Improving Knowledge and Early Detection of Atrial Fibrillation through a Community-Based Opportunistic Screening Program: What’s Your Beat?
Abstract
:1. Introduction
2. Methods
2.1. Study Settings
2.2. Study Population
2.3. Recruitment of Participants
2.4. Procedure
2.5. Follow-Up of Participants with Positive Screening Outcomes
2.6. AF Promotion at Screening Venues
2.7. Outcome Measures
2.8. Statistical Analysis
3. Results
3.1. Participants
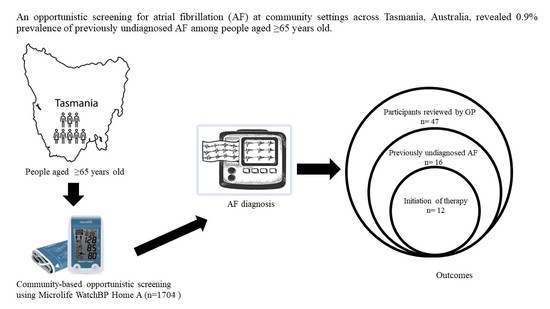
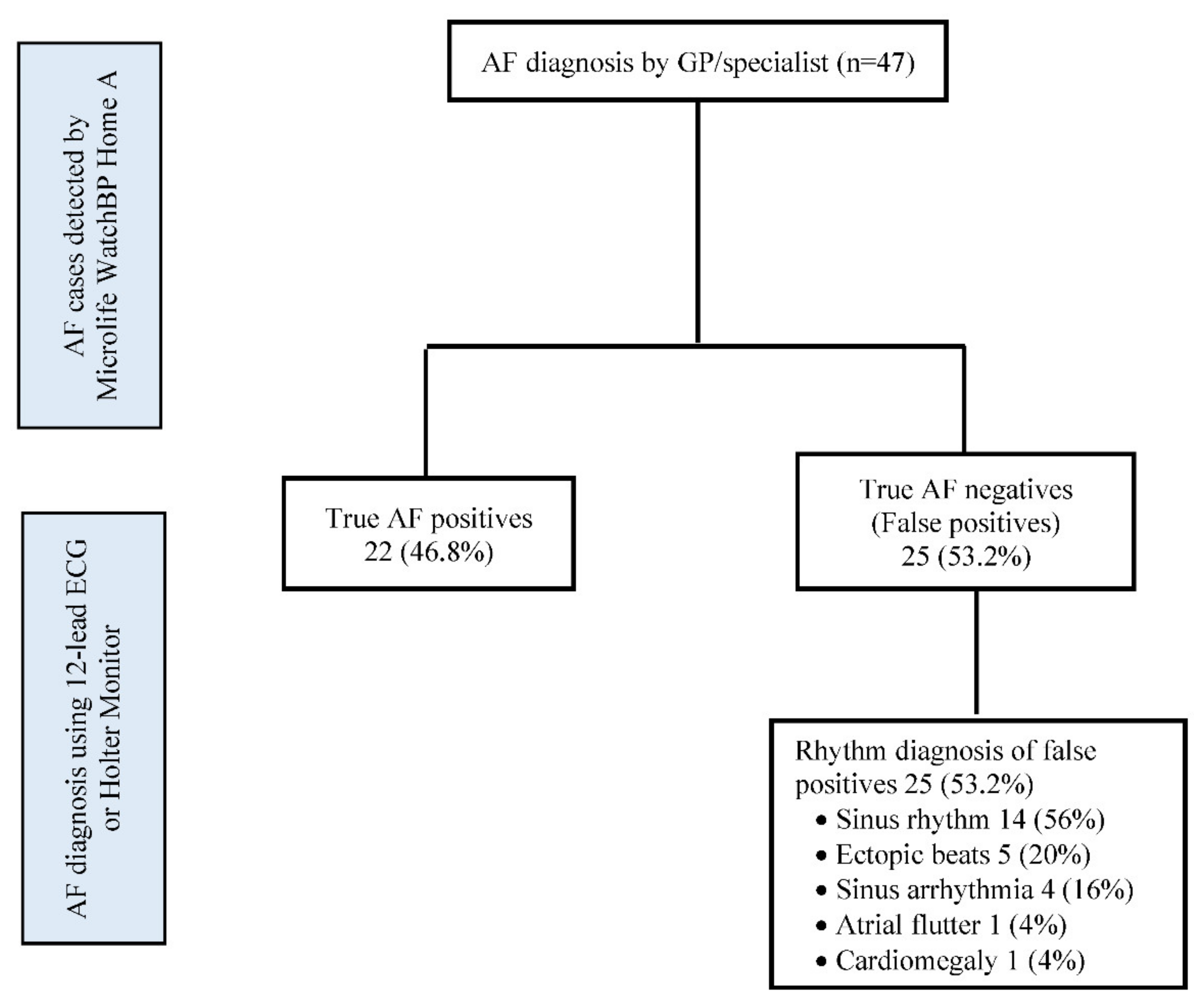
3.2. Prevalence of Previously Undiagnosed AF
3.3. Stroke Risk and Initiation of OAC Therapy
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. -Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef]
- Kumar, K.; Manning, W. Overview of Atrial Fibrillation. Available online: https://www.uptodate.com/contents/overview-of-atrial-fibrillation#H440859983 (accessed on 20 April 2022).
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Tsang, T.S.; Barnes, M.E.; Pellika, P.A.; Gin, K.; Miyasaka, Y.; Seward, J.B.; Gersh, B.J. Silent atrial fibrillation in Olmsted County: A community-based study. Can. J. Cardiol. 2011, 27, S122. [Google Scholar] [CrossRef]
- Leyden, J.M.; Kleinig, T.J.; Newbury, J.; Castle, S.; Cranefield, J.; Anderson, C.S.; Crotty, M.; Whitford, D.; Jannes, J.; Lee, A.; et al. Adelaide Stroke Incidence Study. Stroke 2013, 44, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Brieger, D.; Amerena, J.; Attia, J.R.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.M. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Med. J. Aust. 2018, 209, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- The UK NSC Recommendation on Atrial Fibrillation Screening in Adults. Available online: https://legacyscreening.phe.org.uk/atrialfibrillation (accessed on 20 April 2022).
- Kahwati, L.C.; Asher, G.N.; Kadro, Z.O.; Keen, S.; Ali, R.; Coker-Schwimmer, E.; Jonas, D.E. Screening for Atrial Fibrillation: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B. Screening for Atrial Fibrillation A Report of the AF-SCREEN International Collaboration. Circulation 2017, 135, 1851–1867. [Google Scholar] [CrossRef]
- Chan, N.Y. Systematic Screening for Atrial Fibrillation in the Community: Evidence and Obstacles. Arrhythm. Electrophysiol. Rev. 2018, 7, 39–42. [Google Scholar] [CrossRef]
- Department of Health. Population Based Screening Framework. Available online: https://apo.org.au/node/237996 (accessed on 20 April 2022).
- Gladstone, D.J.; Wachter, R.; Schmalstieg-Bahr, K.; Quinn, F.R.; Hummers, E.; Ivers, N.; Marsden, T.; Thornton, A.; Djuric, A.; Suerbaum, J.; et al. Screening for Atrial Fibrillation in the Older Population: A Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 558–567. [Google Scholar] [CrossRef]
- Microlife WatchBP Home A. Available online: https://www.microlife.com/professional-products/watchbp-home/watchbp-home-a-afib (accessed on 23 April 2022).
- WatchBP Home A for Opportunistically Detecting Atrial Fibrillation during Diagnosis and Monitoring of Hypertension 2013. Available online: https://www.nice.org.uk/guidance/mtg13 (accessed on 20 April 2022).
- Kearley, K.; Selwood, M.; Van Den Bruel, A.; Thompson, M.; Mant, D.; Hobbs, F.D.R.; Fitzmaurice, D.; Heneghan, C. Triage tests for identifying atrial fibrillation in primary care: A diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open 2014, 4, e004565. [Google Scholar] [CrossRef]
- Chan, P.-H.; Wong, C.-K.; Pun, L.; Wong, Y.-F.; Wong, M.M.-Y.; Chu, D.W.-S.; Siu, C.-W. Diagnostic performance of an automatic blood pressure measurement device, Microlife WatchBP Home A, for atrial fibrillation screening in a real-world primary care setting. BMJ Open 2017, 7, e013685. [Google Scholar] [CrossRef] [Green Version]
- Lowres, N.; Neubeck, L.; Salkeld, G.; Krass, I.; McLachlan, A.J.; Redfern, J.; Bennett, A.A.; Briffa, T.; Bauman, A.; Martinez, C.; et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies: The SEARCH-AF study. Thromb. Haemost. 2014, 111, 1167–1176. [Google Scholar] [CrossRef]
- Orchard, J.; Lowres, N.; Freedman, S.B.; Ladak, L.; Lee, W.; Zwar, N.; Peiris, D.; Kamaladasa, Y.; Li, J.; Neubeck, L. Screening for atrial fibrillation during influenza vaccinations by primary care nurses using a smartphone electrocardiograph (iECG): A feasibility study. Eur. J. Prev. Cardiol. 2016, 23, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Heart Foundation. Tasmania’s Deaths from Coronary Heart Disease among Highest in the Country. Available online: https://www.heartfoundation.org.au/media-releases/tasmania%E2%80%99s-deaths-from-coronary-heart-disease-amon (accessed on 20 April 2022).
- Naing, L.; Winn, T.; Rusli, B. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- NHMRC. Statement on Consumer and Community Involvement in Health and Medical Research. Available online: https://www.nhmrc.gov.au/about-us/publications/statement-consumer-and-community-involvement-health-and-medical-research (accessed on 24 March 2022).
- Willits, I.; Keltie, K.; Craig, J.; Sims, A. WatchBP Home A for opportunistically detecting atrial fibrillation during diagnosis and monitoring of hypertension: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2014, 12, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and pitfalls in research and practice. Front. Public Health. 2017, 5, 307. [Google Scholar] [CrossRef]
- National Heart Foundation. Guideline for the Diagnosis and Management of Hypertension in Adults—2016; National Heart Foundation of Australia: Melbourne, Australia, 2016; Available online: https://www.mja.com.au/journal/2016/205/2/guideline-diagnosis-and-management-hypertension-adults-2016 (accessed on 21 April 2022).
- Tasmanian Department of Health. Checking Understanding. Available online: https://www.health.tas.gov.au/professionals/health-literacy/health-literacy-workplace-toolkit/spoken-communication/checking-understanding (accessed on 22 April 2022).
- Lang, T.A.; Altman, D.G. Statistical analyses and methods in the published literature: The SAMPL guidelines. In Guidelines for Reporting Health Research: A User’s Manual; Moher, D., Altman, D., Schulz, K., Simera, I., Wager, E., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 264–274. [Google Scholar]
- Proietti, M.; Mairesse, G.H.; Goethals, P.; Scavee, C.; Vijgen, J.; Blankoff, I.; Vandekerckhove, Y.; Lip, G.Y. A population screening programme for atrial fibrillation: A report from the Belgian Heart Rhythm Week screening programme. Europace 2016, 18, 1779–1786. [Google Scholar] [CrossRef]
- Berge, T.; Brynildsen, J.; Larssen, H.K.N.; Onarheim, S.; Jenssen, G.R.; Ihle-Hansen, H.; Christophersen, I.E.; Myrstad, M.; Røsjø, H.; Smith, P.; et al. Systematic screening for atrial fibrillation in a 65-year-old population with risk factors for stroke: Data from the Akershus Cardiac Examination 1950 study. Europace 2017, 20, f299–f305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, R.B.; Wilde, S.; Wild, P.S.; Munzel, T.; Blankenberg, S. Atrial fibrillation: Its prevalence and risk factor profile in the German general population. Deutsches Ärzteblatt International 2012, 109, 293. [Google Scholar] [PubMed]
- Meschia, J.F.; Merrill, P.; Soliman, E.Z.; Howard, V.J.; Barrett, K.M.; Zakai, N.A.; Kleindorfer, D.; Safford, M.; Howard, G. Racial disparities in awareness and treatment of atrial fibrillation: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke 2010, 41, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lowres, N.; Neubeck, L.; Redfern, J.; Ben Freedman, S. Screening to identify unknown atrial fibrillation: A systematic review. Thromb. Haemost. 2013, 110, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Wiesel, J.; Fitzig, L.; Herschman, Y.; Messineo, F.C. Detection of atrial fibrillation using a modified Microlife blood pressure monitor. Am. J. Hypertens. 2009, 22, 848–852. [Google Scholar] [CrossRef] [Green Version]
- Selder, J.L.; Breukel, L.; Blok, S.; van Rossum, A.C.; Tulevski, I.I.; Allaart, C.P. A mobile one-lead ECG device incorporated in a symptom-driven remote arrhythmia monitoring program. The first 5982 Hartwacht ECGs. Neth. Heart J. 2019, 27, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Verberk, W.J.; Omboni, S.; Kollias, A.; Stergiou, G.S. Screening for atrial fibrillation with automated blood pressure measurement: Research evidence and practice recommendations. Int. J. Cardiol. 2016, 203, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Zink, M.D.; Marx, N.; Crijns, H.J.G.M.; Schotten, U. Opportunities and challenges of large-scale screening for atrial fibrillation. Herzschrittmacherther. Elektrophysiol. 2018, 29, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Orchard, J.; Freedman, S.; Lowres, N.; Peiris, D.; Neubeck, L. iPhone ECG screening by practice nurses and receptionists for atrial fibrillation in general practice: The GP-SEARCH qualitative pilot study. Aust. Fam. Physician. 2014, 43, 315–319. [Google Scholar]
- Anderson, J.R.; Hunter, T.; Dinallo, J.M.; Glaser, D.; Roybal, L.K.; Segovia, A.; Raley, R.; Bleske, B.E. Population screening for atrial fibrillation by student pharmacists at health fairs. J. Am. Pharm. Assoc. 2020, 60, e52–e57. [Google Scholar] [CrossRef] [Green Version]
| Tasmania Regions | Regional Areas | No. of Screening Sessions | Nature of Venue |
|---|---|---|---|
| Hobart and Southern Tasmania | Bruny Island | 1 | Community hall |
| Greater Hobart | 50 | Community hall, festival, shopping centre, street | |
| Huonville | 3 | Community hall, festival | |
| Cygnet | 1 | Community hall | |
| North West Tasmania | Devonport | 1 | Festival |
| Burnie | 1 | Community hall | |
| Sheffield | 1 | Community hall | |
| Ulverstone | 1 | Community hall | |
| Port Sorell | 1 | Community hall | |
| West Coast Tasmania | Queenstown | 1 | Festival |
| Launceston and Northern Tasmania | Launceston | 11 | Community hall, market, festival |
| Deloraine | 1 | Community hall | |
| Carrick | 1 | Community hall | |
| Evandale | 1 | Festival | |
| Bridport | 1 | Community hall | |
| East Coast Tasmania | St Helens | 1 | Community hall |
| Bicheno | 1 | Community hall | |
| Fingal | 1 | Community hall | |
| Total | 79 |
| Variables | Median (IQR) | Frequency (%) | |
|---|---|---|---|
| Age | 71.0 (68.0 to 76.0) | ||
| Age categories (year) | 65 to 69 | 663 (38.9) | |
| 70 to 79 | 782 (45.9) | ||
| ≥80 | 259 (15.2) | ||
| Sex | Female | 1006 (59.0) | |
| Male | 698 (41.0) | ||
| Socioeconomic status | Deciles | 7.0 (4.0 to 9.0) | |
| Remoteness | Inner regional | 1074 (63.0) | |
| Outer regional | 528 (31.0) | ||
| Remote/very remote | 102 (6.0) | ||
| Smoking status | Non-smoker | 1224 (71.8) | |
| Ex-smoker | 422 (24.8) | ||
| Current smoker | 58 (3.4) | ||
| History of a medical condition | Diabetes mellitus 1 | 16 (0.9) | |
| Diabetes mellitus 2 | 199 (11.7) | ||
| Heart failure | 39 (2.3) | ||
| PVD | 60 (3.5) | ||
| High blood pressure | 775 (45.5) | ||
| Heart attack | 110 (6.5) | ||
| Stroke/TIA | 102 (6.0) | ||
| Other medical conditions | 291 (19.1) | ||
| None | 659 (38.7) | ||
| Systolic BP at screening (mmHg) | <20 | 174 (10.2) | |
| 120 to 129 | 271 (15.9) | ||
| 130 to 139 | 403 (23.7) | ||
| 140 to 159 | 597 (35.0) | ||
| ≥160 | 259 (15.2) | ||
| AF Awareness | Yes | 842 (49.4) | |
| No | 862 (50.6) | ||
| Variables | Previously Undiagnosed AF n = 16, n (%) | Negative Screening Outcomes n = 1679, n (%) | p | |
|---|---|---|---|---|
| Median (IQR) deciles | SEIFA-IRSAD | 7.5 (4.25 to 8.75) | 7.0 (4.0 to 9.0) | 0.652 a |
| Median (IQR) age (years) | 79 (70.6 to 85.3) | 71.0 (68.0 to 76.0) | 0.002 a | |
| Age categories (years) | 65 to 69 | 2 (12.5) | 657 (39.1) | <0.001 b |
| 70 to 79 | 6 (37.5) | 773 (46.0) | ||
| 80 and above | 8 (50.0) | 249 (14.8) | ||
| Sex | Female | 9 (56.3) | 992 (59.1) | 0.819 b |
| Male | 7 (43.8) | 687 (40.9) | ||
| Location | Inner regional | 12 (75.0) | 1056 (62.9) | 0.563 b |
| Outer regional | 3 (18.3) | 623 (37.1) | ||
| Remote/very remote | 1 (6.3) | 101 (6.0) | ||
| Smoking status | Non-smoker | 9 (56.3) | 1209 (72.0) | 0.367 b |
| Ex-smoker | 6 (37.5) | 414 (24.7) | ||
| Current smoker | 1 (6.3) | 56 (3.3) | ||
| Medical condition | Yes | 15 (93.8) | 1030 (61.0) | 0.007 b |
| No | 1 (6.3) | 658 (39.0) | ||
| Diabetes mellitus 1 | 1 (6.3) | 15 (0.9) | 0.141 c | |
| Diabetes mellitus 2 | 4 (25.0) | 192 (11.4) | 0.091 b | |
| Heart failure | 0 (0.0) | 38 (2.3) | - | |
| PVD | 3 (18.8) | 55 (3.3) | 0.001 c | |
| High blood pressure | 9 (56.3) | 762 (45.4) | 0.385 b | |
| Heart attack | 2 (12.5) | 106 (6.3) | 0.313 b | |
| Stroke/TIA | 1 (6.3) | 99 (5.9) | 0.952 b | |
| None | 1 (6.3) | 658 (39.0) | 0.008 b | |
| Systolic BP at screening (mmHg) | <120 | 1 (6.3) | 172 (10.2) | 0.110 b |
| 120 to 129 | 2 (12.5) | 269 (16.0) | ||
| 130 to 139 | 1 (6.3) | 399 (23.8) | ||
| 140 to 159 | 8 (50.0) | 585 (34.8) | ||
| ≥160 | 4 (25.0) | 251 (15.1) | ||
| History of symptoms | Yes | 12 (75.0) | 58 (3.5) | <0.001 b |
| No | 4 (25.0) | 1621 (96.5) | ||
| AF Awareness | Yes | 8 (50.0) | 829 (49.4) | 0.960 b |
| No | 8 (50.0) | 850 (50.6) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jatau, A.I.; Bereznicki, L.R.; Wimmer, B.C.; Bezabhe, W.M.; Peterson, G.M. Improving Knowledge and Early Detection of Atrial Fibrillation through a Community-Based Opportunistic Screening Program: What’s Your Beat? Int. J. Environ. Res. Public Health 2022, 19, 6860. https://doi.org/10.3390/ijerph19116860
Jatau AI, Bereznicki LR, Wimmer BC, Bezabhe WM, Peterson GM. Improving Knowledge and Early Detection of Atrial Fibrillation through a Community-Based Opportunistic Screening Program: What’s Your Beat? International Journal of Environmental Research and Public Health. 2022; 19(11):6860. https://doi.org/10.3390/ijerph19116860
Chicago/Turabian StyleJatau, Abubakar Ibrahim, Luke R. Bereznicki, Barbara C. Wimmer, Woldesellassie M. Bezabhe, and Gregory M. Peterson. 2022. "Improving Knowledge and Early Detection of Atrial Fibrillation through a Community-Based Opportunistic Screening Program: What’s Your Beat?" International Journal of Environmental Research and Public Health 19, no. 11: 6860. https://doi.org/10.3390/ijerph19116860
APA StyleJatau, A. I., Bereznicki, L. R., Wimmer, B. C., Bezabhe, W. M., & Peterson, G. M. (2022). Improving Knowledge and Early Detection of Atrial Fibrillation through a Community-Based Opportunistic Screening Program: What’s Your Beat? International Journal of Environmental Research and Public Health, 19(11), 6860. https://doi.org/10.3390/ijerph19116860