Factors Associated with Treatment Outcomes and Pathological Features in Patients with Osteoradionecrosis: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definition and Classification of ORN
2.3. Data Collection
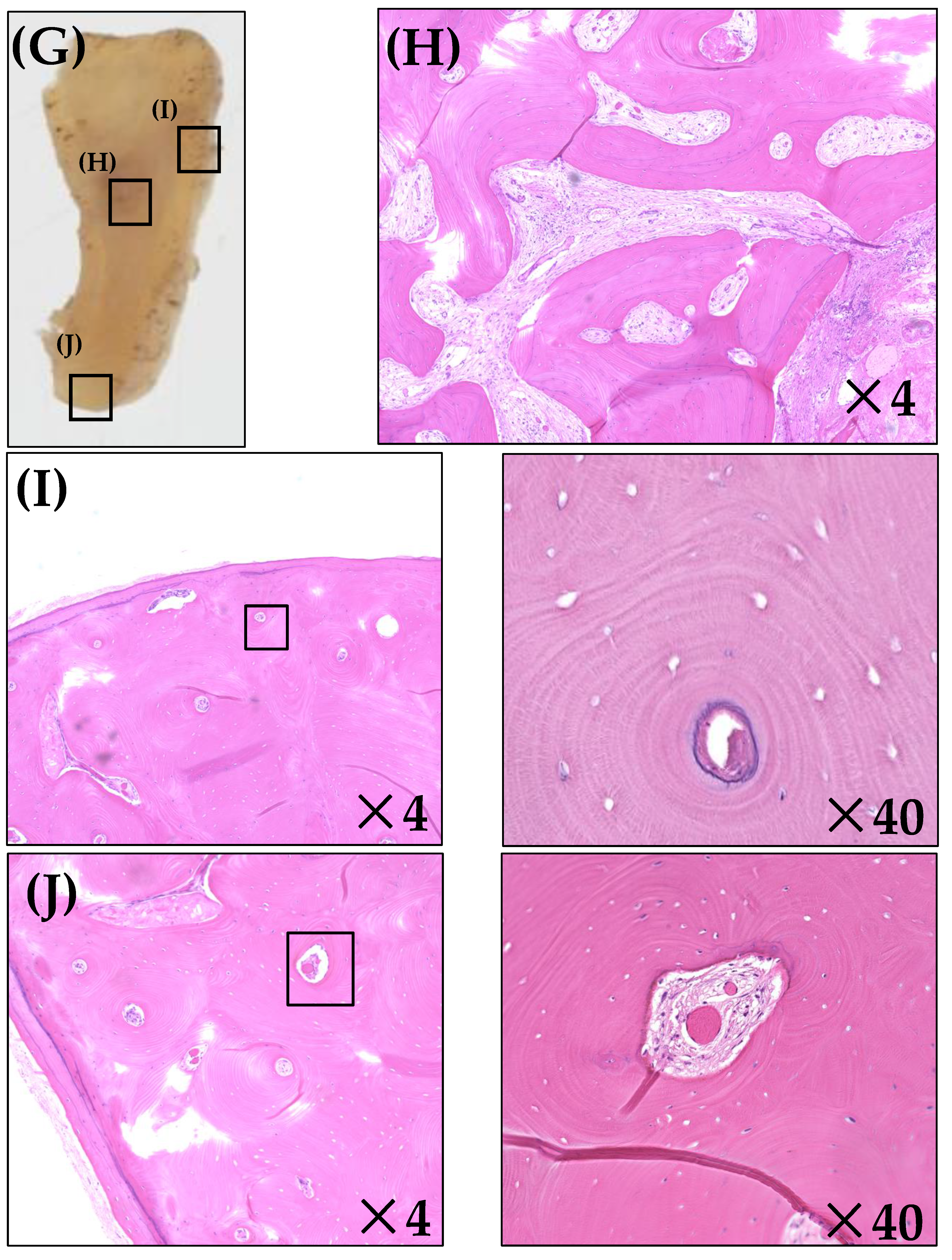
2.4. Histopathological Analysis
2.5. Statistical Analysis
3. Results
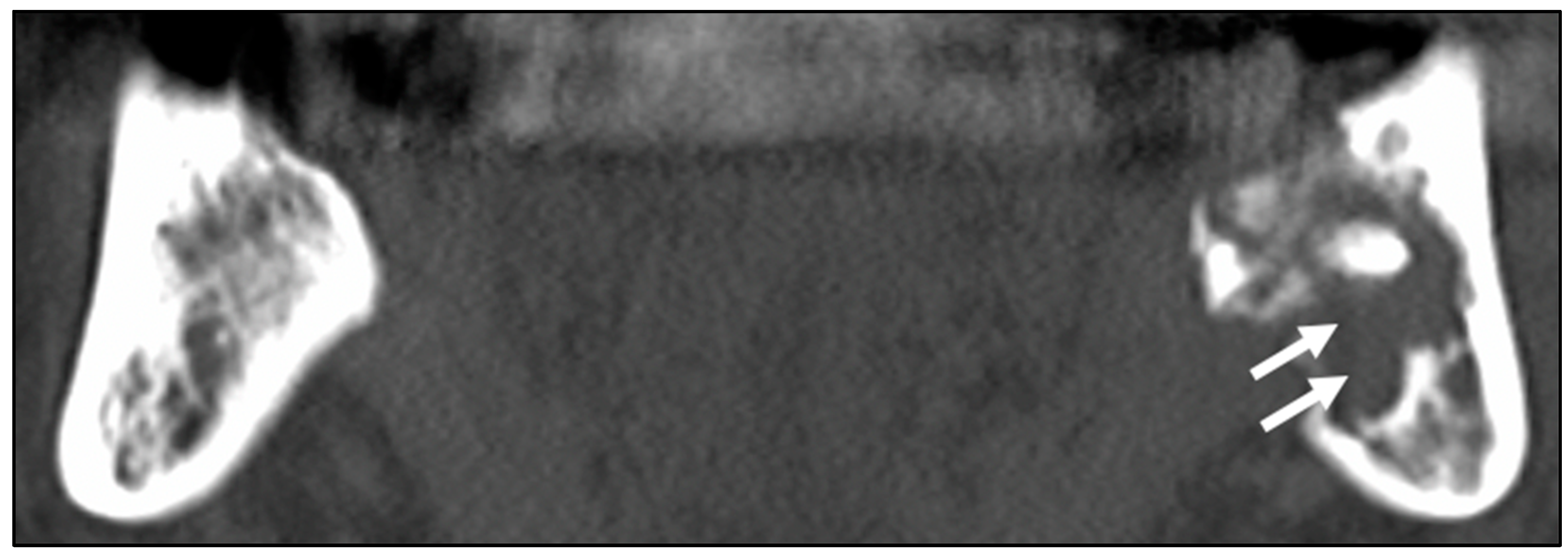
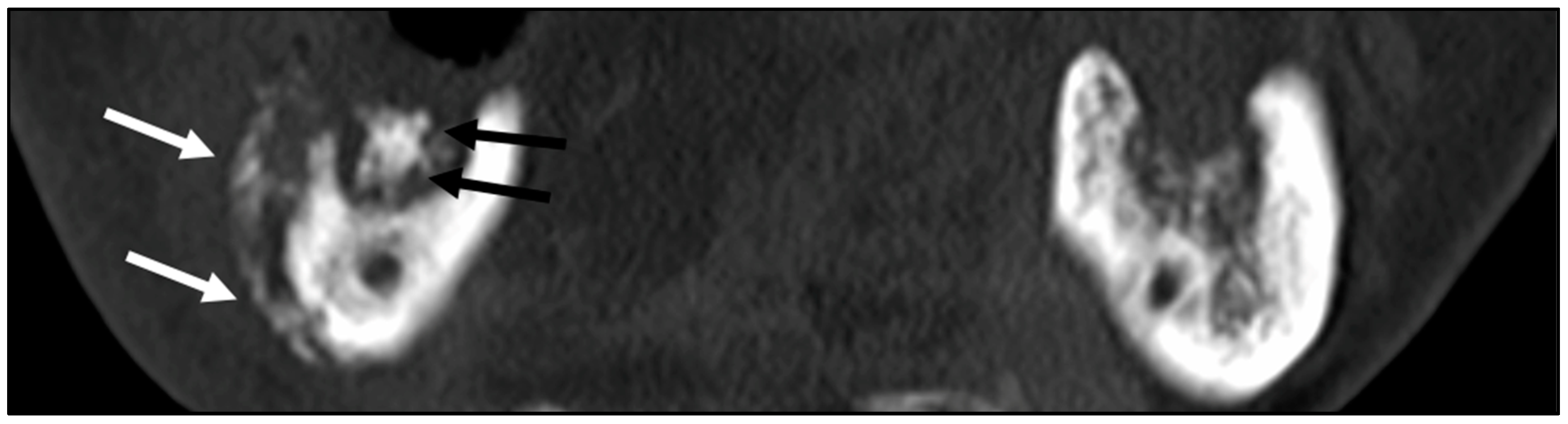
3.1. Patients’ Clinical Characteristics and CT Image Findings
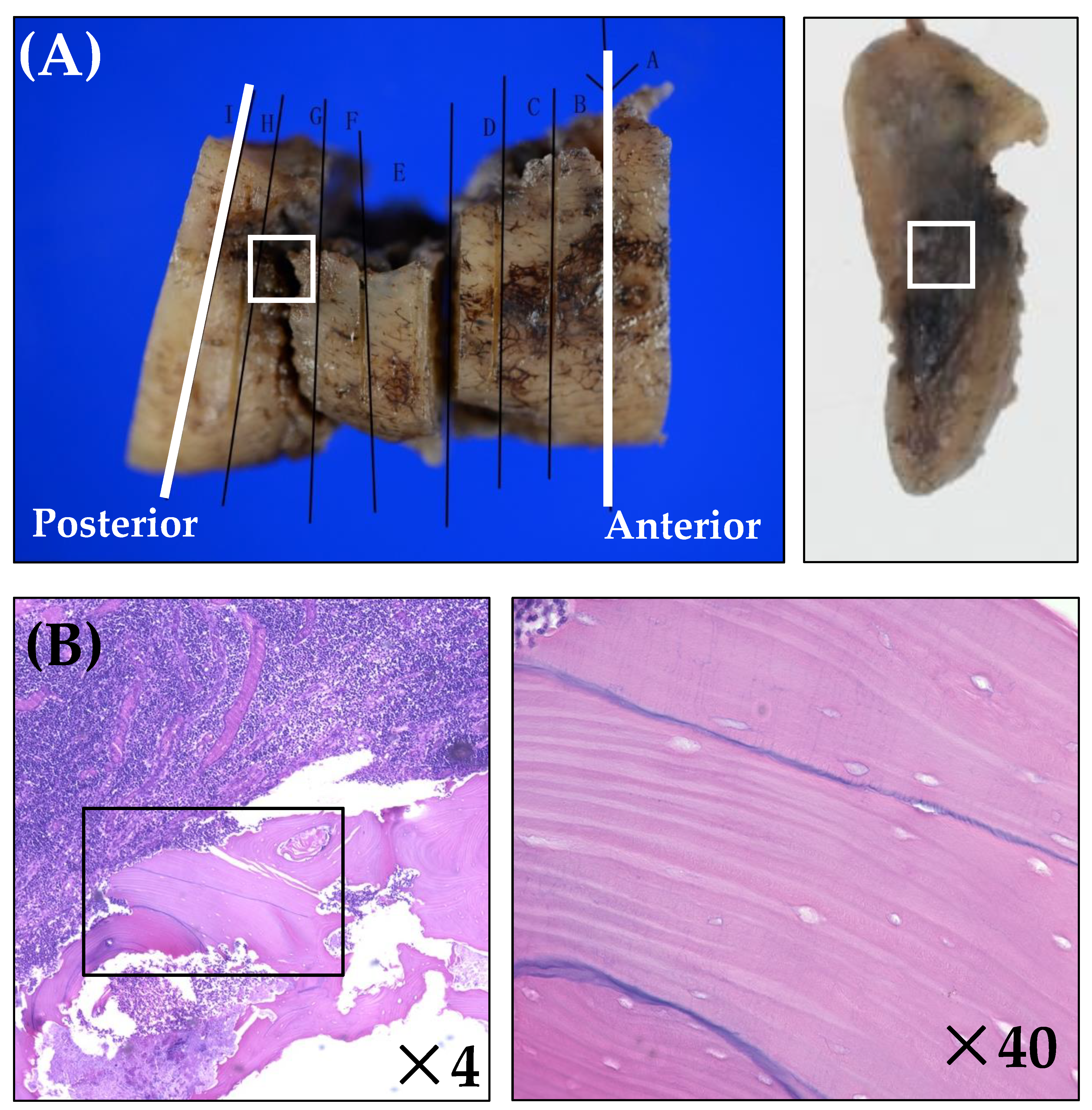
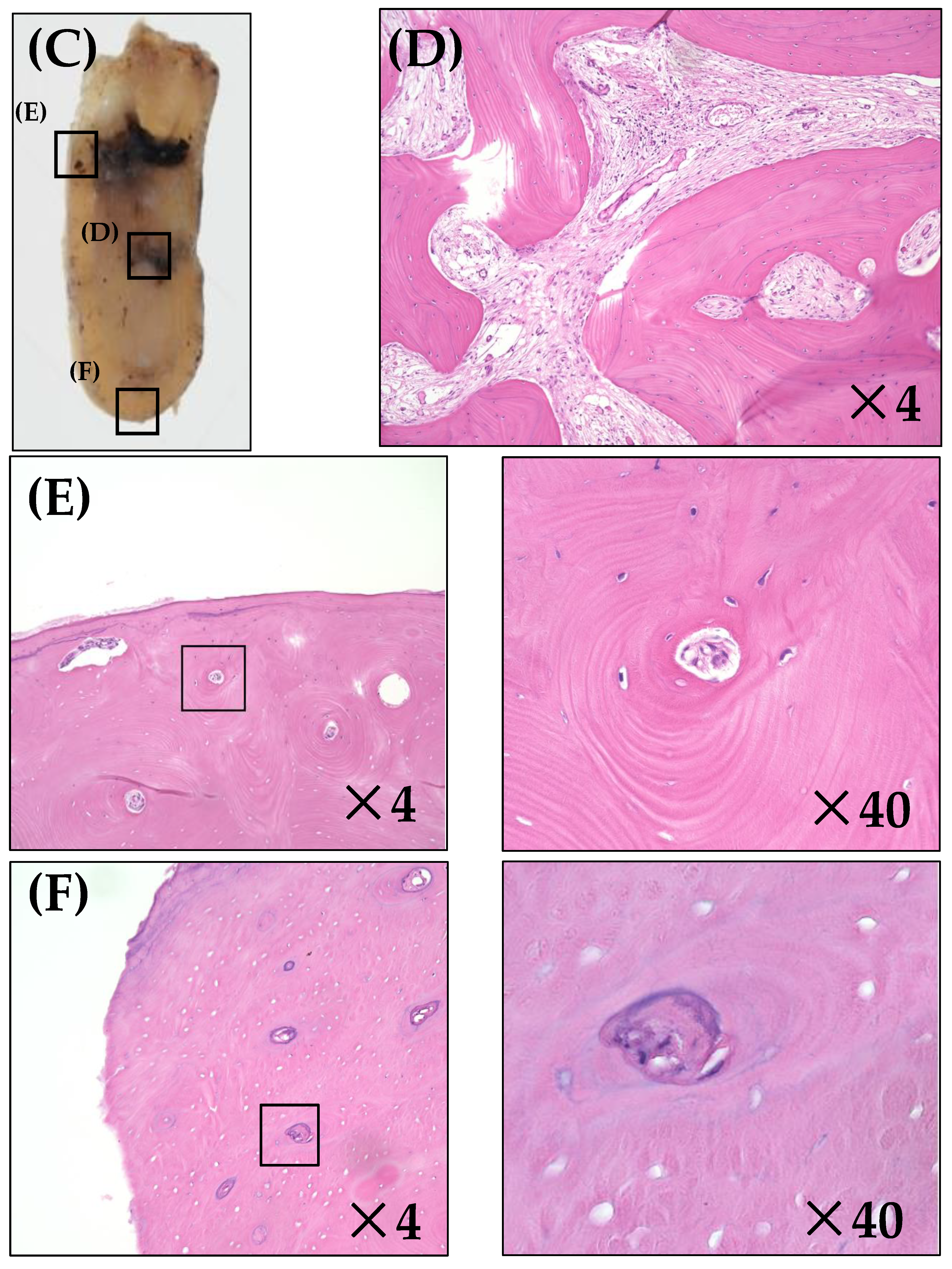
3.2. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borras, J.M.; Barton, M.; Grau, C.; Corral, J.; Verhoeven, R.; Lemmens, V.; van Eycken, L.; Henau, K.; Primic-Zakelj, M.; Strojan, P.; et al. The impact of cancer incidence and stage on optimal utilization of radiotherapy: Methodology of a population based analysis by the ESTRO-HERO project. Radiother. Oncol. 2015, 116, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Reuther, T.; Schuster, T.; Mende, U.; Kübler, A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—A report of a thirty year retrospective review. Int. J. Oral Maxillofac. Surg. 2003, 32, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Wong, F.L.; Stevenson-Moore, P. Osteoradionecrosis: Clinical experience and a proposal for classification. J. Oral Maxillofac. Surg. 1987, 45, 104–110. [Google Scholar] [CrossRef]
- Marx, R.E. Osteoradionecrosis: A new concept of its pathophysiology. J. Oral Maxillofac. Surg. 1983, 41, 283–288. [Google Scholar] [CrossRef]
- Jacobson, A.S.; Buchbinder, D.; Hu, K.; Urken, M.L. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010, 46, 795–801. [Google Scholar] [CrossRef]
- Støre, G.; Boysen, M. Mandibular osteoradionecrosis: Clinical behaviour and diagnostic aspects. Clin. Otolaryngol. Allied Sci. 2000, 25, 378–384. [Google Scholar] [CrossRef]
- Lyons, A.; Osher, J.; Warner, E.; Kumar, R.; Brennan, P.A. Osteoradionecrosis—A review of current concepts in defining the extent of the disease and a new classification proposal. Br. J. Oral Maxillofac. Surg. 2014, 52, 392–395. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Brown, J.S.; Rogers, S.N.; Bekiroglu, F.; Schache, A.; Shaw, R.J. Outcomes of microvascular composite reconstruction for mandibular osteoradionecrosis. Br. J. Oral Maxillofac. Surg. 2021, 59, 1031–1035. [Google Scholar] [CrossRef]
- Forner, L.E.; Dieleman, F.J.; Shaw, R.J.; Kanatas, A.; Butterworth, C.J.; Kjeller, G.; Alsner, J.; Overgaard, J.; Hillerup, S.; Hyldegaard, O.; et al. Hyperbaric oxygen treatment of mandibular osteoradionecrosis: Combined data from the two randomized clinical trials DAHANCA-21 and NWHHT2009-1. Radiother. Oncol. 2022, 166, 137–144. [Google Scholar] [CrossRef]
- Owosho, A.A.; Tsai, C.J.; Lee, R.S.; Freymiller, H.; Kadempour, A.; Varthis, S.; Sax, A.Z.; Rosen, E.B.; Yom, S.K.; Randazzo, J.; et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): The Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017, 64, 44–51. [Google Scholar] [CrossRef]
- Oh, H.K.; Chambers, M.S.; Martin, J.W.; Lim, H.J.; Park, H.J. Osteoradionecrosis of the mandible: Treatment outcomes and factors influencing the progress of osteoradionecrosis. J. Oral Maxillofac. Surg. 2009, 67, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Alam, D.S.; Nuara, M.; Christian, J. Analysis of outcomes of vascularized flap reconstruction in patients with advanced mandibular osteoradionecrosis. Otolaryngol. Head Neck Surg. 2009, 141, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Johnson, R.P. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 379–390. [Google Scholar] [CrossRef]
- Alhilali, L.; Reynolds, A.R.; Fakhran, S. Osteoradionecrosis after radiation therapy for head and neck cancer: Differentiation from recurrent disease with CT and PET/CT imaging. AJNR Am. J. Neuroradiol. 2014, 35, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.; Thakur, M.H.; Dholam, K.; Mahajan, A.; Arya, S.; Juvekar, S. Osteoradionecrosis of the mandible: Through a radiologist’s eyes. Clin. Radiol. 2015, 70, 197–205. [Google Scholar] [CrossRef]
- Chong, J.; Hinckley, L.K.; Ginsberg, L.E. Masticator space abnormalities associated with mandibular osteoradionecrosis: MR and CT findings in five patients. AJNR Am. J. Neuroradiol. 2000, 21, 175–178. [Google Scholar]
- Baumann, D.P.; Yu, P.; Hanasono, M.M.; Skoracki, R.J. Free flap reconstruction of osteoradionecrosis of the mandible: A 10-year review and defect classification. Head Neck 2011, 33, 800–807. [Google Scholar] [CrossRef]
- Annane, D.; Depondt, J.; Aubert, P.; Villart, M.; Géhanno, P.; Gajdos, P.; Chevret, S. Hyperbaric oxygen therapy for radionecrosis of the jaw: A randomized, placebo-controlled, double-blind trial from the ORN96 study group. J. Clin. Oncol. 2004, 22, 4893–4900. [Google Scholar] [CrossRef]
- Shaha, A.R.; Cordeiro, P.G.; Hidalgo, D.A.; Spiro, R.H.; Strong, E.W.; Zlotolow, I.; Huryn, J.; Shah, J.P. Resection and immediate microvascular reconstruction in the management of osteoradionecrosis of the mandible. Head Neck 1997, 19, 406–411. [Google Scholar] [CrossRef]
- Vahidi, N.; Lee, T.S.; Daggumati, S.; Shokri, T.; Wang, W.; Ducic, Y. Osteoradionecrosis of the Midface and Mandible: Pathogenesis and Management. Semin. Plast. Surg. 2020, 34, 232–244. [Google Scholar] [CrossRef]
- Matsuo, M.; Rikimaru, F.; Higaki, Y.; Tomita, K. Clinical analysis of mandibular osteoradionecrosis. Nihon Jibiinkoka Gakkai Kaiho 2010, 113, 907–913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Notani, K.; Yamazaki, Y.; Kitada, H.; Sakakibara, N.; Fukuda, H.; Omori, K.; Nakamura, M. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck 2003, 25, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zaghi, S.; Miller, M.; Blackwell, K.; Palla, B.; Lai, C.; Nabili, V. Analysis of surgical margins in cases of mandibular osteoradionecrosis that progress despite extensive mandible resection and free tissue transfer. Am. J. Otolaryngol. 2012, 33, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Hashikawa, K.; Wanifuchi, S.; Kusumoto, J.; Shigeoka, M.; Furudoi, S.; Terashi, H.; Komori, T. Heterogeneity of Necrotic Changes between Cortical and Cancellous Bone in Mandibular Osteoradionecrosis: A Histopathological Analysis of Resection Margin after Segmental Mandibulectomy. BioMed Res. Int. 2017, 2017, 3125842. [Google Scholar] [CrossRef]
- Mitsimponas, K.T.; Moebius, P.; Amann, K.; Stockmann, P.; Schlegel, K.A.; Neukam, F.W.; Wehrhan, F. Osteo-radio-necrosis (ORN) and bisphosphonate-related osteonecrosis of the jaws (BRONJ): The histopathological differences under the clinical similarities. Int. J. Clin. Exp Pathol. 2014, 7, 496–508. [Google Scholar]
- He, Y.; Liu, Z.; Tian, Z.; Dai, T.; Qiu, W.; Zhang, Z. Retrospective analysis of osteoradionecrosis of the mandible: Proposing a novel clinical classification and staging system. Int. J. Oral Maxillofac. Surg. 2015, 44, 1547–1557. [Google Scholar] [CrossRef]
- Santamaria, E.; Wei, F.C.; Chen, H.C. Fibula osteoseptocutaneous flap for reconstruction of osteoradionecrosis of the mandible. Plast Reconstr. Surg. 1998, 101, 921–929. [Google Scholar] [CrossRef]
- Lee, I.J.; Koom, W.S.; Lee, C.G.; Kim, Y.B.; Yoo, S.W.; Keum, K.C.; Kim, G.E.; Choi, E.C.; Cha, I.H. Risk factors and dose-effect relationship for mandibular osteoradionecrosis in oral and oropharyngeal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1084–1091. [Google Scholar] [CrossRef]
- Dieleman, F.J.; Phan, T.T.T.; van den Hoogen, F.J.A.; Kaanders, J.; Merkx, M.A.W. The efficacy of hyperbaric oxygen therapy related to the clinical stage of osteoradionecrosis of the mandible. Int. J. Oral Maxillofac. Surg. 2017, 46, 428–433. [Google Scholar] [CrossRef]
- Chang, D.T.; Sandow, P.R.; Morris, C.G.; Hollander, R.; Scarborough, L.; Amdur, R.J.; Mendenhall, W.M. Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck 2007, 29, 528–536. [Google Scholar] [CrossRef]
- Studer, G.; Studer, S.P.; Zwahlen, R.A.; Huguenin, P.; Grätz, K.W.; Lütolf, U.M.; Glanzmann, C. Osteoradionecrosis of the mandible: Minimized risk profile following intensity-modulated radiation therapy (IMRT). Strahlenther. Onkol. 2006, 182, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Estilo, C.L.; Wolden, S.L.; Zelefsky, M.J.; Kraus, D.H.; Wong, R.J.; Shaha, A.R.; Shah, J.P.; Mechalakos, J.G.; Lee, N.Y. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensity-modulated radiation therapy for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e207–e213. [Google Scholar] [CrossRef] [PubMed]
- Bender, I.B.; Seltzer, S. The oral fistula: Its diagnosis and treatment. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 1367–1376. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, W.S.; Kim, H.K.; Bae, T.H. Multiple foreign bodies causing an orocutaneous fistula of the cheek. Arch. Craniofac. Surg. 2018, 19, 139–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhanda, J.; Pasquier, D.; Newman, L.; Shaw, R. Current Concepts in Osteoradionecrosis after Head and Neck Radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2016, 28, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, C.; Narasimhan, K.; Gursel, T.; Jackson, O.; Schaffner, A. Closure of orocutanous fistula using a pedicled expanded deltopectoral flap. Can. J. Plast. Surg. 2008, 16, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Hermans, R.; Fossion, E.; Ioannides, C.; Van den Bogaert, W.; Ghekiere, J.; Baert, A.L. CT findings in osteoradionecrosis of the mandible. Skeletal. Radiol. 1996, 25, 31–36. [Google Scholar] [CrossRef]
- Miyamoto, I.; Tanaka, R.; Kogi, S.; Yamaya, G.; Kawai, T.; Ohashi, Y.; Takahashi, N.; Izumisawa, M.; Yamada, H. Clinical Diagnostic Imaging Study of Osteoradionecrosis of the Jaw: A Retrospective Study. J. Clin. Med. 2021, 10, 4704. [Google Scholar] [CrossRef]
- Obinata, K.; Shirai, S.; Ito, H.; Nakamura, M.; Carrozzo, M.; Macleod, I.; Carr, A.; Yamazaki, Y.; Tei, K. Image findings of bisphosphonate related osteonecrosis of jaws comparing with osteoradionecrosis. Dentomaxillofacial Radiol. 2017, 46, 20160281. [Google Scholar] [CrossRef]
- Macdougall, J.A.; Evans, A.M.; Lindsay, R.K. Osteoradionecrosis of the mandible And Its Treatment. Am. J. Surg. 1963, 106, 816–818. [Google Scholar] [CrossRef]
- Epstein, J.B.; Wong, F.L.; Dickens, A.; Szasz, I.; Lepawsky, M. Bone and gallium scans in postradiotherapy osteonecrosis of the jaw. Head Neck 1992, 14, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Curi, M.M.; Dib, L.L. Osteoradionecrosis of the jaws: A retrospective study of the background factors and treatment in 104 cases. J. Oral Maxillofac. Surg. 1997, 55, 540–544. [Google Scholar] [CrossRef]
- Delanian, S.; Lefaix, J.L. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004, 73, 119–131. [Google Scholar] [CrossRef] [PubMed]
- McGregor, A.D.; MacDonald, D.G. Post-irradiation changes in the blood vessels of the adult human mandible. Br. J. Oral Maxillofac. Surg. 1995, 33, 15–18. [Google Scholar] [CrossRef]
- Hamparian, A.M. Blood supply of the human fetal mandible. Am. J. Anat. 1973, 136, 67–75. [Google Scholar] [CrossRef]
- Dekker, H.; Bravenboer, N.; van Dijk, D.; Bloemena, E.; Rietveld, D.H.F.; Ten Bruggenkate, C.M.; Schulten, E. The irradiated human mandible: A quantitative study on bone vascularity. Oral Oncol. 2018, 87, 126–130. [Google Scholar] [CrossRef]
| Variables | Prognosis | p-Value | |
|---|---|---|---|
| Good n (%) | Poor n (%) | ||
| Sex | <0.550 * | ||
| Male | 27 (84.4) | 28 (80.9) | |
| Female | 5 (15.6) | 8 (19.1) | |
| Age | 0.431 ** | ||
| Range (years) | 54–84 | 21–90 | |
| Mean ± SD | 69.1 ± 8.0 | 69.3 ± 13.3 | |
| ORN Stage | <0.013 *** | ||
| Stage 1 | 10 (31.3) | 6 (16.7) | |
| Stage 2 | 0 (0.0) | 3 (8.3) | |
| Stage 3 | 5 (15.6) | 16 (44.4) | |
| Stage 4 | 17 (53.1) | 11(30.6) | |
| Jawbone | <0.886 *** | ||
| Maxilla | 4 (12.5) | 6 (16.7) | |
| Mandible | 25 (78.1) | 27 (75.0) | |
| Maxilla and Mandible | 3 (9.4) | 3 (8.3) | |
| Onset Site | 0.166 * | ||
| Molar region | 27 (84.4) | 25 (69.4) | |
| Incisal region | 5 (15.6) | 11 (30.6) | |
| Onset Trigger | 0.257 *** | ||
| Spontaneous onset | 20 (62.5) | 18 (50.0) | |
| Tooth extraction | 7 (21.9) | 8 (22.2) | |
| Infection | 5 (15.6) | 6 (16.7) | |
| Others | 0 (0.0) | 4 (11.1) | |
| Primary Tumor Site | 0.401 *** | ||
| Oral | 8 (25.0) | 14 (38.9) | |
| Pharynx | 22 (68.8) | 19 (52.8) | |
| Others | 2 (6.3) | 3 (8.3) | |
| Radiation Dose | 0.003 ** | ||
| Range (Gy) | 30–70 | 60–81 | |
| Mean ± SD | 63.7 ± 9.0 | 66.4 ± 6.9 | |
| Observation Period | <0.390 ** | ||
| Range (months) | 1–144 | 0–144 | |
| Mean ± SD | 80.8 ± 39.4 | 71.2 ± 40.6 | |
| Medical History | |||
| Hypertension | <0.550 * | ||
| No | 24 (75.0) | 30 (83.3) | |
| Yes | 8 (25.0) | 6 (16.7) | |
| Diabetes | 1.000 * | ||
| No | 31 (96.9) | 35 (97.2) | |
| Yes | 1 (3.1) | 1 (2.8) | |
| Steroid Therapy | |||
| No | 29 (90.6) | 36 (95.6) | |
| Yes | 3 (9.4) | 0 (4.4) | |
| Clinical Symptoms | 1.000 * | ||
| Pain | |||
| No | 6 (18.8) | 6 (16.7) | |
| Yes | 26 (81.3) | 30 (83.3) | |
| Nerve Paralysis | 0.182 * | ||
| No | 21 (65.6) | 29 (80.6) | |
| Yes | 11 (34.4) | 7 (19.4) | |
| Pus Discharge | 1.000 * | ||
| No | 13 (40.6) | 14 (38.9) | |
| Yes | 19 (59.4) | 22 (61.1) | |
| Pathological Fracture | 0.461 * | ||
| No | 27 (84.4) | 33 (91.7) | |
| Yes | 5 (15.6) | 3 (8.3) | |
| Orocutaneous Fistula | 0.021 * | ||
| No | 29 (90.6) | 24 (77.9) | |
| Yes | 3 (9.4) | 12 (33.3) | |
| Trismus | 0.331 * | ||
| No | 18 (56.3) | 15 (41.7) | |
| Yes | 14 (43.8) | 21 (58.3) | |
| Treatment | 0.016 *** | ||
| Conservative Therapy | 12 (37.5) | 24 (66.7) | |
| Minimal Debridement | 2 (6.3) | 4 (11.1) | |
| Resection | 18 (56.3) | 8 (22.2) | |
| Osteolysis | 1.000 * | ||
| No | 4 (12.5) | 5 (13.9) | |
| Yes | 28 (87.5) | 31 (86.1) | |
| Osteosclerosis | 0.443 * | ||
| No | 9 (28.1) | 14 (38.9) | |
| Yes | 23 (71.9) | 22 (61.1) | |
| Periosteal Reaction | 0.616 * | ||
| No | 31 (96.9) | 33 (91.7) | |
| Yes | 1 (4.0) | 3 (3.1) | |
| Sequestration | 0.047 * | ||
| No | 15 (46.9) | 26 (72.2) | |
| Yes | 17 (53.1) | 10 (27.8) | |
| 95% CI | ||||
|---|---|---|---|---|
| Variable | p-Value | Hazard Ratio | Lower | Upper |
| Radiation Dose | 0.005 | 1.148 | 1.042 | 1.264 |
| Orocutaneous fistula | 0.005 | 2.929 | 1.374 | 6.246 |
| Non-Sequestration | 0.028 | 2.493 | 1.106 | 5.624 |
| Case Number | Anterior Margin | Medial Area (Central Area of Bone Destruction) | Posterior Margin | ||||
|---|---|---|---|---|---|---|---|
| Cortical Bone Inferior Border | Cortical Bone Middle Level | Cancellous Bone | Cancellous Bone | Cortical Bone Inferior Border | Cortical Bone Middle Level | Cancellous Bone | |
| (A) | |||||||
| 1 | V | V | V | N | V | V | V |
| 2 | N | V | V | V | V | V | V |
| 3 | V | V | V | N | V | V | V |
| 4 | V | V | V | N | N | V | V |
| 5 | V | V | V | N | V | V | V |
| 6 | N | V | V | N | V | V | V |
| 7 | N | V | V | N | V | V | V |
| 8 | N | V | V | V | N | N | V |
| 9 | V | V | V | V | V | V | V |
| 10 | N | V | V | N | V | V | V |
| 11 | N | V | V | N | N | N | N |
| 12 | V | V | V | N | V | V | N |
| Necrosis rate (%) | 50 | 0 | 0 | 75 | 25 | 16.7 | 16.7 |
| (B) | |||||||
| 13 | N | N | V | N | N | N | V |
| 14 | N | N | V | N | V | V | N |
| 15 | N | V | V | N | N | V | N |
| 16 | N | N | V | N | N | N | V |
| Necrosis rate (%) | 100 | 75 | 0 | 100 | 75 | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadokoro, Y.; Hasegawa, T.; Takeda, D.; Murakami, A.; Yatagai, N.; Iwata, E.; Saito, I.; Kusumoto, J.; Akashi, M. Factors Associated with Treatment Outcomes and Pathological Features in Patients with Osteoradionecrosis: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 6565. https://doi.org/10.3390/ijerph19116565
Tadokoro Y, Hasegawa T, Takeda D, Murakami A, Yatagai N, Iwata E, Saito I, Kusumoto J, Akashi M. Factors Associated with Treatment Outcomes and Pathological Features in Patients with Osteoradionecrosis: A Retrospective Study. International Journal of Environmental Research and Public Health. 2022; 19(11):6565. https://doi.org/10.3390/ijerph19116565
Chicago/Turabian StyleTadokoro, Yoshiaki, Takumi Hasegawa, Daisuke Takeda, Aki Murakami, Nanae Yatagai, Eiji Iwata, Izumi Saito, Junya Kusumoto, and Masaya Akashi. 2022. "Factors Associated with Treatment Outcomes and Pathological Features in Patients with Osteoradionecrosis: A Retrospective Study" International Journal of Environmental Research and Public Health 19, no. 11: 6565. https://doi.org/10.3390/ijerph19116565
APA StyleTadokoro, Y., Hasegawa, T., Takeda, D., Murakami, A., Yatagai, N., Iwata, E., Saito, I., Kusumoto, J., & Akashi, M. (2022). Factors Associated with Treatment Outcomes and Pathological Features in Patients with Osteoradionecrosis: A Retrospective Study. International Journal of Environmental Research and Public Health, 19(11), 6565. https://doi.org/10.3390/ijerph19116565






