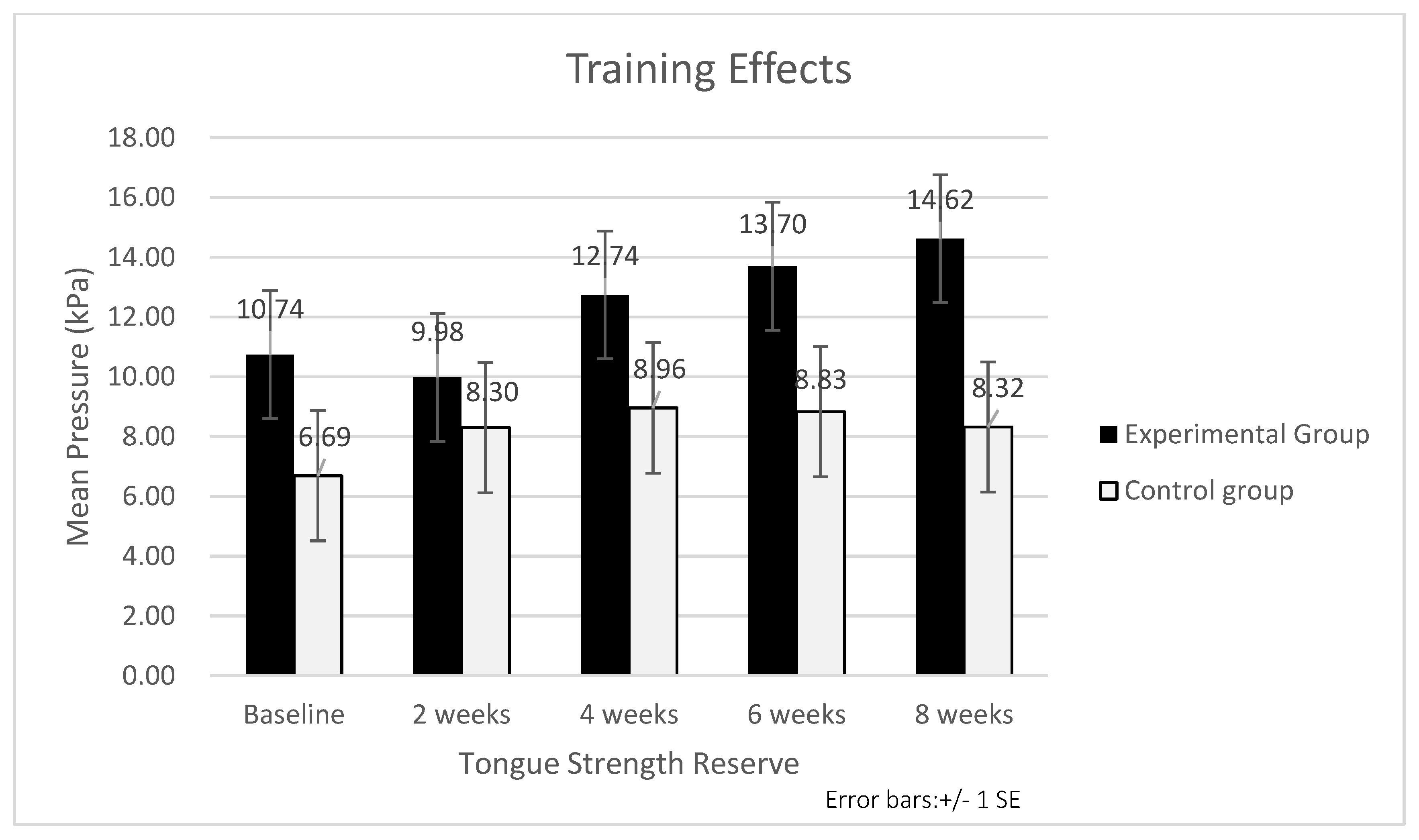
3.2. Training Effects
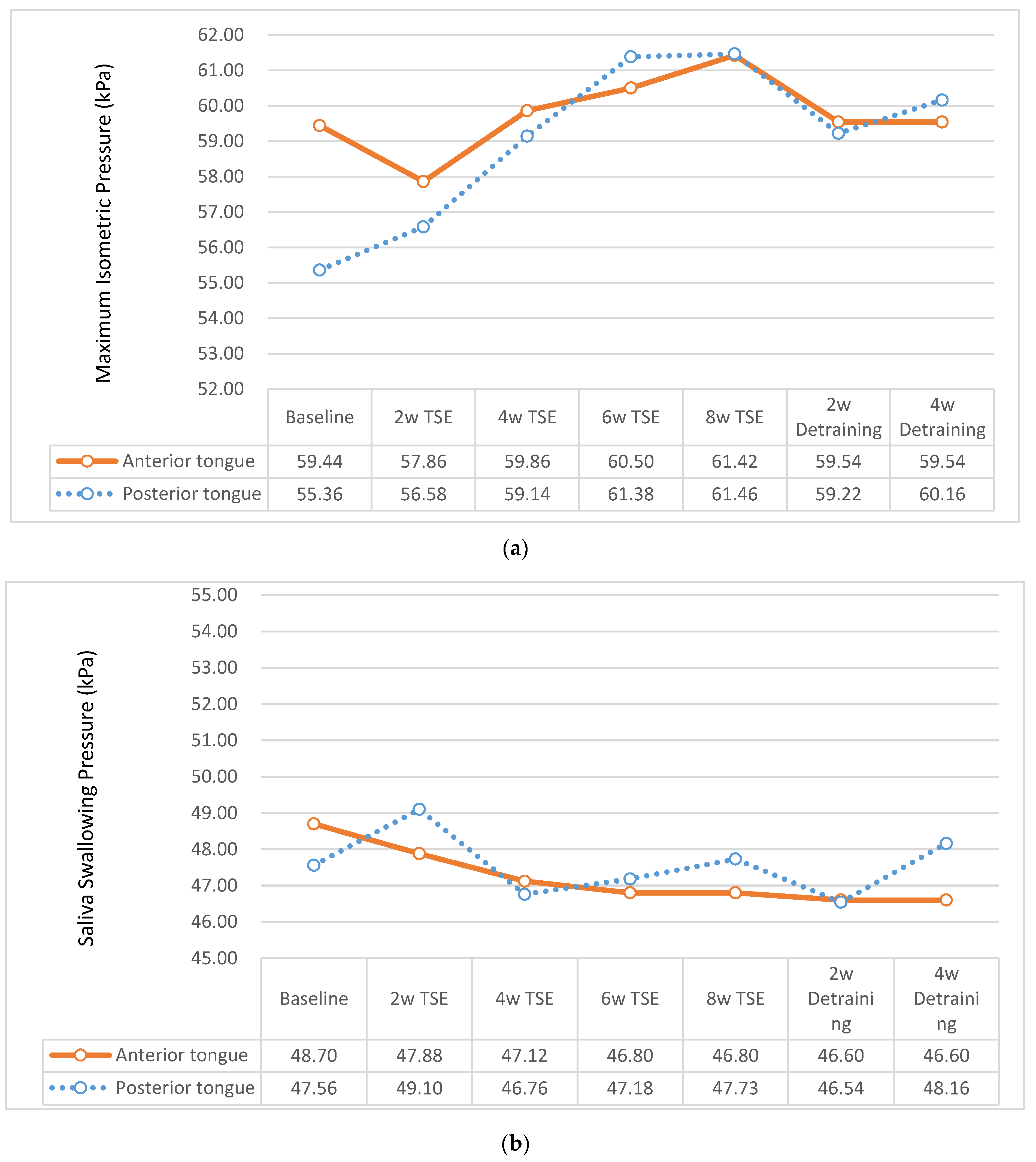
3.2.1. MIP of Tongue
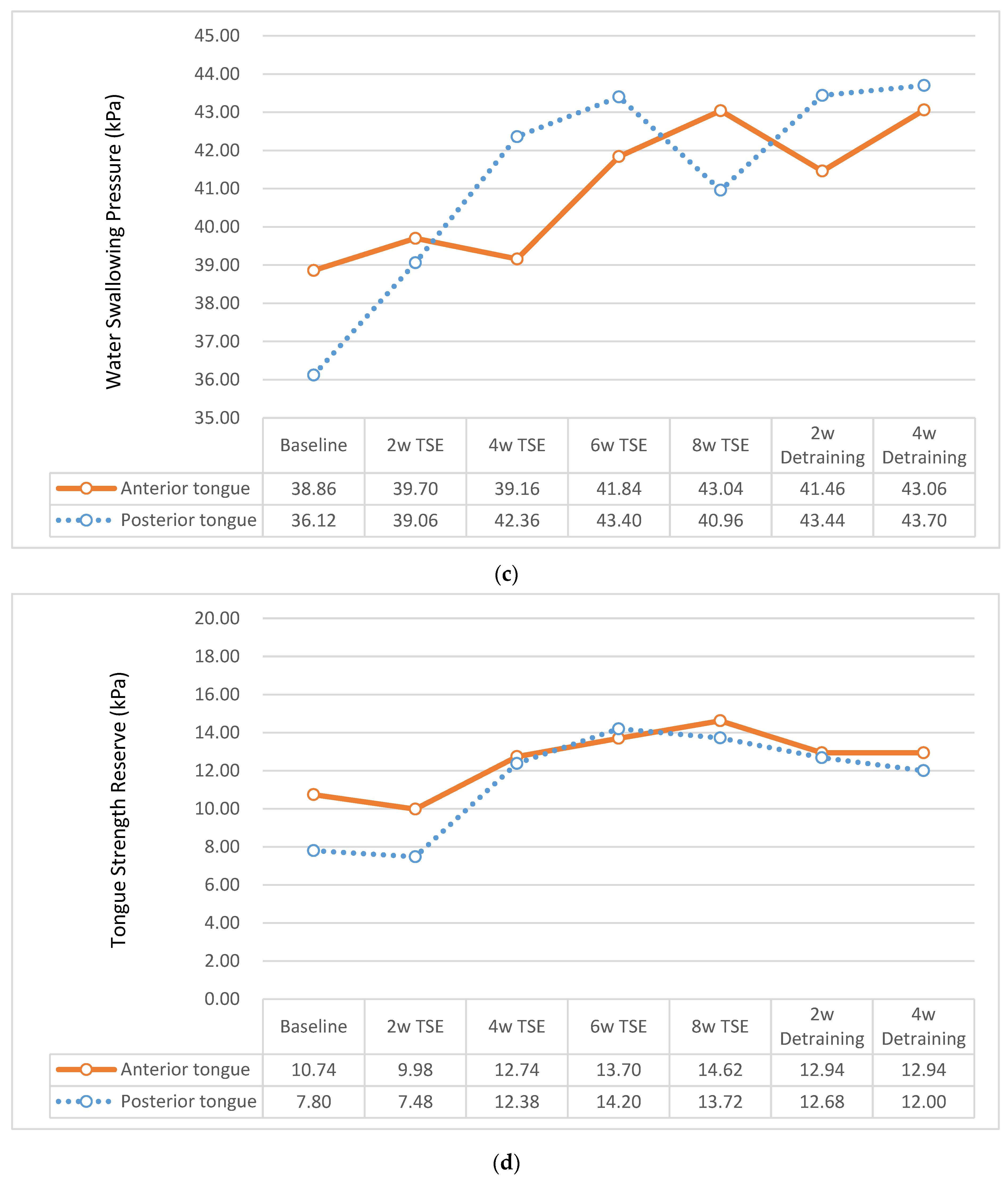
3.2.2. Swallowing Pressure of Tongue
3.2.3. Tongue Strength Reserve
4. Discussion
4.1. Effects of TSE on Tongue Strength Reserve
4.2. Effects of TSE on Tongue Swallowing Pressure
4.3. Limitations
4.4. Suggestions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section/Topic | Item No | Checklist Item | Reported on Page No |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 1–2 |
| 2b | Specific objectives or hypotheses | 2 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 3–4; Figure 1 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 3 | |
| Participants | 4a | Eligibility criteria for participants | 3 |
| 4b | Settings and locations where the data were collected | 3 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 3–4 |
| Outcomes | 6a | Completely defined prespecified primary and secondary outcome measures, including how and when they were assessed | 3–4 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | 3 | |
| Sample size | 7a | How the sample size was determined | 4 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | NA | |
| Randomisation: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 3 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 3 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 3 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 3 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, and those assessing outcomes) and how | 3 |
| 11b | If relevant, description of the similarity of interventions | NA | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 4 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 4 | |
| Results | |||
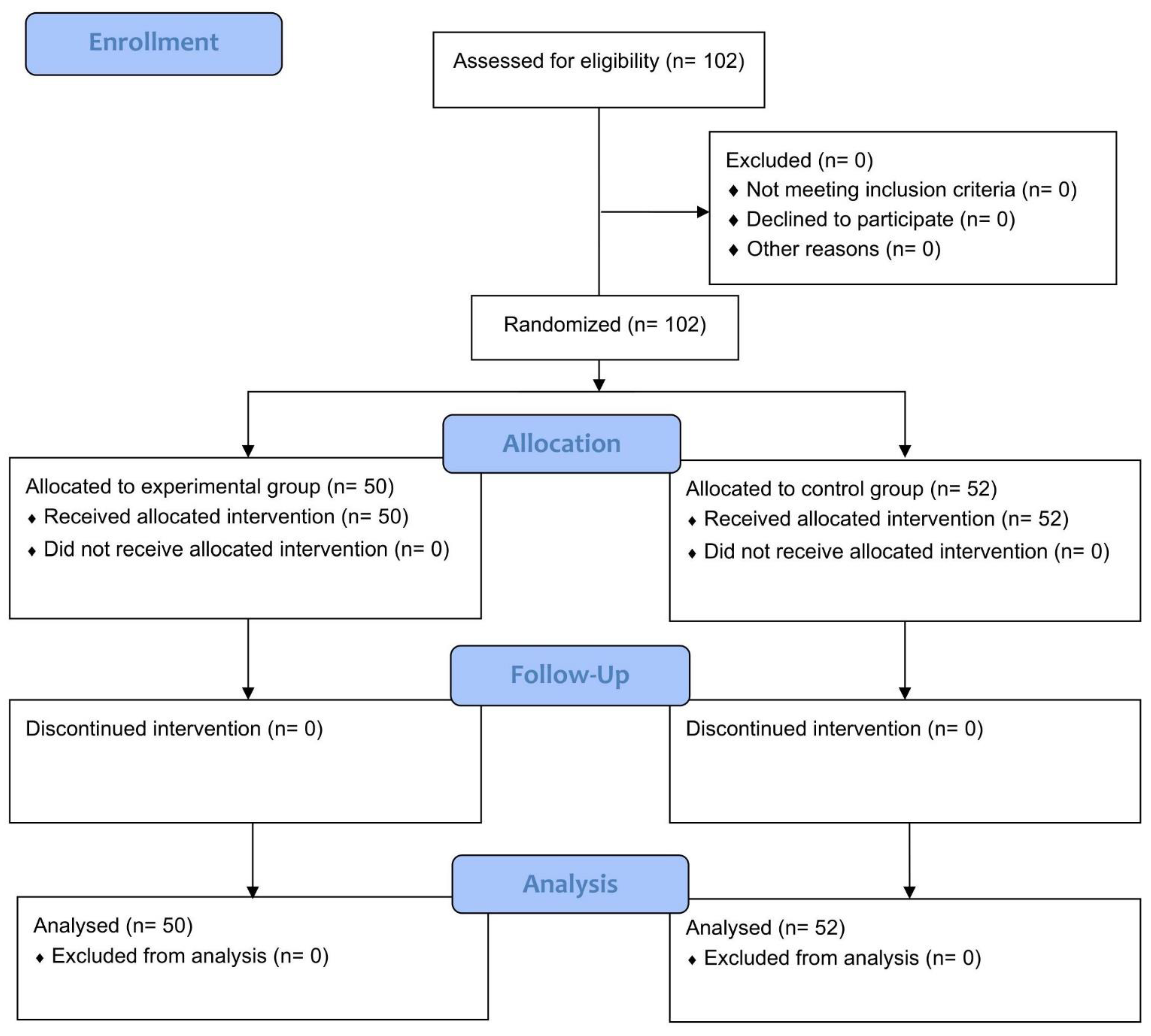
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 5; Figure 1 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 5; Figure 1 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 3 |
| 14b | Why the trial ended or was stopped | 3 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 6–7 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 3, 5–6; Figure 1 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 6–10; Table 2 and Table 3; Figure 2 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | NA | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing prespecified from exploratory | 6–14; Table 2, Table 3 and Table 4; Figure 2 and Figure 3 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance, see CONSORT for harms) | 3 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 18 |
| Generalisability | 21 | Generalisability (external validity and applicability) of the trial findings | 14–18 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits, and harms, and considering other relevant evidence | 14–18 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | NA |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 2–4 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 19 |
References
- Burkhead, L.M.; Sapienza, C.M.; Rosenbek, J.C. Strength-training exercise in dysphagia rehabilitation: Principles, procedures, and directions for future research. Dysphagia 2007, 22, 251–265. [Google Scholar] [CrossRef]
- Stål, P.; Marklund, S.; Thornell, L.E.; De Paul, R.; Eriksson, P.O. Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs 2003, 173, 147–161. [Google Scholar] [CrossRef]
- Zaidi, F.N.; Meadows, P.; Jacobowitz, O.; Davidson, T.M. Tongue anatomy and physiology, the scientific basis for a novel targeted neurostimulation system designed for the treatment of obstructive sleep apnea. Neuromodulation 2013, 16, 376–386. [Google Scholar] [CrossRef]
- Peladeau-Pigeon, M.; Steele, C.M. Age-Related Variability in Tongue Pressure Patterns for Maximum Isometric and Saliva Swallowing Tasks. J. Speech Lang. Hear. Res. 2017, 60, 3177–3184. [Google Scholar] [CrossRef] [Green Version]
- Adams, V.; Mathisen, B.; Baines, S.; Lazarus, C.; Callister, R. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI). Dysphagia 2013, 28, 350–369. [Google Scholar] [CrossRef]
- Clark, H.M.; Solomon, N.P. Age and sex differences in orofacial strength. Dysphagia 2012, 27, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Vanderwegen, J.; Guns, C.; Van Nuffelen, G.; Elen, R.; De Bodt, M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy belgian adults. Dysphagia 2013, 28, 159–166. [Google Scholar] [CrossRef]
- Fei, T.; Polacco, R.C.; Hori, S.E.; Molfenter, S.M.; Peladeau-Pigeon, M.; Tsang, C.; Steele, C.M. Age-related differences in tongue-palate pressures for strength and swallowing tasks. Dysphagia 2013, 28, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Nicosia, M.A.; Hind, J.A.; Roecker, E.B.; Carnes, M.; Doyle, J.; Dengel, G.A.; Robbins, J. Age effects on the temporal evolution of isometric and swallowing pressure. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M634–M640. [Google Scholar] [CrossRef]
- Youmans, S.R.; Youmans, G.L.; Stierwalt, J.A. Differences in tongue strength across age and gender: Is there a diminished strength reserve? Dysphagia 2009, 24, 57–65. [Google Scholar] [CrossRef]
- Lin, C.H.; Chung, S.Y.; Lin, C.T.; Hwu, Y.J. Effect of tongue-to-palate resistance training on tongue strength in healthy adults. Auris Nasus Larynx 2021, 48, 116–123. [Google Scholar] [CrossRef]
- Steele, C.M.; Bailey, G.L.; Polacco, R.E.; Hori, S.F.; Molfenter, S.M.; Oshalla, M.; Yeates, E.M. Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. Int. J. Speech Lang. Pathol. 2013, 15, 492–502. [Google Scholar] [CrossRef]
- McKenna, V.S.; Zhang, B.; Haines, M.B.; Kelchner, L.N. A Systematic Review of Isometric Lingual Strength-Training Programs in Adults With and Without Dysphagia. Am. J. Speech Lang. Pathol. 2017, 26, 524–539. [Google Scholar] [CrossRef] [Green Version]
- Van den Steen, L.; Schellen, C.; Verstraelen, K.; Beeckman, A.S.; Vanderwegen, J.; De Bodt, M.; Van Nuffelen, G. Tongue-Strengthening Exercises in Healthy Older Adults: Specificity of Bulb Position and Detraining Effects. Dysphagia 2018, 33, 337–344. [Google Scholar] [CrossRef]
- Van den Steen, L.; Vanderwegen, J.; Guns, C.; Elen, R.; De Bodt, M.; Van Nuffelen, G. Tongue-Strengthening Exercises in Healthy Older Adults: Does Exercise Load Matter? A Randomized Controlled Trial. Dysphagia 2019, 34, 315–324. [Google Scholar] [CrossRef]
- Van den Steen, L.; De Bodt, M.; Guns, C.; Elen, R.; Vanderwegen, J.; Van Nuffelen, G. Tongue-Strengthening Exercises in Healthy Older Adults: Effect of Exercise Frequency—A Randomized Trial. Folia Phoniatr. Logop. 2021, 73, 109–116. [Google Scholar] [CrossRef]
- Oh, J.C. Effects of Tongue Strength Training and Detraining on Tongue Pressures in Healthy Adults. Dysphagia 2015, 30, 315–320. [Google Scholar] [CrossRef]
- Clark, H.M.; O’Brien, K.; Calleja, A.; Corrie, S.N. Effects of directional exercise on lingual strength. J. Speech Lang. Hear. Res. 2009, 52, 1034–1047. [Google Scholar] [CrossRef]
- Pitts, L.L.; Stierwalt, J.A.G.; Hageman, C.F.; LaPointe, L.L. The Influence of Oropalatal Dimensions on the Measurement of Tongue Strength. Dysphagia 2017, 32, 759–766. [Google Scholar] [CrossRef]
- Jeon, S.R.; Nam, D.; Kim, T.H. Dropouts in randomized clinical trials of Korean medicine interventions: A systematic review and meta-analysis. Trials 2021, 22, 176. [Google Scholar] [CrossRef]
- Doeltgen, S.H.; Macrae, P.; Huckabee, M.L. Pharyngeal pressure generation during tongue-hold swallows across age groups. Am. J. Speech. Lang. Pathol. 2011, 20, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Van den Steen, L.; Baudelet, M.; Tomassen, P.; Bonte, K.; De Bodt, M.; Van Nuffelen, G. Effect of tongue-strengthening exercises on tongue strength and swallowing-related parameters in chronic radiation-associated dysphagia. Head Neck 2020, 42, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Tohara, H.; Minakuchi, S. Treatment and evaluation of dysphagia rehabilitation especially on suprahyoid muscles as jaw-opening muscles. Jpn. Dent. Sci. Rev. 2018, 54, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Humpal, N.S.; Banaszynski, K.; Hind, J.; Rogus-Pulia, N. Age-Related Differences in Pressures Generated During Isometric Presses and Swallows by Healthy Adults. Dysphagia 2016, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Namiki, C.; Hara, K.; Tohara, H.; Kobayashi, K.; Chantaramanee, A.; Nakagawa, K.; Saitou, T.; Yamaguchi, K.; Yoshimi, K.; Nakane, A.; et al. Tongue-pressure resistance training improves tongue and suprahyoid muscle functions simultaneously. Clin. Interv. Aging 2019, 14, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.; Levine, R.; Wood, J.; Roecker, E.B.; Luschei, E. Age effects on lingual pressure generation as a risk factor for dysphagia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, M257–M262. [Google Scholar] [CrossRef]
- Steele, C.M. Optimal approaches for measuring tongue-pressure functional reserve. J. Aging Res. 2013, 2013, 542909. [Google Scholar] [CrossRef] [Green Version]
- Palmer, P.M.; Jaffe, D.M.; McCulloch, T.M.; Finnegan, E.M.; Van Daele, D.J.; Luschei, E.S. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J. Speech Lang. Hear. Res. 2008, 51, 828–835. [Google Scholar] [CrossRef]
- Intrinsic and Extrinsic Muscles of the Tongue. Available online: https://3d4medical.com/blog/intrinsic-and-extrinsic-muscles-of-the-tongue (accessed on 3 April 2022).
- Clark, H.M. Specificity of training in the lingual musculature. J. Speech Lang. Hear. Res. 2012, 55, 657–667. [Google Scholar] [CrossRef]
- Garin, O. Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
| Task | Bulb-Position | Repetitions |
|---|---|---|
| Isometric | Anterior | 3 presses |
| Posterior | 3 presses | |
| <5 min break> | ||
| Saliva Swallowing | Anterior | 3 presses |
| Posterior | 3 presses | |
| <5 min break> | ||
| Water (5 mL) Swallowing | Anterior | 3 presses |
| Posterior | 3 presses | |
| Variable | Total (N = 102) | Experimental Group (n = 50) | Control Group (n = 52) | t/χ2 | p |
|---|---|---|---|---|---|
| N (%)/Mean ± SD | n (%)/Mean ± SD | n (%)/Mean ± SD | |||
| Gender | 0.31 | 0.575 | |||
| Male | 36 (35.3) | 19(38.0) | 17(32.7) | ||
| Female | 66 (64.7) | 31(62.0) | 35(67.3) | ||
| Age (years) | 37.24 ± 14.74 | 37.68 ± 16.25 | 36.81 ± 13.28 | 0.30 | 0.767 |
| Body Height (cm) | 163.36 ± 8.27 | 163.41 ± 8.84 | 163.32 ± 7.75 | 0.05 | 0.957 |
| Body Weight (kg) | 64.76 ± 13.54 | 64.92 ± 14.83 | 64.60 ± 12.32 | 0.12 | 0.906 |
| BMI | 24.12 ± 4.01 | 24.14 ± 4.37 | 24.10 ± 3.68 | 0.05 | 0.960 |
| MaximumIsometric Pressure (MIP, kPa) | |||||
| Anterior tongue | 56.41 ± 14.17 | 59.44 ± 11.97 | 53.50 ± 15.57 | 2.15 | 0.034 |
| Posterior tongue | 52.76 ±13.09 | 55.36 ± 13.53 | 50.27 ± 12.28 | 1.99 | 0.049 |
| Saliva Swallowing Pressure (kPa) | |||||
| Anterior tongue | 47.74 ± 15.91 | 48.70 ± 17.71 | 46.81 ± 14.07 | 0.60 | 0.551 |
| Posterior tongue | 47.27 ± 15.25 | 47.56 ± 16.56 | 47.00 ± 14.03 | 0.19 | 0.854 |
| Water Swallowing Pressure (kpa) | |||||
| Anterior tongue | 43.22 ± 16.90 | 38.86 ± 17.07 | 47.42 ± 15.79 | −2.63 | 0.010 |
| Posterior tongue | 41.07 ± 15.89 | 36.12 ± 16.74 | 45.83 ± 13.55 | −3.22 | 0.002 |
| Tongue Strength Reserve (kPa) | |||||
| Anterior tongue | 8.68 ± 2.10 | 10.74 ± 2.06 | 6.69 ± 2.14 | 1.36 | 0.177 |
| Posterior tongue | 5.49 ± 1.77 | 7.80 ± 1.92 | 3.27 ± 1.63 | 1.80 | 0.074 |
| Variables | Sphericity Test (p) | Mean Square | Degree of Freedom | F | p | LSD Test b |
|---|---|---|---|---|---|---|
| Maximum Isometric Pressure (MIP, kPa) | ||||||
| Anterior tongue | <0.001 | |||||
| Group | 2774.81 | 1 | 4.87 | 0.030 | ||
| Time (1, 2, 3, 4, 5) a | 209.30 | 3.16 | 3.41 | 0.016 | 5 > 3 > 2 > 1 | |
| Group × Time | 23.49 | 3.16 | 0.38 | 0.776 | ||
| Posterior tongue | <0.001 | |||||
| Group | 2093.85 | 1 | 4.67 | 0.033 | ||
| Time (1, 2, 3, 4, 5) | 1018.65 | 3.19 | 18.56 | <0.001 | 5 > 3 > 2 > 1 | |
| Group × Time | 24.56 | 3.19 | 0.45 | 0.731 | ||
| Saliva Swallowing Pressure (kPa) | ||||||
| Anterior tongue | <0.001 | |||||
| Group | 36.06 | 1 | 0.05 | 0.827 | ||
| Time (1, 2, 3, 4, 5) | 32.22 | 3.44 | 0.31 | 0.846 | ||
| Group × Time | 75.75 | 3.44 | 0.72 | 0.558 | ||
| Posterior tongue | <0.001 | |||||
| Group | 16.53 | 1 | 0.02 | 0.883 | ||
| Time (1, 2, 3, 4, 5) | 33.43 | 3.25 | 0.34 | 0.814 | ||
| Time × Group | 112.45 | 3.25 | 1.13 | 0.338 | ||
| Water Swallowing Pressure (kPa) | ||||||
| Anterior tongue | <0.001 | |||||
| Group | 6819.09 | 1 | 8.06 | 0.005 | ||
| Time (1, 2, 3, 4, 5) | 199.17 | 3.03 | 1.42 | 0.238 | ||
| Group × Time | 54.02 | 3.03 | 0.38 | 0.767 | ||
| Posterior tongue | <0.001 | |||||
| Group | 6517.02 | 1 | 7.32 | 0.008 | ||
| Time (1, 2, 3, 4, 5) | 528.41 | 3.04 | 4.63 | 0.003 | 4 > 2 > 1, 3 > 1, 5 > 1 | |
| Group × Time | 166.78 | 3.04 | 1.48 | 0.220 | ||
| Tongue strength reserve | ||||||
| Anterior tongue | <0.001 | |||||
| Group | 2178.21 | 1 | 2.93 | 0.090 | ||
| Time (1, 2, 3, 4, 5) | 189.39 | 3.47 | 1.46 | 0.219 | ||
| Group × Time | 83.93 | 3.47 | 0.65 | 0.607 | ||
| Posterior tongue | 0.002 | |||||
| Group | 2482.45 | 1 | 4.92 | 0.029 | ||
| Time (1, 2, 3, 4, 5) | 684.41 | 3.54 | 6.26 | <0.001 | 3 > 1, 4 > 1, 5 >1, 4 > 2, 5 > 2 | |
| Group × Time | 149.57 | 3.54 | 1.37 | 0.249 |
| Variable | M ± SE | Mean Square | df | F | p | LSD Test |
|---|---|---|---|---|---|---|
| Maximum Isometric Pressure (MIP, kPa) | ||||||
| Anterior tongue | 80.56 | 4.42 | 1.60 | 0.168 | ||
| 59.44 ± 1.69 | |||||
| 57.86 ± 1.94 | |||||
| 59.86 ± 1.53 | |||||
| 60.50 ± 1.70 | |||||
| 61.42 ± 1.58 | |||||
| 59.54 ± 1.66 | |||||
| 59.54 ± 1.45 | |||||
| Posterior tongue | 393.01 | 4.10 | 7.07 | <0.001 | 6 > 1, 6 > 2, 6 > 4, 6 > 5, 7 > 1, 7 > 2 | |
| 55.36 ± 1.91 | |||||
| 56.58 ± 1.79 | |||||
| 59.14 ± 1.58 | |||||
| 61.38 ± 1.49 | |||||
| 61.46 ± 1.42 | |||||
| 59.22 ± 1.42 | |||||
| 60.16 ± 1.32 | |||||
| Saliva Swallow Pressure (kPa) | ||||||
| Anterior tongue | 31.31 | 6 | 0.30 | 0.937 | ||
| 48.70 ± 2.50 | |||||
| 47.88 ± 2.24 | |||||
| 47.12 ± 2.12 | |||||
| 46.80 ± 2.34 | |||||
| 46.80 ± 2.24 | |||||
| 46.60 ± 2.31 | |||||
| 46.60 ± 2.26 | |||||
| Posterior tongue | 49.53 | 4.63 | 0.39 | 0.845 | ||
| 47.56 ± 2.34 | |||||
| 49.10 ± 2.17 | |||||
| 46.76 ± 2.02 | |||||
| 47.18 ± 2.09 | |||||
| 47.73 ± 2.12 | |||||
| 46.54 ± 2.13 | |||||
| 48.16 ± 1.96 | |||||
| Water Swallow Pressure (kPa) | ||||||
| Anterior tongue | 259.92 | 3.65 | 1.41 | 0.236 | ||
| 38.86 ± 2.41 | |||||
| 39.70 ± 2.13 | |||||
| 39.16 ± 2.58 | |||||
| 41.84 ± 2.45 | |||||
| 43.04 ± 2.56 | |||||
| 41.46 ± 2.57 | |||||
| 43.06 ± 2.34 | |||||
| Posterior tongue | 558.67 | 4.28 | 4.03 | 0.003 | 6 > 1, 6 > 2, 7 > 1, 7 > 2 | |
| 36.12 ± 2.36 | |||||
| 39.06 ± 2.28 | |||||
| 42.36 ± 2.58 | |||||
| 43.40 ± 2.42 | |||||
| 40.96 ± 2.61 | |||||
| 43.44 ± 2.23 | |||||
| 43.70 ± 2.28 | |||||
| Tongue strength reserve | ||||||
| Anterior tongue | 162.60 | 4.87 | 1.12 | 0.351 | ||
| 10.74 ± 2.06 | |||||
| 9.98 ± 2.21 | |||||
| 12.74 ± 1.98 | |||||
| 13.70 ± 2.62 | |||||
| 14.62 ± 2.22 | |||||
| 12.94 ± 2.29 | |||||
| 12.94 ± 2.21 | |||||
| Posterior tongue | 452.66 | 4.92 | 3.34 | 0.006 | 6 > 1, 6 > 2 | |
| 7.80 ± 1.92 | |||||
| 7.48 ± 2.01 | |||||
| 12.38 ± 1.95 | |||||
| 14.20 ± 1.97 | |||||
| 13.72 ± 1.97 | |||||
| 12.68 ± 2.11 | |||||
| 12.00 ± 1.85 |
| Study/Method/Aim | Participants | Interventions | Outcomes |
|---|---|---|---|
| Clark et al. [18]; Randomization of assignment to the sequential TSE group (n = 29) or concurrent TSE group (n = 10) Aim: To verify the effects of TSE on tongue strength, and whether the strength gains maintain after exercise discontinued | 39 healthy adults; 17 males and 22 females; Mean = 37.8 years, range = 18–67 years |
|
|
| Oh [17]; pre-experimental research design Aim: To assess the effects of TSE and detraining effects on tongue strength and tongue pressure during effortful swallowing. | 10 young healthy volunteers; 3 males and 7 females; Mean = 25.8 years, range = 21–35 years |
|
|
| Van den Steen et al. [14]; Assignment to anterior TSE group (n = 7) or posterior TSE group (n = 9) using convenience sampling Aim: To explore the training effects of anterior and posterior TSE on tongue strength and the detraining effects. | 16 older adults in nursing home; 8 males and 8 females; Mean = 84 years, range = 70–95 years |
|
|
| Present study; Randomization of assignment to the experimental group (n = 50) or control group (n = 52) Aim: To explore the effects of TSE on MIPs of tongue strength, tongue pressure during saliva and water swallowing, tongue pressure reserve and to measure possible detraining effects. | 102 healthy adults; 36 males and 66 females; Mean = 37.2 years, range = 20–59 years |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, H.-L.; Lou, J.-H.; Wang, C.-C.; Lai, Y.-J.; Wu, S.-J.; Hwu, Y.-J. Effects of Tongue-Strengthening Exercise on Tongue Strength Reserve and Detraining Effects among Healthy Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 6878. https://doi.org/10.3390/ijerph19116878
Hsiao H-L, Lou J-H, Wang C-C, Lai Y-J, Wu S-J, Hwu Y-J. Effects of Tongue-Strengthening Exercise on Tongue Strength Reserve and Detraining Effects among Healthy Adults: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(11):6878. https://doi.org/10.3390/ijerph19116878
Chicago/Turabian StyleHsiao, Hui-Ling, Jiunn-Horng Lou, Chun-Chieh Wang, Yun-Ju Lai, Shang-Jung Wu, and Yueh-Juen Hwu. 2022. "Effects of Tongue-Strengthening Exercise on Tongue Strength Reserve and Detraining Effects among Healthy Adults: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 11: 6878. https://doi.org/10.3390/ijerph19116878
APA StyleHsiao, H.-L., Lou, J.-H., Wang, C.-C., Lai, Y.-J., Wu, S.-J., & Hwu, Y.-J. (2022). Effects of Tongue-Strengthening Exercise on Tongue Strength Reserve and Detraining Effects among Healthy Adults: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(11), 6878. https://doi.org/10.3390/ijerph19116878






