Epidemiological Study of Risk Factors for Lung Cancer in KwaZulu-Natal, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
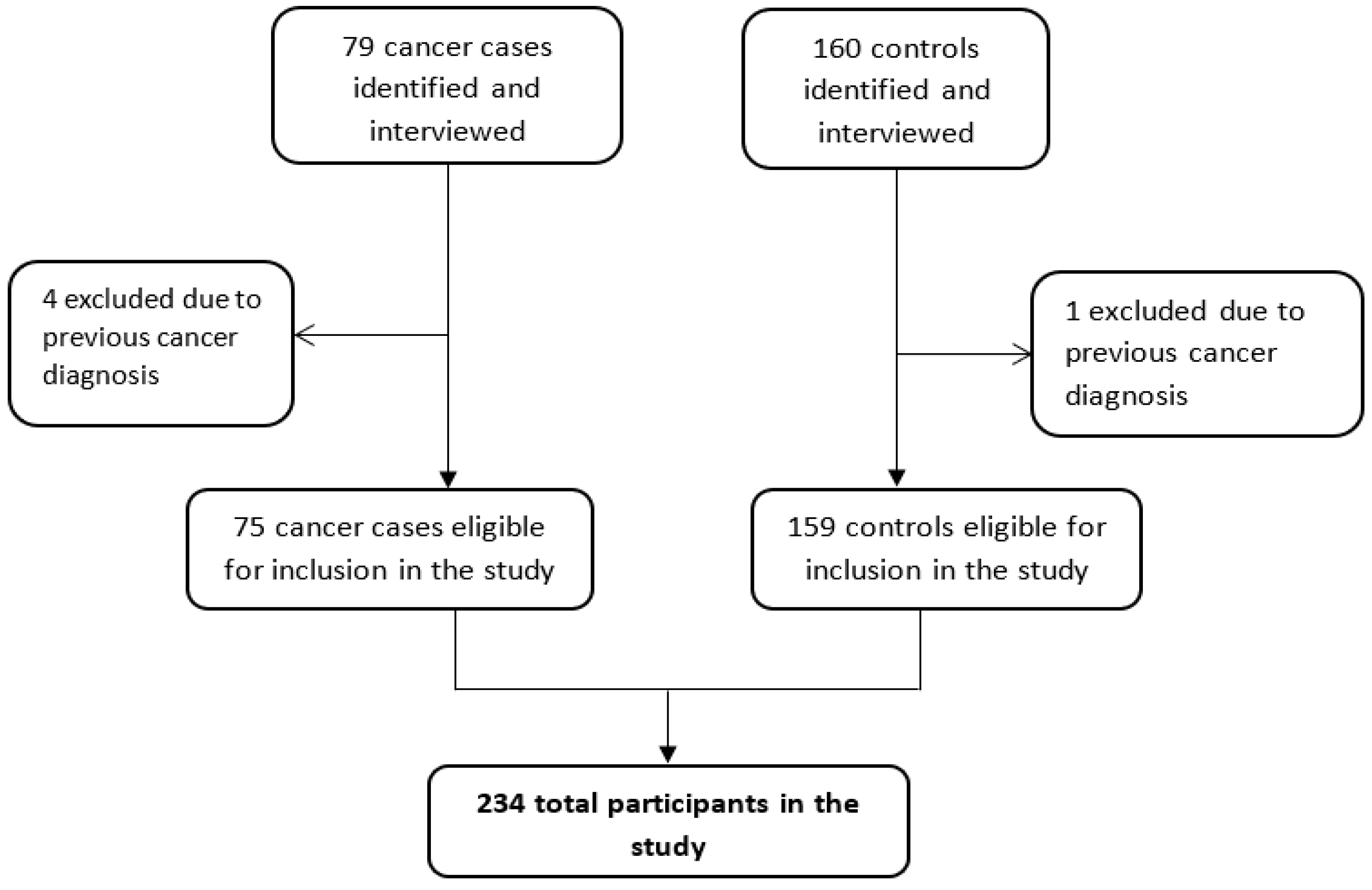
2.2. Identification and Recruitment of Participants
2.3. Data Collection Using Questionnaire
2.4. Data Analysis
2.5. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IARC | International Agency for Research on Cancer |
| KZN | KwaZulu-Natal |
| OR | Odds Ratio |
| WHO | World Health Organization |
| Stats-SA | Statistics South Africa |
References
- Arkin, D.M.P.; Ray, F.B.; Erlay, J.F.; Isani, P.P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hashim, D.; Boffetta, P. Occupational and environmental exposures and cancers in developing countries. Ann. Glob. Health 2014, 80, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Koegelenberg, C.F.N.; Society, O.B.O.T.S.A.T.; Dorfman, S.; Schewitz, I.; Richards, G.A.; Maasdorp, S.; Smith, C.; Dheda, K. Recommendations for lung cancer screening in Southern Africa. J. Thorac. Dis. 2019, 11, 3696–3703. [Google Scholar] [CrossRef]
- Luis, F.; Moncayo, G. NCR Pathalogy 2018 Full Report 1; National Health Laboratory Service: Johannesburg, South Africa, 2021; pp. 1–38. [Google Scholar]
- van Eeden, R.; Tunmer, M.; Geldenhuys, A.; Nayler, S.; Rapoport, B.L. Lung Cancer in South Africa. J. Thorac. Oncol. 2020, 15, 22–28. [Google Scholar] [CrossRef]
- WHO-IARC; Stewart, B.W.; Kleihues, P. World Cancer Report; IACR: Lyon, France, 2003. [Google Scholar]
- Khuder, S.A. Effect of cigarette smoking on major histological types of lung cancer: A meta-analysis. Lung Cancer 2001, 31, 139–148. [Google Scholar] [CrossRef]
- WHO-IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Tobacco Smoke and Involuntary Smoking; IACR: Lyon, France, 2004; Volume 83. [Google Scholar]
- Inoue-Choi, M.; Hartge, P.; Liao, L.M.; Caporaso, N.; Freedman, N.D. Association between long-term low- intensity cigarette smoking and incidence of smoking-related cancer in the National Institutes of Health-AARP cohort. Int. J. Cancer 2018, 142, 271–280. [Google Scholar] [CrossRef]
- Jafri, S.H.; Ali, F.; Mollaeian, A.; Hasan, S.M.; Hussain, R.; Akkanti, B.; Williams, J.; Shoukier, M.; El-Osta, H.; Akanti, B. Major Stressful Life Events and Risk of Developing Lung Cancer: A Case-Control Study. Clin. Med. Insights Oncol. 2019, 13, 117955491983579. [Google Scholar] [CrossRef]
- Driscoll, T.; Nelson, D.I.; Steenland, K.; Leigh, J.; Concha-Barrientos, M.; Fingerhut, M.; Prüss-Üstün, A. The global burden of disease due to occupational carcinogens. Am. J. Ind. Med. 2005, 48, 419–431. [Google Scholar] [CrossRef]
- Ngamwong, Y.; Tangamornsuksan, W.; Lohitnavy, O.; Chaiyakunapruk, N.; Scholfield, C.N.; Reisfeld, B.; Lohitnavy, M. Additive synergism between asbestos and smoking in lung cancer risk: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0135798. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef]
- Matakidou, A.; Eisen, T.; Houlston, R.S. Systematic review of the relationship between family history and lung cancer risk. Br. J. Cancer 2005, 93, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Jemal, A. Cancer in Africa 2012. Cancer Epidemiol. Biomark. Prev. 2014, 23, 953–966. [Google Scholar] [CrossRef] [PubMed]
- (NDoH) National Department of Health; (Stats SA) Statistics South Africa; (SAMRC) South African Medical Research Council; ICF. South Africa Demographic and Health Survey 2016; National Department of Health: Cape Town, South Africa, 2019. [Google Scholar]
- Stats, S.A. Census. 2001. Available online: http://www.statssa.gov.za/?page_id=4519 (accessed on 14 July 2019).
- Smith, C.J.; Perfetti, T.A.; Rumple, M.A.; Rodgman, A.; Doolittle, D.J. ‘IARC Group 2B carcinogens’ reported in cigarette mainstream smoke. Food Chem. Toxicol. 2001, 39, 183–205. [Google Scholar] [CrossRef]
- Kim, A.; Ko, H.; Kwon, J.; Lee, J. Exposure to secondhand Smoke and risk of cancer in never smokers: A meta-analysis of epidemiologic studies. Int. J. Environ. Res. Public Health 2018, 15, 1981. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Bansal, J.G. Risk factors of Lung Cancer in nonsmoker. Curr. Probl. Cancer 2017, 41, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Furge, L.L.; Guengerich, F.P. Mini-series: Modern metabolic concepts cytochrome P450 enzymes in drug metabolism and chemical toxicology. Biochem. Mol. Biol. Educ. 2006, 3, 66–74. [Google Scholar] [CrossRef]
- Massion, P.P.; Sequist, L.V.; Cancer, L. Biology of lung cancer. In Neoplasms of the Lung; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; pp. 912–926. [Google Scholar]
- Bandera, E.V.; Freudenheim, J.L.; Vena, J.E. Alcohol Consumption and Lung Cancer: A Review of the Epidemiologic Evidence. Am. Assoc. Cancer Res. 2001, 10, 813–821. [Google Scholar]
- Carpenter, C.L.; Morgenstern, H.; London, S.J. Alcoholic Beverage Consumption and Lung Cancer Risk among Residents of Los Angeles County 1–6. Am. Soc. Nutr. Sci. 1998, 128, 694–700. [Google Scholar] [CrossRef]
- Grønbaek, M.; Jensen, M.K.; Johansen, D.; Becker, U. Intake of beer, wine and spirits and risk of heavy drinking and alcoholic cirrhosis. Biol. Res. 2004, 37, 195–200. [Google Scholar] [CrossRef]
- Nachiappan, V.; Si, M.; Chakravarti, A.; Cd, E.; Rajasekharan, R. Lipid peroxidation and ethanol-related tumor promotion in Fischer- 344 rats treated with tobacco-specific nitrosamines. Alcohol Alcohol. 1994, 29, 7811340. [Google Scholar]
- Fang, J.; Vaca, C.E. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis 1997, 18, 627–632. [Google Scholar] [CrossRef]
- Corrales, L.; Rosell, R.; Cardona, A.F.; Martín, C.; Zatarain-Barrón, Z.L.; Arrieta, O. Lung cancer in never smokers: The role of different risk factors other than tobacco smoking. Crit. Rev. Oncol. Hematol. 2020, 148, 102895. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Fedewa, S.A.; Henley, S.J.; Pollack, L.A.; Jemal, A. Proportion of Never Smokers among Men and Women with Lung Cancer in 7 US States. JAMA Oncol. 2021, 7, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-L.; Hsiao, C.-F.; Chang, G.-C.; Tsai, Y.-H.; Huang, M.-S.; Su, W.-C.; Chen, Y.-M.; Hsin, C.-W.; Chang, C.-H.; Yang, P.-C.; et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control 2013, 24, 567–576. [Google Scholar] [CrossRef]
- Couraud, S.; Zalcman, G.; Milleron, B.; Morin, F.; Souquet, P.-J. Lung cancer in never smokers—A review. J. Clin. Oncol. 2017, 5, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Boffetta, P.; Duell, E.J.; Bickeböller, H.; Rosenberger, A.; McCormack, V.; Muscat, J.E.; Yang, P.; Wichmann, H.-E.; Brueske-Hohlfeld, I.; et al. Previous lung diseases and lung cancer risk: A pooled analysis from the international lung cancer consortium. Am. J. Epidemiol. 2012, 176, 573–585. [Google Scholar] [CrossRef]
- Nalbandian, A.; Yan, B.; Pichugin, A.; Bronson, R.T.; Kramnik, I. Lung carcinogenesis induced by chronic tuberculosis infection: The experimental model and genetic control. Oncogene 2009, 28, 1928–1938. [Google Scholar] [CrossRef]
- Gomperts, B.; Spira, A.; Massion, P.; Walser, T.; Wistuba, I.; Minna, J.; Dubinett, S. Evolving concepts in lung carcinogenesis. Natl. Inst. Health 2012, 32, 32–43. [Google Scholar] [CrossRef]
- Shu, C.; Chang, S.; Lai, Y.; Chang, C. Factors for the early revision of misdiagnosed Tuberculosis to lung cancer: A multicenter study in a Tuberculosis-prevalent area. J. Clin. Med. 2019, 8, 700. [Google Scholar] [CrossRef]
- Parker, C.S.; Siracuse, C.G.; Litle, V.R. Identifying lung cancer in patients with active pulmonary tuberculosis. J. Thorac. Dis. 2018, 10, 3392–3397. [Google Scholar] [CrossRef]
- Hammen, I. Tuberculosis mimicking lung cancer. Respir. Med. Case Rep. 2015, 16, 45–47. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.S.; Harris, M.A.; Tjepkema, M.; Peters, P.A.; Demers, P.A. Cancer risks among welders and occasional welders in a national population-based cohort study: Canadian census health and environmental cohort. Saf. Health Work 2017, 8, 258–266. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man; ICAR: Lyon, France, 1973; Volume 2, pp. 17–47. [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chromium, Nickel and Welding; IARC: Lyon, France, 1990; Volume 49, p. 677. [Google Scholar]
- Leonard, S.S.; Chen, B.T.; Stone, S.G.; Schwegler-Berry, D.; Kenyon, A.J.; Frazer, D.; Antonini, J.M. Comparison of stainless and mild steel welding fumes in generation of reactive oxygen species. Part. Fibre Toxicol. 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Hughes, J.M.; Weill, H. Asbestos exposure, asbestosis, and asbestos-attributable lung cancer. Thorax 1996, 51, 9–15. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1090700&tool=pmcentrez&rendertype=abstract (accessed on 1 February 2022). [CrossRef] [PubMed]
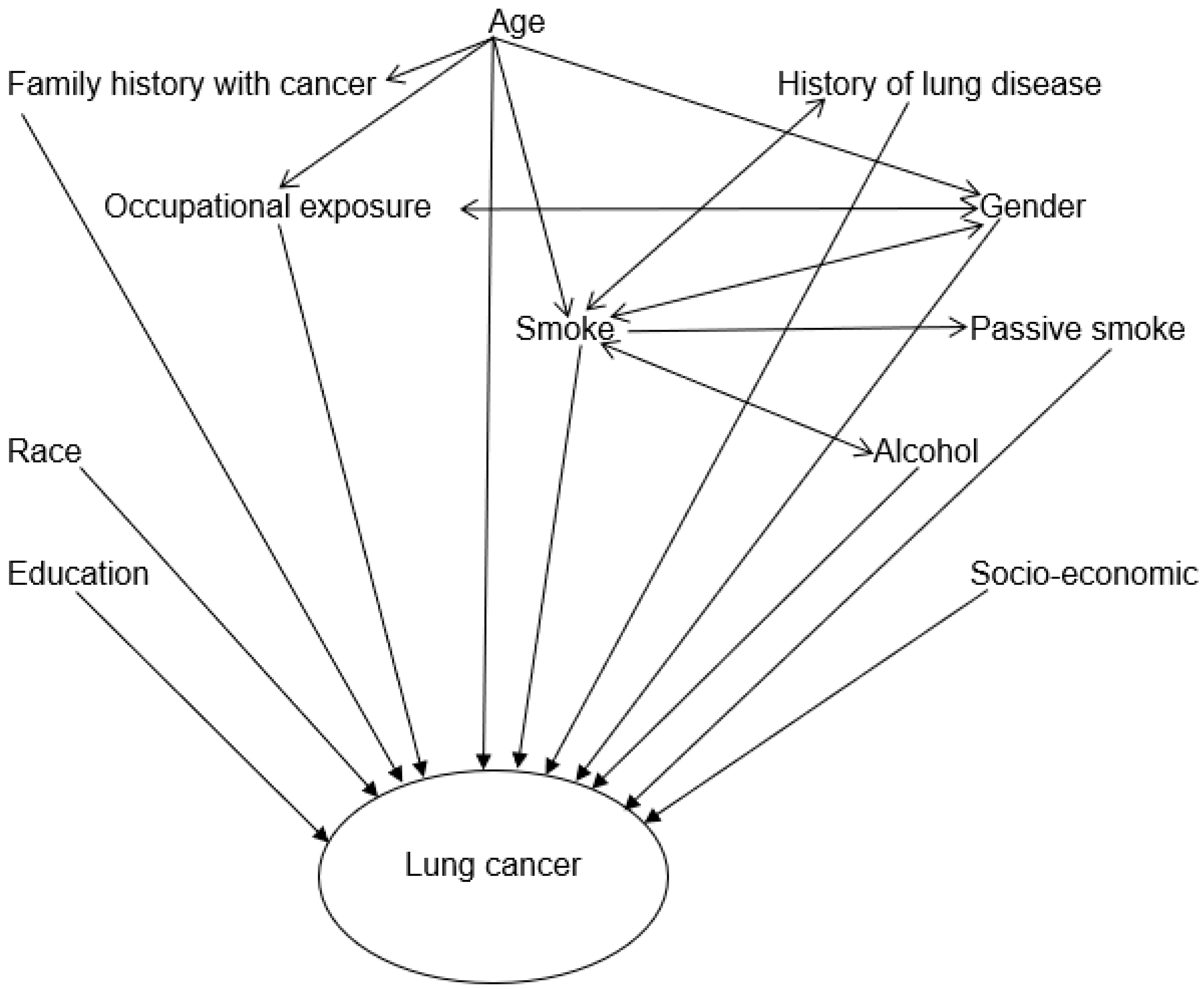
| Characteristics/Category | Cases N (%) | Controls N (%) | Crude OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Total no | 75 | 159 | |||
| Age | |||||
| Mean ± SD (range) | ±61.8 (13.9) | ±54.8 (14.4) | |||
| 21–39 | 7 (9.3) | 16 (10.1) | - | ||
| 40–49 | 8 (10.7) | 48 (30.2) | 0.38 (0.12–1.22) | <0.001 | |
| 50–59 | 12 (16.0) | 40 (25.2) | 0.69 (0.23–2.06) | ||
| 60–69 | 25 (33.3) | 32 (20.1) | 1.79 (0.64–5.01) | ||
| 70+ | 23 (30.7) | 23 (14.5) | 2.29 (0.79–6.60) | ||
| Gender | |||||
| Female | 27(36.0) | 63(39.6) | - | ||
| Male | 48 (64.0) | 96 (60.4) | 1.17 (0.66–2.06) | 0.595 | |
| Race | |||||
| African | 43 (57.3) | 135 (84.9) | - | ||
| Mixed race | 0 (0) | 2 (1.26) | - | ||
| White | 10 (13.3) | 9 (5.7) | 3.49 (1.33–9.14)) | <0.001 | |
| Asian | 22 (29.3) | 13 (8.2) | 5.31 (2.47–11.4) | ||
| a Marital status | |||||
| Single | 17 (23.3) | 69 (44.5) | - | ||
| Married | 42 (57.5) | 67 (43.2) | 2.54 (1.32–4.90) | 0.004 | |
| Divorced + Widowed | 14 (19.2) | 19 (12.3) | 2.99 (1.25–7.14) | ||
| b Education | |||||
| No education—Primary | 20 (30.3) | 40 (29.0) | - | ||
| High school—Higher education | 46 (69.7) | 98 (71.0) | 0.94 (0.49–1.78) | 0.847 | |
| c Monthly household income | 0–R4000 | 44 (71.0) | 77 (74.0) | - | |
| R4 001+ | 18 (29.0) | 27 (26.0) | 1.17 (0.58–2.35) | 0.667 | |
| Clinical characteristics of cases | |||||
| Basis of diagnosis | |||||
| Biopsy | 56 (74.7) | - | |||
| x-ray | 10 (13.4) | - | - | - | |
| CT Scan | 6 (8.00) | - | |||
| Cytology | 3 (4.00) | ||||
| d Histological classification | - | - | |||
| Adenocarcinoma | 16 (21.3) | - | |||
| Squamous Cell Carcinoma | 11 (14.7) | - | |||
| Other | 9 (12) | - | |||
| Staging | |||||
| 1 | 1 (1.33) | - | |||
| 2 | 0 (0.00) | - | - | - | |
| 3 | 2 (2.67) | - | |||
| 4 | 28 (37.3) | - | |||
| Unknown | 44 (58.7) | - | |||
| Variables | Cases n = 75 (%) | Controls n = 159 (%) | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Lifestyle | |||||
| Passive smoke exposure | No | 34 (45.3) | 112 (70.4) | - | |
| Yes | 41 (54.7) | 47 (29.6) | 2.87 (1.63–5.07) | <0.001 | |
| Have you ever smoked | No | 26 (34.7) | 102 (64.2) | - | |
| Yes | 49 (65.3) | 57 (35.9) | 3.37 (1.90–6.00) | <0.001 | |
| Have you ever consumed alcohol? | No | 50 (66.7) | 93 (58.5) | Ref | |
| Yes | 25 (33.3) | 66 (41.5) | 2.82 (1.59–5.00) | <0.001 | |
| Family history with cancer | |||||
| Biological father | No | 73 (97.3) | 158 (99.4) | - | |
| Yes | 2 (2.67) | 1 (0.63) | 4.33 (0.39–48.50) | 0.235 | |
| Biological mother | No | 69 (92.0) | 155 (97.5) | - | |
| Yes | 6 (8.00) | 4 (2.52) | 3.37 (0.92–12.30) | 0.066 | |
| Siblings | No | 64 (85.3) | 156 (98.1) | - | |
| Yes | 11 (14.7) | 3 (1.89) | 8.93 (2.41–33.10) | 0.001 | |
| * History of lung disease | No | 53 (70.7) | 151 (95.0) | - | |
| Yes | 22 (29.3) | 8 (5.03) | 7.83 (3.29–18.7) | <0.001 | |
| Employment sector | |||||
| Office and Household | No | 29 (38.7) | 51 (32.1) | - | |
| Yes | 46 (61.3) | 108 (67.9) | 0.75 (0.42–1.33) | (0.322) | |
| Transport, Storage and Repair of motor vehicles | No | 59 (78.7) | 146 (91.8) | - | |
| Yes | 16 (21.3) | 13 (8.18) | 3.05 (1.38–6.72) | (0.006) | |
| * Mining, Construction and Manufacturing | No | 40 (53.3) | 113 (71.1) | - | |
| Yes | 35 (46.7) | 46 (28.9) | 2.15 (1.22–3.79) | (0.008) | |
| Agriculture, Forestry and Fishing | No | 65 (86.7) | 151 (95.0) | - | |
| Yes | 10 (13.3) | 8 (5.03) | 2.90 (1.09–7.69) | (0.032) | |
| Occupational exposures | |||||
| Soot | No | 68 (90.7) | 157 (98.7) | - | |
| Yes | 7 (9.33) | 2 (1.26) | 8.08 (1.64–39.9) | (0.010) | |
| Iron and Steel | No | 67 (89.3) | 157 (98.7) | - | |
| Yes | 8 (10.7) | 2 (1.26) | 9.37 (1.94–45.3) | (0.005) |
| Variable | Univariate OR (95%CI) | Multivariate Models aOR (95% CI) | ||
|---|---|---|---|---|
| Tobacco Smoking | Passive Smoke Exposure | |||
| Tobacco smoking | No | - | ||
| Yes | * 3.37 (1.90–6.00) (<0.001) | ** 2.86 (1.21–6.77) (0.017) | - | |
| Passive smoke exposure | No | - | ||
| Yes | * 2.87 (1.63–5.07) (<0.001) | - | ** 3.28 (1.48–7.30) (0.004) | |
| Alcohol consumption | No | |||
| Yes | * 2.82 (1.59–5.01) (<0.001) | ** 2.79 (1.21–6.41) (0.016) | ** 3.35 (1.44–7.75) (0.005) | |
| Sibling with cancer | No | |||
| Yes | ** 8.94 (2.41–33.1) (0.001) | 3.83 (0.53–27.4) (0.181) | 3.79 (0.49–29.2) (0.201) | |
| * History of lung disease | No | |||
| Yes | * 7.83 (3.29–18.7) (<0.001) | * 9.91 (3.04–32.3) (<0.001) | * 12.1 (3.72–39.7) (<0.001) | |
| Variables | Univariate OR (95% CI) | Multivariate aOR (95%CI) | ||||
|---|---|---|---|---|---|---|
| Office and Household | Transport, Storage and Repair of Motor Vehicles | Mining, Construction and Manufacturing | Agriculture, Forestry and Fishing | |||
| Office and Household | No | - | - | - | ||
| Yes | 0.75 (0.42–1.33) (0.322) | 0.69 (0.30–1.62) (0.395) | - | - | - | |
| Transport, Storage and repair of motor vehicles | No | - | - | - | ||
| Yes | * 3.05 (1.38–6.72) (0.006) | - | 1.63 (0.54–4.94) (0.387) | - | - | |
| Mining, Construction and Manufacturing | No | - | - | - | ||
| Yes | * 2.15 (1.22–3.80) (0.008) | - | - | * 2.64 (1.16–6.01) (0.020) | - | |
| Agriculture, forestry and fishing | No | - | - | |||
| Yes | * 2.90 (1.10–7.69) (0.032) | - | - | 3.69 (0.95–14.3) (0.059) | ||
| Alcohol consumption | No | |||||
| Yes | ** 2.82 (1.59–5.01) (<0.001) | * 2.85 (1.23–6.60) (0.015) | * 2.72 (1.18–6.29) (0.019) | * 2.81 (1.19–6.60) (0.018) | * 2.76 (1.19–6.43) | |
| Sibling with cancer | No | |||||
| Yes | * 8.94 (2.41–33.1) (0.001) | 3.55 (0.51–24.8) (0.202) | 3.30 (0.44–24.9) (0.246) | 4.25 (0.61–29.6) (0.144) | 4.23 (0.59–30.1) (0.150) | |
| a History of Lung disease | No | |||||
| Yes | * 7.83 (3.29–18.7) (<0.001) | * 10.6 (3.20–35.2) (<0.001) | * 9.63 (2.98–31.1) (<0.001) | * 9.73 (2.96–32.0) (<0.001) | * 9.98 (3.02–33.0) (<0.001) | |
| Tobacco smoking | No | |||||
| Yes | * 3.37 (1.90–6.00) (<0.001) | ** 2.83 (1.19–6.70) (0.018) | ** 2.86 (1.20–6.82) (0.017) | ** 2.90 (1.20–7.00) (0.018) | ** 2.86 (1.19–6.83) (0.018) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbeje, N.P.; Ginindza, T.; Jafta, N. Epidemiological Study of Risk Factors for Lung Cancer in KwaZulu-Natal, South Africa. Int. J. Environ. Res. Public Health 2022, 19, 6752. https://doi.org/10.3390/ijerph19116752
Mbeje NP, Ginindza T, Jafta N. Epidemiological Study of Risk Factors for Lung Cancer in KwaZulu-Natal, South Africa. International Journal of Environmental Research and Public Health. 2022; 19(11):6752. https://doi.org/10.3390/ijerph19116752
Chicago/Turabian StyleMbeje, Noluthando P., Themba Ginindza, and Nkosana Jafta. 2022. "Epidemiological Study of Risk Factors for Lung Cancer in KwaZulu-Natal, South Africa" International Journal of Environmental Research and Public Health 19, no. 11: 6752. https://doi.org/10.3390/ijerph19116752
APA StyleMbeje, N. P., Ginindza, T., & Jafta, N. (2022). Epidemiological Study of Risk Factors for Lung Cancer in KwaZulu-Natal, South Africa. International Journal of Environmental Research and Public Health, 19(11), 6752. https://doi.org/10.3390/ijerph19116752







