Automated Machine Learning (AutoML)-Derived Preconception Predictive Risk Model to Guide Early Intervention for Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Machine Learning Methodology and Statistical Analyses
3. Results
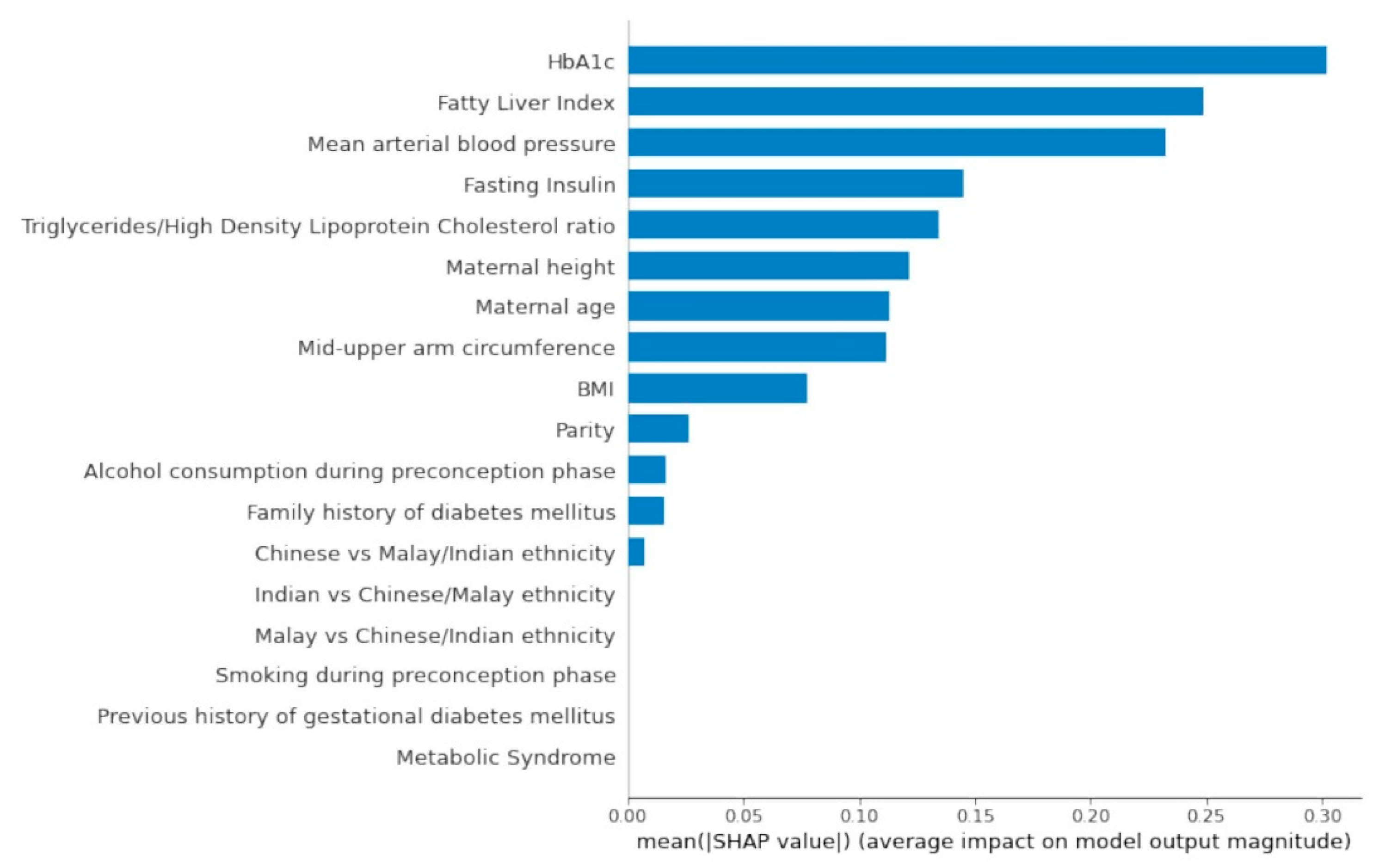
3.1. Top Predictors from AutoML Feature Selection Model
3.2. Preconception Predictive Risk Model
3.3. Associations of Top Predictors and GDM Outcome
3.4. Associations of Top Predictors and Adverse Birth Outcomes (Preterm Birth, Low Birthweight at Term and Large for Gestational Age Infant)
4. Discussion
Primary Findings
5. Limitations
Comparison with Prior Work
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Metzger, B.E.; Coustan, D.R. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 1998, 21, B161–B167. [Google Scholar] [PubMed]
- American Diabetes Association. Gestational Diabetes Mellitus. Diabetes Care 2003, 26, s103–s105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Chu, A.H.Y.; Godfrey, K.M. Gestational Diabetes Mellitus and Developmental Programming. Ann. Nutr. Metab. 2020, 76, 4–15. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- International Diabetes Federation. IDF GDM Model of Care; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Moholdt, T.; Hawley, J.A. Maternal Lifestyle Interventions: Targeting Preconception Health. Trends Endocrinol. Metab. 2020, 31, 561–569. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Barker, M.; Dombrowski, S.U.; Colbourn, T.; Fall, C.H.D.; Kriznik, N.M.; Lawrence, W.T.; Norris, S.A.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef] [Green Version]
- Artzi, N.S.; Shilo, S.; Hadar, E.; Rossman, H.; Barbash-Hazan, S.; Ben-Haroush, A.; Balicer, R.D.; Feldman, B.; Wiznitzer, A.; Segal, E. Prediction of gestational diabetes based on nationwide electronic health records. Nat. Med. 2020, 26, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Zhang, C.-J.; Mol, B.W.; Kawai, A.; Li, C.; Chen, L.; Wang, Y.; Sheng, J.-Z.; Fan, J.-X.; Shi, Y.; et al. Early Prediction of Gestational Diabetes Mellitus in the Chinese Population via Advanced Machine Learning. J. Clin. Endocrinol. Metab. 2020, 106, e1191–e1205. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.X.L.; Soh, S.E.; Loy, S.L.; Ng, S.; Tint, M.T.; Chan, S.Y.; Huang, J.Y.; Yap, F.; Tan, K.H.; Chern, B.S.M.; et al. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO). Eur. J. Epidemiol. 2021, 36, 129–142. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization; International Diabetes Federation (IDF). Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia; World Health Organization: Geneva, Switzerland; International Diabetes Federation: Brussels, Belgium, 2006. [Google Scholar]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Ding, C.; Chan, Z.; Chooi, Y.C.; Choo, J.; Sadananthan, S.A.; Michael, N.; Velan, S.S.; Leow, M.K.-S.; Magkos, F. Association between Serum Vitamin D Metabolites and Metabolic Function in Healthy Asian Adults. Nutrients 2020, 12, 3706. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Reaven, G.; Strom, T.K.; Fox, B. Syndrome X, The Silent Killer: The New Heart Disease Risk; Simon and Schuster: New York, NY, USA, 2001. [Google Scholar]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Health Promotion Board, Metabolic Syndrome. Available online: https://www.hpb.gov.sg/article/metabolic-syndrome (accessed on 7 March 2022).
- Eiben, A.E.; Smith, J.E. Introduction to Evolutionary Computing; Springer: Berlin/Heidelberg, Germany, 2003; Volume 53. [Google Scholar]
- Banzhaf, W.; Nordin, P.; Keller, R.E.; Francone, F.D. Genetic Programming: An Introduction: On the Automatic Evolution of Computer Programs and Its Applications; Morgan Kaufmann Publishers Inc.: Burlington, MA, USA, 1998. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Le, T.T.; Fu, W.; Moore, J.H. Scaling tree-based automated machine learning to biomedical big data with a feature set selector. Bioinformatics 2019, 36, 250–256. [Google Scholar] [CrossRef] [Green Version]
- WHO. Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253. [Google Scholar]
- Soh, S.-E.; Tint, M.T.; Gluckman, P.D.; Godfrey, K.M.; Rifkin-Graboi, A.; Chan, Y.H.; Stünkel, W.; Holbrook, J.D.; Kwek, K.; Chong, Y.-S.; et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int. J. Epidemiol. 2013, 43, 1401–1409. [Google Scholar] [CrossRef]
- Mikolajczyk, R.T.; Zhang, J.; Betran, A.P.; Souza, J.P.; Mori, R.; Gülmezoglu, A.M.; Merialdi, M. A global reference for fetal-weight and birthweight percentiles. Lancet 2011, 377, 1855–1861. [Google Scholar] [CrossRef]
- Lurie, S.; Mamet, Y. Red blood cell survival and kinetics during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 93, 185–192. [Google Scholar] [CrossRef]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Bonora, E.; Tuomilehto, J. The Pros and Cons of Diagnosing Diabetes With A1C. Diabetes Care 2011, 34, S184–S190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van’t Riet, E.; Alssema, M.; Rijkelijkhuizen, J.M.; Kostense, P.J.; Nijpels, G.; Dekker, J.M. Relationship between A1C and glucose levels in the general Dutch population: The new Hoorn study. Diabetes Care 2010, 33, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Neovius, M.; Söderling, J.; Gudbjörnsdottir, S.; Svensson, A.M.; Franzén, S.; Stephansson, O.; Pasternak, B. Maternal Glycemic Control in Type 1 Diabetes and the Risk for Preterm Birth: A Population-Based Cohort Study. Ann. Intern. Med. 2019, 170, 691–701. [Google Scholar] [CrossRef]
- An-Na, C.; Man-Li, Y.; Jeng-Hsiu, H.; Pesus, C.; Shin-Kuo, S.; Heung-Tat, N. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci. 1995, 56, 2367–2375. [Google Scholar] [CrossRef]
- Toescu, V.; Nuttall, S.L.; Martin, U.; Nightingale, P.; Kendall, M.J.; Brydon, P.; Dunne, F. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin. Sci. 2004, 106, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Chen, Y.; Zhou, M.; Liu, M.; Zhang, L.; Liang, Z.; Chen, D. An early prediction model for gestational diabetes mellitus based on genetic variants and clinical characteristics in China. Diabetol. Metab. Syndr. 2022, 14, 15. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, L.; Chen, Y.; Salerno, S.; Li, Y.; Zeng, X.; Li, H. Prediction of gestational diabetes mellitus in the first 19 weeks of pregnancy using machine learning techniques. J. Matern. Neonatal Med. 2022, 35, 2457–2463. [Google Scholar] [CrossRef]

| S-PRESTO (n = 222) | |
|---|---|
| Demographics | |
| Age (years), mean ± SD | 30.51 ± 3.11 |
| Ethnicity, n (%) | |
| Chinese | 176 (79.28) |
| Malay | 30 (13.51) |
| Indian | 16 (7.21) |
| Medical/Obstetric History | |
| Family history of diabetes mellitus, n (%) | |
| Yes | 63 (28.38) |
| No | 159 (71.62) |
| History of GDM, n (%) | |
| Yes | 6 (2.70) |
| No | 216 (97.30) |
| Parity, n (%) | |
| Nulliparous | 140 (63.06) |
| Multiparous | 82 (36.94) |
| Medical history of high blood pressure, n (%) | |
| Yes | 0 (0.00) |
| No | 222 (100.00) |
| Physical Measures at Preconception | |
| Pre-pregnancy weight (kg), mean ± SD | 59.31 ± 11.80 |
| Maternal height (cm), mean ± SD | 159.96 ± 5.55 |
| Pre-pregnancy BMI (kg/m2), mean ± SD | 23.18 ± 4.52 |
| Waist circumference (cm), mean ± SD | 81.35 ± 10.10 |
| Mid-upper arm circumference (cm), mean ± SD | 27.27 ± 4.08 |
| Systolic blood pressure (mm Hg), mean ± SD | 104.15 ± 8.92 |
| Diastolic blood pressure (mm Hg), mean ± SD | 67.38 ± 7.51 |
| Mean arterial blood pressure (mm Hg), mean ± SD | 79.63 ± 7.48 |
| Blood-Derived Markers at Preconception | |
| HbA1c (mmol/mol), mean ± SD | 31.80 ± 2.73 |
| Fasting glucose (mmol/L), mean ± SD | 4.72 ± 0.33 |
| Fasting insulin (mU/L), mean ± SD | 5.97 ± 4.83 |
| Triglycerides (mmol/L), mean ± SD | 0.81 ± 0.38 |
| High density lipoprotein cholesterol (mmol/L), mean ± SD | 1.48 ± 0.28 |
| Gamma-glutamyl transferase (U/L), mean ± SD | 18.99 ± 14.28 |
| Lifestyle Factors at Preconception | |
| Self-reported smoking, n (%) | |
| Yes | 6 (2.70) |
| No | 216 (97.30) |
| Self-reported alcohol consumption, n (%) | |
| Yes | 159 (71.62) |
| No | 63 (28.38) |
| Metabolic Indices at Preconception | |
| Homeostasis model assessment-insulin resistance (HOMA-IR) index, mean ± SD | 1.27 ± 1.08 |
| Triglycerides/high density lipoprotein cholesterol ratio | 0.59 ± 0.41 |
| Fatty liver index, mean ± SD | 5.61 ± 10.38 |
| Metabolic syndrome, n (%) | |
| Yes | 7 (3.15) |
| No | 215 (96.85) |
| Prediabetes Status at Preconception | |
| Impaired fasting glucose (IFG), n (%) | 0 (0.00) |
| Impaired glucose tolerance (IGT), n (%) | 11 (5.00) |
| Type 2 diabetes (T2D), n (%) | 0 (0.00) |
| Normal glucose metabolism, n (%) | 209 (95.00) |
| OGTT at 24+1–28+6 Weeks’ Gestation | |
| Glucose measures (mmol/L), mean ± SD | |
| Fasting glucose | 4.28 ± 0.35 |
| 1-hour glucose | 7.99 ± 1.52 |
| 2-hour glucose | 6.68 ± 1.27 |
| GDM, n (%) | |
| IADPSG/WHO 2013 criteria | 29 (13.06) |
| Adverse Birth Outcomes | |
| Preterm birth, n (%) | |
| Yes | 10 (4.50) |
| No | 212 (95.50) |
| Low birthweight at term, n (%) | |
| Yes | 7 (3.24) |
| No | 209 (96.76) |
| Large for gestational age infant, n (%) | |
| Yes | 34 (15.74) |
| No | 182 (84.26) |
| Features | Optimal Machine Learning Pipeline | AUC |
|---|---|---|
| 1: HbA1c | Gradient boosting classifier. | 0.81 |
| 2: HbA1c + fatty liver index | Stacked ensemble model with logistic regression classifier, multinomial naïve Bayes classifier and multi-layer perceptron classifier. | 0.78 |
| 3: HbA1c + fatty liver index + mean arterial blood pressure | Stacked ensemble model with k-nearest neighbors classifier and decision tree classifier. | 0.82 |
| 4: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin | Stacked ensemble model with k-nearest neighbors classifier and decision tree classifier. | 0.88 |
| 5: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio | Extra trees classifier. | 0.93 |
| 6: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height | Stacked ensemble model with logistic regression classifier (stochastic gradient descent training) and k-nearest neighbors classifier. | 0.89 |
| 7: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age | Multi-layer perceptron classifier. | 0.88 |
| 8: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age + mid-upper arm circumference | Stacked ensemble model with Bernoulli naïve Bayes classifier, gaussian naïve Bayes classifier, multinomial naïve Bayes classifier and linear support vector machine classifier. | 0.93 |
| 9: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age + mid-upper arm circumference + BMI | Stacked ensemble model with extra trees classifier, Bernoulli naïve Bayes classifier and gaussian naïve Bayes classifier. | 0.85 |
| 10: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age + mid-upper arm circumference + BMI + parity | Stacked ensemble model with k-nearest neighbors classifier and multi-layer perceptron classifier. | 0.85 |
| 11: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age + mid-upper arm circumference + BMI + parity + alcohol consumption | Stacked ensemble model with gradient boosting classifier, multi-layer perceptron classifier and linear support vector machine classifier. | 0.90 |
| 12: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age + mid-upper arm circumference + BMI + parity + alcohol consumption + family history of diabetes mellitus | Stacked ensemble model with multinomial naïve Bayes classifier and multi-layer perceptron classifier. | 0.87 |
| 13: HbA1c + fatty liver index + mean arterial blood pressure + fasting insulin + TG/HDL ratio + height + age + mid-upper arm circumference + BMI + parity + alcohol consumption + family history of diabetes mellitus + Chinese ethnicity | Stacked ensemble model with multinomial naïve Bayes classifier and multi-layer perceptron classifier. | 0.87 |
| 14: Mean arterial blood pressure + height + age + mid-upper arm circumference + BMI + parity + alcohol consumption + family history of diabetes mellitus + Chinese ethnicity | Stacked ensemble model with linear support vector machine classifier (stochastic gradient descent training), Bernoulli naïve Bayes classifier, multinomial naïve Bayes classifier, multi-layer perceptron classifier and linear support vector machine classifier. | 0.81 |
| Feature | GDM (n = 222) |
|---|---|
| OR (95% CI) p-Value | |
| HbA1c (mmol/mol) | OR: 1.31 (1.12–1.53) p-value = 0.001 * |
| Fatty liver index | OR: 1.01 (0.98–1.05) p-value = 0.458 |
| Mean arterial blood pressure (mm Hg) | OR: 0.99 (0.94–1.04) p-value = 0.584 |
| Fasting insulin (mU/L) | OR: 1.05 (0.99–1.12) p-value = 0.119 |
| Triglycerides/high density lipoprotein cholesterol ratio | OR: 1.45 (0.65–3.28) p-value = 0.365 |
| Maternal height (cm) | OR: 0.96 (0.90–1.04) p-value = 0.311 |
| Age (years) | OR: 0.97 (0.86–1.10) p-value = 0.673 |
| Mid-upper arm circumference (cm) | OR: 1.05 (0.96–1.15) p-value = 0.290 |
| BMI (kg/m2) | OR: 1.05 (0.97–1.13) p-value = 0.241 |
| Parity | OR: 0.74 (0.32–1.71) p-value = 0.481 |
| Self-reported alcohol consumption | OR: 2.06 (0.75–5.67) p-value = 0.161 |
| Family history of diabetes mellitus | OR: 1.39 (0.61–3.18) p-value = 0.436 |
| Chinese vs. Malay/Indian ethnicity | OR: 1.29 (0.47–3.60) p-value = 0.621 |
| Feature | GDM (n = 222) |
| OR (95% CI) p-value | |
| HbA1c (mmol/mol) ^ | OR: 1.34 (1.13–1.60) p-value = 0.001 * |
| Feature | GDM (n = 211) |
| OR (95% CI) p-value | |
| HbA1c (mmol/mol) #,^ | OR: 1.32 (1.10–1.59) p-value = 0.003 * |
| Feature | Preterm Birth (n = 222) | Low Birthweight at Term (n = 216) | Large for Gestational Age Infant (n = 216) |
|---|---|---|---|
| OR (95% CI) p-Value | OR (95% CI) p-Value | OR (95% CI) p-Value | |
| HbA1c (mmol/mol) | OR: 1.28 (1.01–1.62) p-value = 0.042 * | OR: 1.13 (0.86–1.49) p-value = 0.381 | OR: 1.06 (0.92–1.21) p-value = 0.416 |
| Fatty liver index | OR: 1.00 (0.94–1.06) p-value = 0.951 | OR: 0.89 (0.68–1.16) p-value = 0.386 | OR: 1.06 (1.03–1.10) p-value < 0.001 * |
| Mean arterial blood pressure (mm Hg) | OR: 1.02 (0.94–1.11) p-value = 0.688 | OR: 0.96 (0.86–1.06) p-value = 0.403 | OR: 1.03 (0.98–1.08) p-value = 0.253 |
| Fasting insulin (mU/L) | OR: 1.04 (0.96–1.14) p-value = 0.317 | OR: 1.05 (0.95–1.15) p-value = 0.359 | OR: 1.08 (1.01–1.16) p-value = 0.019 * |
| Triglycerides/high density lipoprotein cholesterol ratio | OR: 0.79 (0.13–4.76) p-value = 0.797 | OR: 1.42 (0.34–6.00) p-value = 0.630 | OR: 2.85 (1.30–6.21) p-value = 0.009 * |
| Maternal height (cm) | OR: 0.95 (0.84–1.07) p-value = 0.363 | OR: 0.91 (0.78–1.05) p-value = 0.192 | OR: 0.99 (0.93–1.06) p-value = 0.794 |
| Age (years) | OR: 1.05 (0.86–1.29) p-value = 0.629 | OR: 0.97 (0.76–1.24) p-value = 0.799 | OR: 1.07 (0.95–1.20) p-value = 0.267 |
| Mid-upper arm circumference (cm) | OR: 0.97 (0.82–1.15) p-value = 0.737 | OR: 0.90 (0.71–1.14) p-value = 0.362 | OR: 1.22 (1.12–1.33) p-value < 0.001 * |
| BMI (kg/m2) | OR: 1.00 (0.88–1.16) p-value = 0.914 | OR: 0.84 (0.64–1.10) p-value = 0.206 | OR: 1.18 (1.09–1.27) p-value < 0.001 * |
| Parity | OR: 1.75 (0.49–6.25) p-value = 0.387 | OR: 1.29 (0.28–5.90) p-value = 0.746 | OR: 1.89 (0.90–3.95) p-value = 0.091 |
| Self-reported alcohol consumption | OR: 0.38 (0.11–1.35) p-value = 0.134 | OR: 2.47 (0.29–20.97) p-value = 0.407 | OR: 0.44 (0.21–0.94) p-value = 0.033 * |
| Family history of diabetes mellitus | OR: 1.73 (0.47–6.35) p-value = 0.409 | OR: 1.91 (0.41–8.78) p-value = 0.407 | OR: 1.95 (0.92–4.17) p-value = 0.083 |
| Chinese vs. Malay/Indian ethnicity | OR: 0.59 (0.15–2.39) p-value = 0.463 | OR: 0.33 (0.07–1.51) p-value = 0.152 | OR: 0.55 (0.24–1.26) p-value = 0.158 |
| Feature | Preterm Birth (n = 185) | ||
| OR (95% CI) p-value | |||
| HbA1c (mmol/mol) ^ | OR: 1.63 (1.12–2.38) p-value = 0.011 * | ||
| Feature | Preterm Birth (n = 154) | ||
| OR (95% CI) p-value | |||
| HbA1c (mmol/mol) #,^ | OR: 1.75 (1.14–2.67) p-value = 0.010 * | ||
| Feature | Large for Gestational Age Infant (n = 198) | ||
| OR (95% CI) p-value | |||
| Fatty liver index ^ | OR: 1.02 (0.96–1.08) p-value = 0.473 | ||
| Feature | Large for Gestational Age Infant (n = 198) | ||
| OR (95% CI) p-value | |||
| Fasting insulin (mU/L) ^ | OR: 1.01 (0.92–1.10) p-value = 0.825 | ||
| Feature | Large for Gestational Age Infant (n = 198) | ||
| OR (95% CI) p-value | |||
| Triglycerides/high density lipoprotein cholesterol ratio ^ | OR: 1.98 (0.76–5.10) p-value = 0.160 | ||
| Feature | Large for Gestational Age Infant (n = 198) | ||
| OR (95% CI) p-value | |||
| Mid-upper arm circumference (cm) ^ | OR: 1.21 (0.93–1.58) p-value = 0.162 | ||
| Feature | Large for Gestational Age Infant (n = 198) | ||
| OR (95% CI) p-value | |||
| BMI (kg/m2) ~ | OR: 1.20 (1.10–1.31) p-value < 0.001 * | ||
| Feature | Large for Gestational Age Infant (n = 198) | ||
| OR (95% CI) p-value | |||
| Self-reported alcohol consumption ^ | OR: 0.47 (0.17–1.28) p-value = 0.138 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Ang, L.T.; Png, H.; Ng, M.; Tan, K.; Loy, S.L.; Tan, K.H.; Chan, J.K.Y.; Godfrey, K.M.; Chan, S.-y.; et al. Automated Machine Learning (AutoML)-Derived Preconception Predictive Risk Model to Guide Early Intervention for Gestational Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 6792. https://doi.org/10.3390/ijerph19116792
Kumar M, Ang LT, Png H, Ng M, Tan K, Loy SL, Tan KH, Chan JKY, Godfrey KM, Chan S-y, et al. Automated Machine Learning (AutoML)-Derived Preconception Predictive Risk Model to Guide Early Intervention for Gestational Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2022; 19(11):6792. https://doi.org/10.3390/ijerph19116792
Chicago/Turabian StyleKumar, Mukkesh, Li Ting Ang, Hang Png, Maisie Ng, Karen Tan, See Ling Loy, Kok Hian Tan, Jerry Kok Yen Chan, Keith M. Godfrey, Shiao-yng Chan, and et al. 2022. "Automated Machine Learning (AutoML)-Derived Preconception Predictive Risk Model to Guide Early Intervention for Gestational Diabetes Mellitus" International Journal of Environmental Research and Public Health 19, no. 11: 6792. https://doi.org/10.3390/ijerph19116792
APA StyleKumar, M., Ang, L. T., Png, H., Ng, M., Tan, K., Loy, S. L., Tan, K. H., Chan, J. K. Y., Godfrey, K. M., Chan, S.-y., Chong, Y. S., Eriksson, J. G., Feng, M., & Karnani, N. (2022). Automated Machine Learning (AutoML)-Derived Preconception Predictive Risk Model to Guide Early Intervention for Gestational Diabetes Mellitus. International Journal of Environmental Research and Public Health, 19(11), 6792. https://doi.org/10.3390/ijerph19116792






