Evaluation of the Microbiological Effectiveness of Three Accessible Mask Decontamination Methods and Their Impact on Filtration, Air Permeability and Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Respiratory Protective Devices (RPD)
2.2. Decontamination Treatments
2.2.1. Contact with Nebulized Hydrogen Peroxide (H2O2)
2.2.2. Immersion in Commercial Bleach (NaClO)
2.2.3. Contact with Steam in Microwave Steam-Sanitizing Bags (Steam Bags)
2.3. Microbiological Assays
2.3.1. Endospores Production
2.3.2. Mask Contamination Protocol
2.3.3. Microbiological Analysis
2.4. Filtration Efficiency and Air Permeability of RPD
2.4.1. Filtration Efficiency
2.4.2. Air Permeability
2.5. Physicochemical Characterization of RPD
2.5.1. Fourier Transform Infrared Spectroscopy Coupled with Attenuated Total Reflection (FTIR-ATR) Analysis
2.5.2. Contact angle Measurements
2.5.3. Water Vapor Transmission Rates
2.5.4. Thermogravimetric Analysis (TGA)
2.5.5. Modulated Differential Scanning Calorimetry (MDSC)
2.5.6. Mercury Intrusion Porosimetry (MIP)
2.5.7. Optical and Electronic Microscopy
2.6. Data Analysis and Statistics
3. Results and Discussion
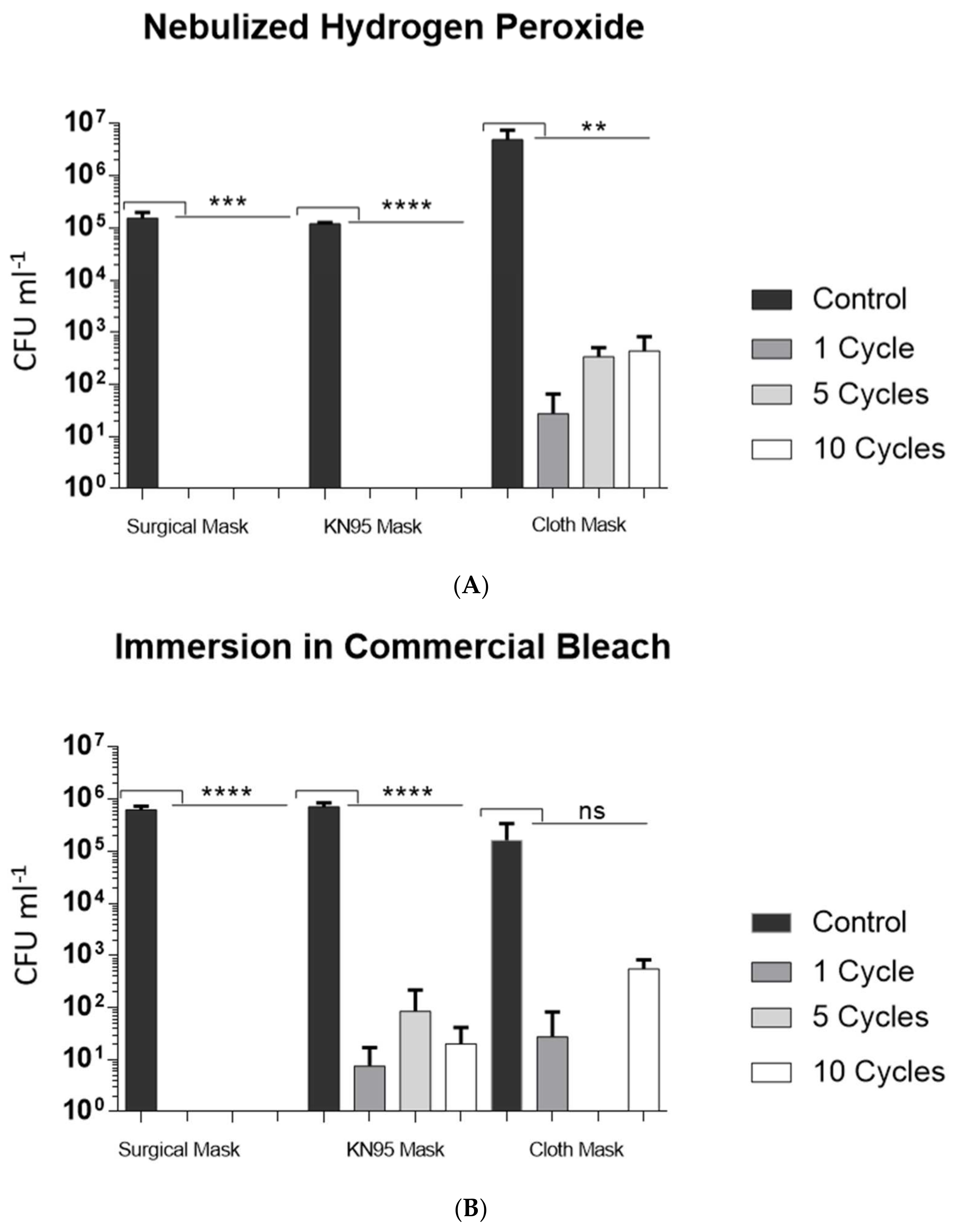
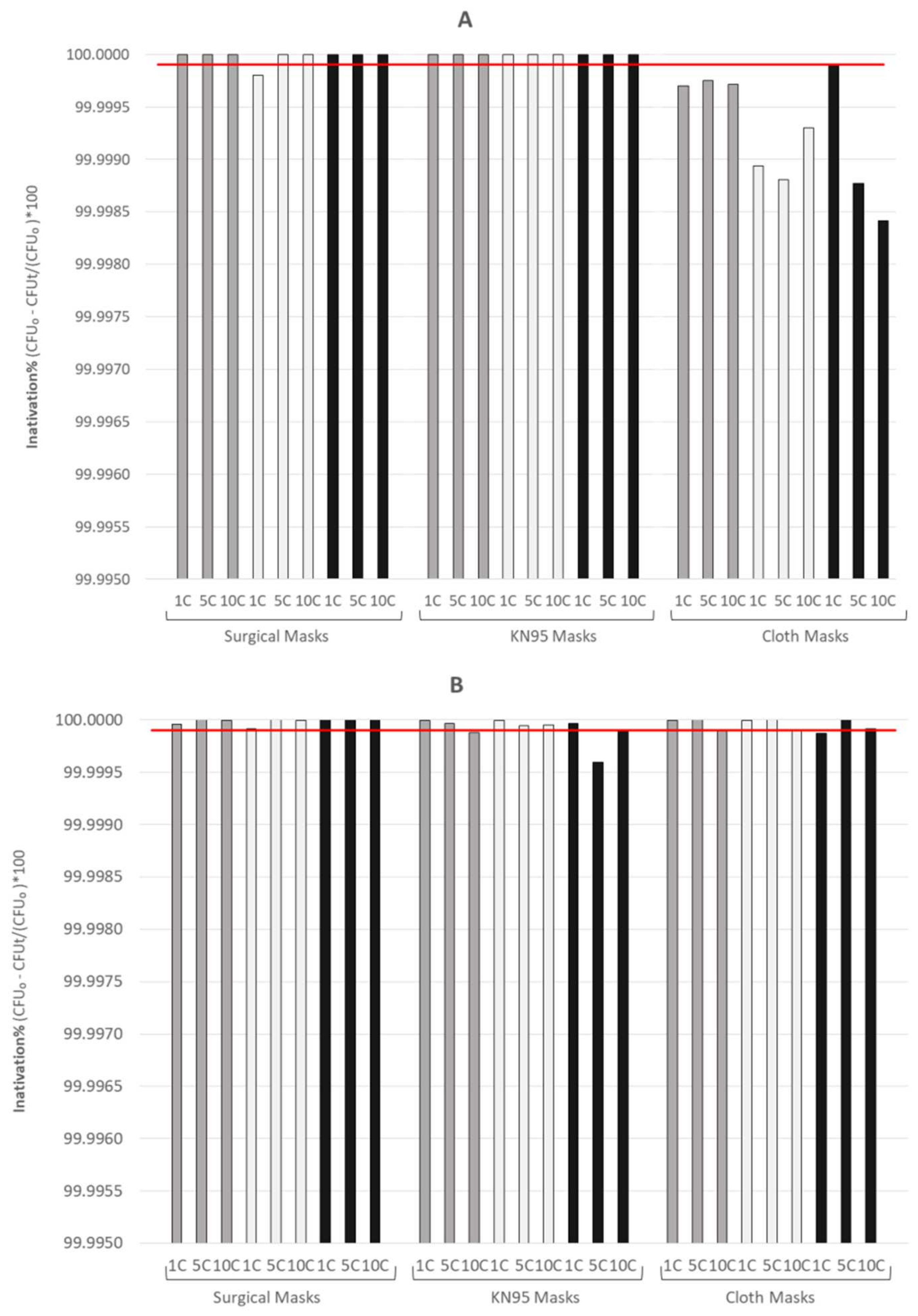
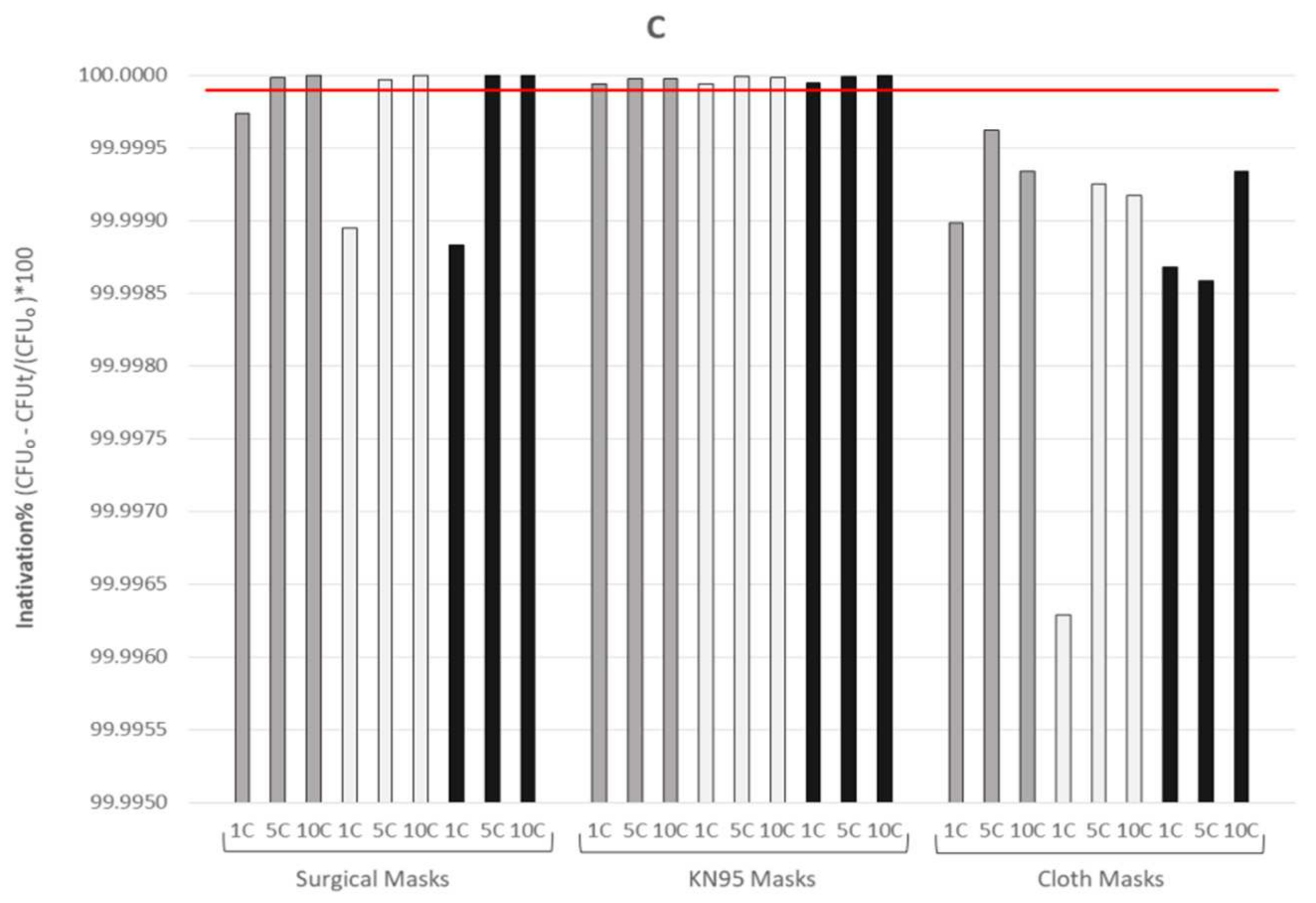
3.1. Assessment of the Microbiological Effectiveness
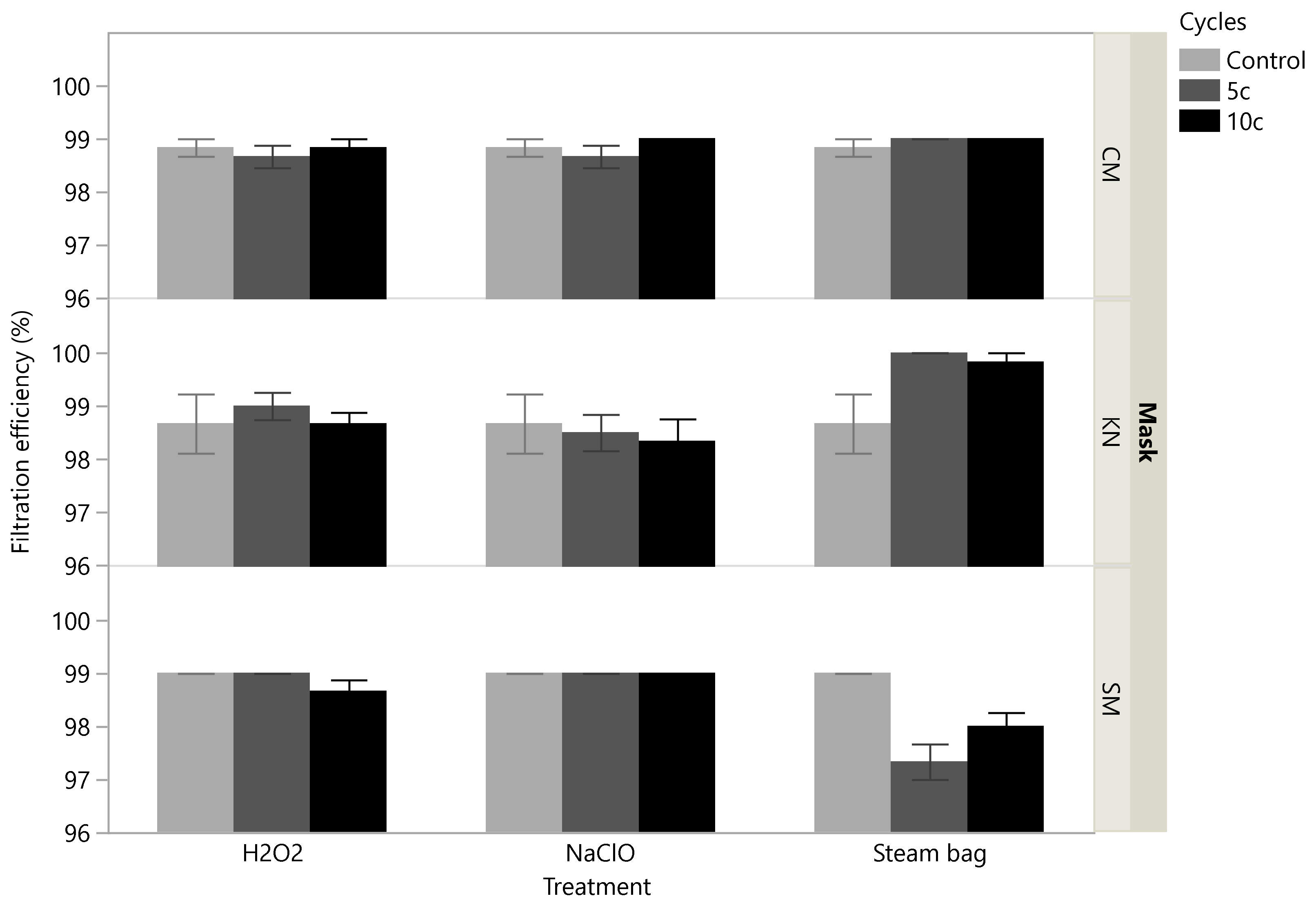
3.2. Effects of Treatments on Filtration Efficiency
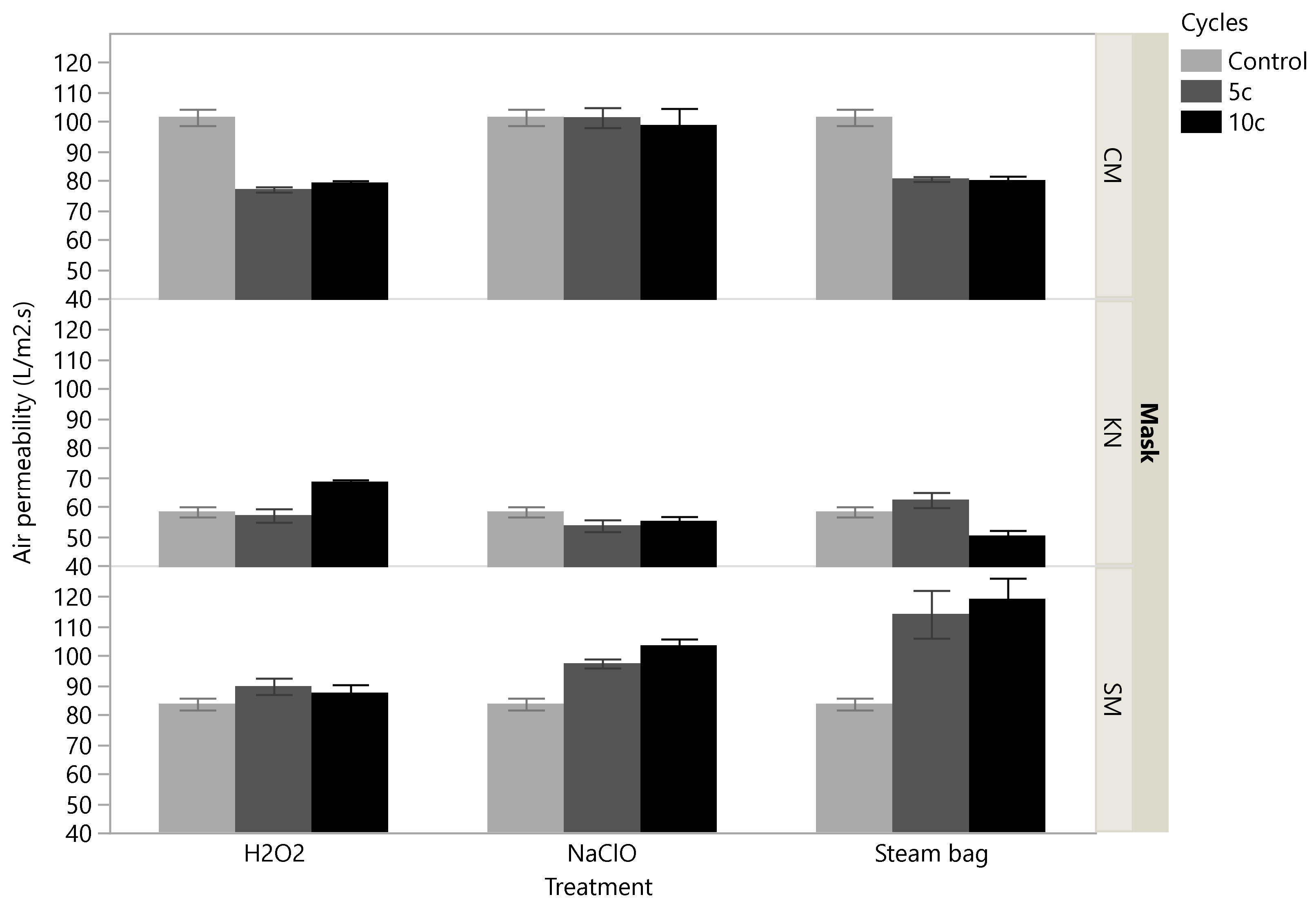
3.3. Air Permeability Tests
3.4. Effects of Treatments on the RPD’s Physicochemical Properties and Structure
3.4.1. FTIR-ATR Analysis
3.4.2. Contact Angle Measurements
3.4.3. Water Vapour Transmission Rate (WVTR)
3.4.4. Thermogravimetric Analysis (TGA)
3.4.5. Modulated Differential Scanning Calorimetry (MDSC)
3.4.6. Mercury Intrusion Porosimetry (MIP)
3.4.7. Optical and Electronic Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.; Sambol, A.R.; Shaffer, R.E. Evaluation of Multiple (3-Cycle) Decontamination Processing for Filtering Facepiece Respirators. J. Eng. Fibers Fabr. 2010, 5, 155892501000500405. [Google Scholar] [CrossRef]
- Viscusi, D.J.; Bergman, M.S.; Novak, D.A.; Faulkner, K.A.; Palmiero, A.; Powell, J.; Shaffer, R.E. Impact of Three Biological Decontamination Methods on Filtering Facepiece Respirator Fit, Odor, Comfort, and Donning Ease. J. Occup. Environ. Hyg. 2011, 8, 426–436. [Google Scholar] [CrossRef]
- Heimbuch, B.K.; Wallace, W.H.; Kinney, K.; Lumley, A.E.; Wu, C.-Y.; Woo, M.-H.; Wander, J. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control 2011, 39, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Rengasamy, S.; Viscusi, D.; Vo, E.; Shaffer, R. Development of a Test System To Apply Virus-Containing Particles to Filtering Facepiece Respirators for the Evaluation of Decontamination Procedures. Appl. Environ. Microbiol. 2009, 75, 1500–1507. [Google Scholar] [CrossRef][Green Version]
- Fisher, E.M.; Williams, J.L.; Shaffer, R.E. Evaluation of Microwave Steam Bags for the Decontamination of Filtering Facepiece Respirators. PLoS ONE 2011, 6, e18585. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef]
- Rubio-Romero, J.C.; del Carmen Pardo-Ferreira, M.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef]
- Zorko, D.; Gertsman, S.; O’Hearn, K.; Timmerman, N.; Ambu-Ali, N.; Dinh, T.; Sampson, M.; Sikora, L.; McNally, J.; Choong, K. Decontamination interventions for the reuse of surgical mask personal protective equipment: A systematic review. J. Hosp. Infect. 2020, 106, 283–294. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Wielick, C.; Ludwig-Begall, L.F.; Dams, L.; Razafimahefa, R.M.; Demeuldre, P.F.; Napp, A.; Laperre, J.; Jolois, O.; Farnir, F.; Haubruge, E.; et al. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J. Hosp. Infect. 2020, 106, 577–584. [Google Scholar] [CrossRef]
- Zulauf, K.E.; Green, A.B.; Ba, A.N.N.; Jagdish, T.; Reif, D.; Seeley, R.; Dale, A.; Kirby, J.E. Microwave-Generated Steam Decontamination of N95 Respirators Utilizing Universally Accessible Materials. mBio 2020, 11, e00997-20. [Google Scholar] [CrossRef] [PubMed]
- Kotzekidou, P. BACILLUS|Geobacillus stearothermophilus (Formerly Bacillus stearothermophilus). In Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 129–134. [Google Scholar] [CrossRef]
- Delbrück, A.I.; Zhang, Y.; Heydenreich, R.; Mathys, A. Bacillus spore germination at moderate high pressure: A review on underlying mechanisms, influencing factors, and its comparison with nutrient germination. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4159–4181. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.; Davies, D.J.G.; Woodson, L.P.; Soper, C.J. Biological indicators for steam sterilization: Characterization of a rapid biological indicator utilizing Bacillus stearothermophilusspore-associated alpha-glucosidase enzyme. J. Appl. Microbiol. 1998, 85, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Marceneiro, S.; Lobo, I.; Dias, I.; de Pinho, E.; Dias, A.M.A.; de Sousa, H.C. Eco-friendlier and sustainable natural-based additives for poly(vinyl chloride)-based composites. J. Ind. Eng. Chem. 2022, 110, 248–261. [Google Scholar] [CrossRef]
- Kanaan, A.F.; Piedade, A.P.; de Sousa, H.C.; Dias, A.M.A. Effect of mold assemblies-induced interfaces in the mechanical actuation of electro-responsive ionic liquid-based polycationic hydrogels. Appl. Mater. Today 2020, 20, 100711. [Google Scholar] [CrossRef]
- Su-Velez, B.M.; Maxim, T.; Long, J.L.; St John, M.A.; Holliday, M.A. Decontamination Methods for Reuse of Filtering Facepiece Respirators. JAMA Otolaryngol. Neck Surg. 2020, 146, 734. [Google Scholar] [CrossRef]
- Tsai, P. Performance of Masks and Discussion of the Inactivation of SARS-CoV-2. Eng. Sci. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Bessesen, M.T.; Adams, J.C.; Radonovich, L.; Anderson, J. Disinfection of reusable elastomeric respirators by health care workers: A feasibility study and development of standard operating procedures. Am. J. Infect. Control 2015, 43, 629–634. [Google Scholar] [CrossRef]
- Pascoe, M.J.; Robertson, A.; Crayford, A.; Durand, E.; Steer, J.; Castelli, A.; Wesgate, R.; Evans, S.L.; Porch, A.; Maillard, J.Y. Dry heat and microwave-generated steam protocols for the rapid decontamination of respiratory personal protective equipment in response to COVID-19-related shortages. J. Hosp. Infect. 2020, 106, 10–19. [Google Scholar] [CrossRef]
- Eun, J.; Lee, H.; Jeon, S. Regeneration of an electret filter by contact electrification. RSC Adv. 2021, 11, 4610–4615. [Google Scholar] [CrossRef] [PubMed]
- Parlin, A.F.; Stratton, S.M.; Culley, T.M.; Guerra, P.A. A laboratory-based study examining the properties of silk fabric to evaluate its potential as a protective barrier for personal protective equipment and as a functional material for face coverings during the COVID-19 pandemic. PLoS ONE 2020, 15, e0239531. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Abbasnezhad, N.; Seydani, M.Z.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration. Bioact. Mater. 2020, 6, 106–122. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Agrawal, A. Tailoring surface wettability to reduce chances of infection of COVID-19 by a respiratory droplet and to improve the effectiveness of personal protection equipment. Phys. Fluids 2020, 32, 081702. [Google Scholar] [CrossRef]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K.I.; Cha, H.J.; Kim, I.S. Reusability Comparison of Melt-Blown vs Nanofiber Face Mask Filters for Use in the Coronavirus Pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- Wibisono, Y.; Fadila, C.; Saiful, S.; Bilad, M. Facile Approaches of Polymeric Face Masks Reuse and Reinforcements for Micro-Aerosol Droplets and Viruses Filtration: A Review. Polymers 2020, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- De-La-Torre, G.E.; Rakib, R.J.; Pizarro-Ortega, C.I.; Dioses-Salinas, D.C. Occurrence of personal protective equipment (PPE) associated with the COVID-19 pandemic along the coast of Lima, Peru. Sci. Total Environ. 2021, 774, 145774. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Antenucci, S.; Ortenzi, M.A.; Parolini, M. Laundering of face masks represents an additional source of synthetic and natural microfibers to aquatic ecosystems. Sci. Total Environ. 2021, 806, 150495. [Google Scholar] [CrossRef]
- Charvet, A.; Bardin-Monnier, N.; Thomas, D.; Dufaud, O.; Pfrimmer, M.; Barrault, M.; Bourrous, S.; Mocho, V.; Ouf, F.-X.; Poirier, S.; et al. Impact of washing cycles on the performances of face masks. J. Aerosol Sci. 2021, 160, 105914. [Google Scholar] [CrossRef]
- Ju, J.T.; Boisvert, L.N.; Zuo, Y.Y. Face masks against COVID-19: Standards, efficacy, testing and decontamination methods. Adv. Colloid Interface Sci. 2021, 292, 102435. [Google Scholar] [CrossRef]
- Lee, K.-P.; Yip, J.; Kan, C.-W.; Chiou, J.-C.; Yung, K.-F. Reusable Face Masks as Alternative for Disposable Medical Masks: Factors that Affect their Wear-Comfort. Int. J. Environ. Res. Public Health 2020, 17, 6623. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M. Comparison of Facemask Characteristics with User Assessment of Comfort. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2019. [Google Scholar]
- Yang, A.; Cai, L.; Zhang, R.; Wang, J.; Hsu, P.-C.; Wang, H.; Zhou, G.; Xu, J.; Cui, Y. Thermal Management in Nanofiber-Based Face Mask. Nano Lett. 2017, 17, 3506–3510. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A.; Mekonnen, B.A. Current plastics pollution threats due to COVID-19 and its possible mitigation techniques: A waste-to-energy conversion via Pyrolysis. Environ. Syst. Res. 2021, 10, 8. [Google Scholar] [CrossRef]
- Arık, B.; Bozacı, E.; Demir, A.; Özdoğan, E. Thermogravimetric, microscopic and mechanical analyses of PBT and pet yarns. Text. Appar. 2013, 23, 101–106. [Google Scholar]
- Larsen, G.S.; Cheng, Y.; Daemen, L.L.; Lamichhane, T.N.; Hensley, D.K.; Hong, K.; Meyer, I.H.M.; Monaco, S.J.; Levine, A.M.; Lee, R.J.; et al. Polymer, Additives, and Processing Effects on N95 Filter Performance. ACS Appl. Polym. Mater. 2021, 3, 1022–1031. [Google Scholar] [CrossRef]
- Pereira, A.P.D.S.; Silva, M.H.P.D.; Lima, É.P.; Paula, A.D.S.; Tommasini, F.J. Processing and Characterization of PET Composites Reinforced With Geopolymer Concrete Waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef]
- Suzuki, S.; Nakamura, Y.; Hasan, A.K.; Liu, B.; Terano, M.; Nakatani, H. Dependence of tacticity distribution in thermal oxidative degradation of polypropylene. Polym. Bull. 2005, 54, 311–319. [Google Scholar] [CrossRef]
- Üreyen, M.E.; Kaynak, E. Effect of Zinc Borate on Flammability of PET Woven Fabrics. Adv. Polym. Technol. 2019, 2019, 7150736. [Google Scholar] [CrossRef]
- Battegazzore, D.; Cravero, F.; Frache, A. Is it Possible to Mechanical Recycle the Materials of the Disposable Filtering Masks? Polymers 2020, 12, 2726. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Moradi, T.; Esfahani, M.S.; Ebrahimi, H.; Khosroshahi, A.; Safapour, S. Study on bonding ability of melt-spun polypropylene/polyethylene blend fibers. J. Ind. Text. 2020, 1528083720955210. [Google Scholar] [CrossRef]
- Nayak, R.; Kyratzis, I.L.; Truong, Y.B.; Padhye, R.; Arnold, L.; Peeters, G.; O’Shea, M.; Nichols, L. Fabrication and characterisation of polypropylene nanofibres by meltblowing process using different fluids. J. Mater. Sci. 2012, 48, 273–281. [Google Scholar] [CrossRef]
- Rodrigues, A.; Figueiredo, L.; Diogo, H.; Bordado, J. Mechanical behavior of PET fibers and textiles for Stent-Grafts using video extensometry and image analysis. Sci. Technol. Mater. 2018, 30, 23–33. [Google Scholar] [CrossRef]
- Wang, D.; Luo, F.; Shen, Z.; Wu, X.; Qi, Y. A study on the crystallization behavior and mechanical properties of poly(ethylene terephthalate) induced by chemical degradation nucleation. RSC Adv. 2017, 7, 37139–37147. [Google Scholar] [CrossRef]
- Wang, D.; Yang, B.; Chen, Q.-T.; Chen, J.; Su, L.-F.; Chen, P.; Zheng, Z.-Z.; Miao, J.-B.; Qian, J.-S.; Xia, R.; et al. A facile evaluation on melt crystallization kinetics and thermal properties of low-density polyethylene (LDPE)/Recycled polyethylene terephthalate (RPET) blends. Adv. Ind. Eng. Polym. Res. 2019, 2, 126–135. [Google Scholar] [CrossRef]
- Crilley, L.R.; Angelucci, A.A.; Malile, B.; Young, C.J.; VandenBoer, T.C.; Chen, J.I.L. Non-woven materials for cloth-based face masks inserts: Relationship between material properties and sub-micron aerosol filtration. Environ. Sci. Nano 2021, 8, 1603–1613. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; He, B.; Ke, Q.-F. The Effect of Temperature and Humidity on the Filtration Performance of Electret Melt-Blown Nonwovens. Materials 2020, 13, 4774. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhang, X.; Huang, C.; Jin, X. Design of electret polypropylene melt blown air filtration material containing nucleating agent for effective PM2.5 capture. RSC Adv. 2018, 8, 7932–7941. [Google Scholar] [CrossRef]
- da Silva, R.C.L.; Alves, C.; Nascimento, J.H.; Neves, J.R.O.; Teixeira, V. Surface Modification of Polyester Fabric by Non-Thermal Plasma Treatment. J. Phys. Conf. Ser. 2012, 406, 012017. [Google Scholar] [CrossRef]
- Elnagar, K.; Abou Elmaaty, T.; Raouf, S. Dyeing of Polyester and Polyamide Synthetic Fabrics with Natural Dyes Using Ecofriendly Technique. J. Text. 2014, 2014, 363079. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]


| Mask/Treatment. | H2O2 | NaClO | Steam Bag |
|---|---|---|---|
| CM | 0.7382 | 0.3220 | 0.3679 |
| KN | 0.5968 | 0.5750 | 0.0067 * |
| SM | 0.1194 | 1.000 | 0.0024 * |
| Mask/Treatment | H2O2 | NaClO | Steam Bag |
|---|---|---|---|
| CM | 0.0014 * | 0.5792 | 0.0033 * |
| KN | 0.0030 * | 0.1447 | 0.0041 * |
| SM | 0.2501 | 0.0014 * | 0.0096 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordelo, R.; Botelho, J.R.S.; Morais, P.V.; de Sousa, H.C.; Branco, R.; Dias, A.M.A.; Reis, M.S. Evaluation of the Microbiological Effectiveness of Three Accessible Mask Decontamination Methods and Their Impact on Filtration, Air Permeability and Physicochemical Properties. Int. J. Environ. Res. Public Health 2022, 19, 6567. https://doi.org/10.3390/ijerph19116567
Lordelo R, Botelho JRS, Morais PV, de Sousa HC, Branco R, Dias AMA, Reis MS. Evaluation of the Microbiological Effectiveness of Three Accessible Mask Decontamination Methods and Their Impact on Filtration, Air Permeability and Physicochemical Properties. International Journal of Environmental Research and Public Health. 2022; 19(11):6567. https://doi.org/10.3390/ijerph19116567
Chicago/Turabian StyleLordelo, Roberta, José Rafael S. Botelho, Paula V. Morais, Hermínio C. de Sousa, Rita Branco, Ana M. A. Dias, and Marco S. Reis. 2022. "Evaluation of the Microbiological Effectiveness of Three Accessible Mask Decontamination Methods and Their Impact on Filtration, Air Permeability and Physicochemical Properties" International Journal of Environmental Research and Public Health 19, no. 11: 6567. https://doi.org/10.3390/ijerph19116567
APA StyleLordelo, R., Botelho, J. R. S., Morais, P. V., de Sousa, H. C., Branco, R., Dias, A. M. A., & Reis, M. S. (2022). Evaluation of the Microbiological Effectiveness of Three Accessible Mask Decontamination Methods and Their Impact on Filtration, Air Permeability and Physicochemical Properties. International Journal of Environmental Research and Public Health, 19(11), 6567. https://doi.org/10.3390/ijerph19116567








