Searching for the Relationship between the Concentration of Heavy Metals in the Blood and the Clinical Course of Multiple Sclerosis: A Cross-Sectional Study in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement Methodology
2.2. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
- 96.70% (n = 146): relapsing–remitting multiple sclerosis (RRMS);
- 2.60% (n = 4): secondary progressive multiple sclerosis (SPMS);
- 0.70% (n = 1): primary progressive multiple sclerosis (PPMS).
3.2. Levels of the Tested Elements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. Poisoning in the Modern World—New Tricks for an Old Dog? Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/64762 (accessed on 22 February 2022). [CrossRef] [Green Version]
- Bian, B.; Zhou, L.J.; Li, L.; Fan, Y.M. Risk assessment of heavy metals in air, water, vegetables, grains, and related soils irrigated with biogas slurry in Taihu Basin, China. Environ. Sci. Pollut. Res. 2015, 22, 7794–7807. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Shefa, S.T.; Héroux, P. Both physiology and epidemiology support zero tolerable blood lead levels. Toxicol. Lett. 2017, 280, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.M.; Pretorius, C.J.; Ungerer, J.P. Lead toxicity: An Australian perspective. Clin. Biochem. Rev. 2018, 39, 61–98. [Google Scholar] [PubMed]
- Etemadifar, M.; Mehrabi, B.; Kiani-Peykani, R.; Abtahi, S.H.; NekouieIsfahani, K.; Ramagopalan, S.; Fereidan-Esfahani, M. Soil heavy metals are associated with the distribution of multiple sclerosis in Isfahan, Iran. Acta Neurol. Scand. 2016, 134, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar]
- Yun, S.W.; Hoyer, S. Effects of low-level lead on glycolytic enzymes and pyruvate dehydrogenase of rat brain in vitro: Relevance to sporadic Alzheimer’s disease? J. Neural Transm. 2000, 107, 355–368. [Google Scholar] [CrossRef]
- Khalil, N.; Morrow, L.A.; Needleman, H.; Talbott, E.O.; Wilson, J.W.; Cauley, J.A. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology 2009, 23, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- González-Estecha, M.; Trasobares, E.; Fuentes, M.; Martínez, M.J.; Cano, S.; Vergara, N.; Gaspar, M.J.; González-Revaldería, J.; Barciela, M.C.; Bugarín, Z. Blood lead and cadmium levels in a six hospital employee population. PESA study, 2009. J. Trace Elem. Med. Biol. 2011, 25, S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Dilokthornsakul, P.; Valuck, R.J.; Nair, K.V.; Corboy, J.R.; Allen, R.R.; Campbell, J.D. Multiple sclerosis prevalence in the united states commercially insured population. Neurology 2016, 86, 1014–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Stwardnienie rozsiane—Etiopatogeneza i możliwości diagnostyczne. Postepy Hig. Med. Dosw. 2017, 71, 551–563. [Google Scholar]
- Jakubowski, M. Kadm i jego związki nieorganiczne—W przeliczeniu na Cd. Dokumentacja dopuszczalnych wielkości narażenia zawodowego. Podstawy I Metod. Oceny Sr. Pr. 2012, 2, 111–146. [Google Scholar]
- Jakubowski, M. Ołów i jego związki nieorganiczne, z wyjątkiem arsenianu(V), ołowiu(II) i chromianu(VI) ołowiu(II)—W przeliczeniu na ołów, frakcja wdychalna. Dokumentacja proponowanych dopuszczalnych wielkości narażenia zawodowego. Podstawy I Metod. Oceny Sr. Pr. 2014, 2, 111–144. [Google Scholar] [CrossRef] [Green Version]
- Razavi, Z.; Jokar, M.; Allafchian, A.; Hossinpour, Z.; Berenjani, L.; Shayegan Nejad, V. The relationship between blood lead levels and clinical features among multiple sclerosis patients in isfahan, Iran. IJHSE 2016, 3, 412–420. [Google Scholar]
- Graves, J.S.; Chitnis, T.; Weinstock-Guttman, B.; Rubin, J.; Zelikovitch, A.S.; Nourbakhsh, B.; Simmons, T.; Waltz, M.; Casper, T.C.; Waubant, E.; et al. Maternal and Perinatal Exposures Are Associated with Risk for Pediatric-Onset Multiple Sclerosis. Pediatrics 2017, 139, e20162838. [Google Scholar] [CrossRef] [Green Version]
- Magyari, M.; Koch-Henriksen, N.; Pfleger, C.C.; Sørensen, P.S. Physical and Social Environment and the Risk of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 600–606. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar] [CrossRef] [PubMed]
- Siblerud, R.L.; Kienholz, E. Evidence that mercury from silver dental fillings may be an etiological factor in multiple sclerosis. Sci. Total Environ. 1994, 142, 191–205. [Google Scholar] [CrossRef]
- Attar, A.M.; Kharkhaneh, A.; Etemadifar, M.; Keyhanian, K.; Davoudi, V.; Saadatnia, M. Serum mercury level and multiple sclerosis. Biol. Trace Elem. Res. 2012, 146, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Pachner, A.R. A Primer of Neuroimmunological Disease; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4614-2188-7. [Google Scholar] [CrossRef]
- Ghoreishi, A.; Mohsenib, M.; Amraeic, R.; Alizadehd, A.M.; Mazloomzadehe, S. Investigation the Amount of Copper, Lead, Zinc and Cadmium Levels in Serum of Iranian Multiple Sclerosis Patients. J. Chem. Pharm. Sci. 2015, 8, 40–45. [Google Scholar]
- Kahrizi, F.; Salimi, A.; Noorbakhsh, F.; Faizi, M.; Mehri, F.; Naserzadeh, P.; Naderi, N.; Pourahmad, J. Repeated Administration of Mercury Intensifies Brain Damage in Multiple Sclerosis through Mitochondrial Dysfunction. Iran. J. Pharm. Res. 2016, 15, 834–841. [Google Scholar]
- Moradi, A.; Honarjoo, N.; Etemadifar, M.; Fallahzade, J. Bio-accumulation of some heavy metals in blood serum of residents in Isfahan and Shiraz, Iran. Environ. Monit. Assess 2016, 188, 269. [Google Scholar] [CrossRef]
- Nashmi, A.D.; Hassan, A.F.; Hammady, M.M. Estimation the Level of Metals (Lead, Cadmium, Copper and Zinc) In Multiple Sclerosis Patients in Basra\Iraq. Indian J. Forensic. Med. Toxicol. 2020, 14, 1029–1035. [Google Scholar]
- Aliomrani, M.; Sahraian, M.A.; Shirkhanloo, H.; Sharifzadeh, M.; Khoshayand, M.R.; Ghahremani, M.H. Blood Concentrations of Cadmium and Lead in Multiple Sclerosis Patients from Iran. Iran. J. Pharm. Res. 2016, 15, 825–833. [Google Scholar]
- Aliomrani, M.; Sahraian, M.A.; Shirkhanloo, H.; Sharifzadeh, M.; Khoshayand, M.R.; Ghahremani, M.H. Correlation between heavy metal exposure and GSTM1 polymorphism in Iranian multiple sclerosis patients. Neurol. Sci. 2017, 38, 1271–1278. [Google Scholar] [CrossRef]
- Paknejad, B.; Shirkhanloo, H.; Aliomrani, M. Is There Any Relevance Between Serum Heavy Metal Concentration and BBB Leakage in Multiple Sclerosis Patients? Biol. Trace Elem. Res. 2019, 190, 289–294. [Google Scholar] [CrossRef]
- Alizadeh, A.; Mehrpour, O.; Nikkhah, K.; Bayat, G.; Espandani, M.; Golzari, A.; Jarahi, L.; Foroughipour, M. Comparison of serum Concentration of Se, Pb, Mg, Cu, Zn, between MS patients and healthy controls. Electron. Physician 2016, 8, 2759–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, F.; Umbach, D.M.; Hu, H.; Munsat, T.L.; Shefner, J.M.; Taylor, J.A.; Sandler, D.P. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener. Dis. 2005, 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.P.; Lee, C.T.C. Multiple sclerosis incidence associated with the soil lead and arsenic concentrations in Taiwan. PLoS ONE 2013, 8, e65911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, E.; Houdaji, M.; Etemadifar, M. The relationship of concentrations of lead and zinc and multiple sclerosis in Isfahan Province, Iran. J. Isfahan Med. Sch. 2014, 32, 1–10. [Google Scholar]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 2007, 28, 450–456. [Google Scholar] [CrossRef]
- Turabelidze, G.; Schootman, M.; Zhu, B.-P.; Malone, J.L.; Horowitz, S.; Weidinger, J.; Williamson, D.; Simoes, E. Multiple sclerosis prevalence and possible lead exposure. J. Neurol. Sci. 2008, 269, 158–162. [Google Scholar] [CrossRef]
- Sarihi, S.; Niknam, M.; Mahjour, S.; Hosseini-Bensenjan, M.; Moazzen, F.; Soltanabadi, S.; Akbari, H. Toxic heavy metal concentrations in multiple sclerosis patients: A systematic review and meta-analysis. EXCLI J. 2021, 20, 1571–1584. [Google Scholar]


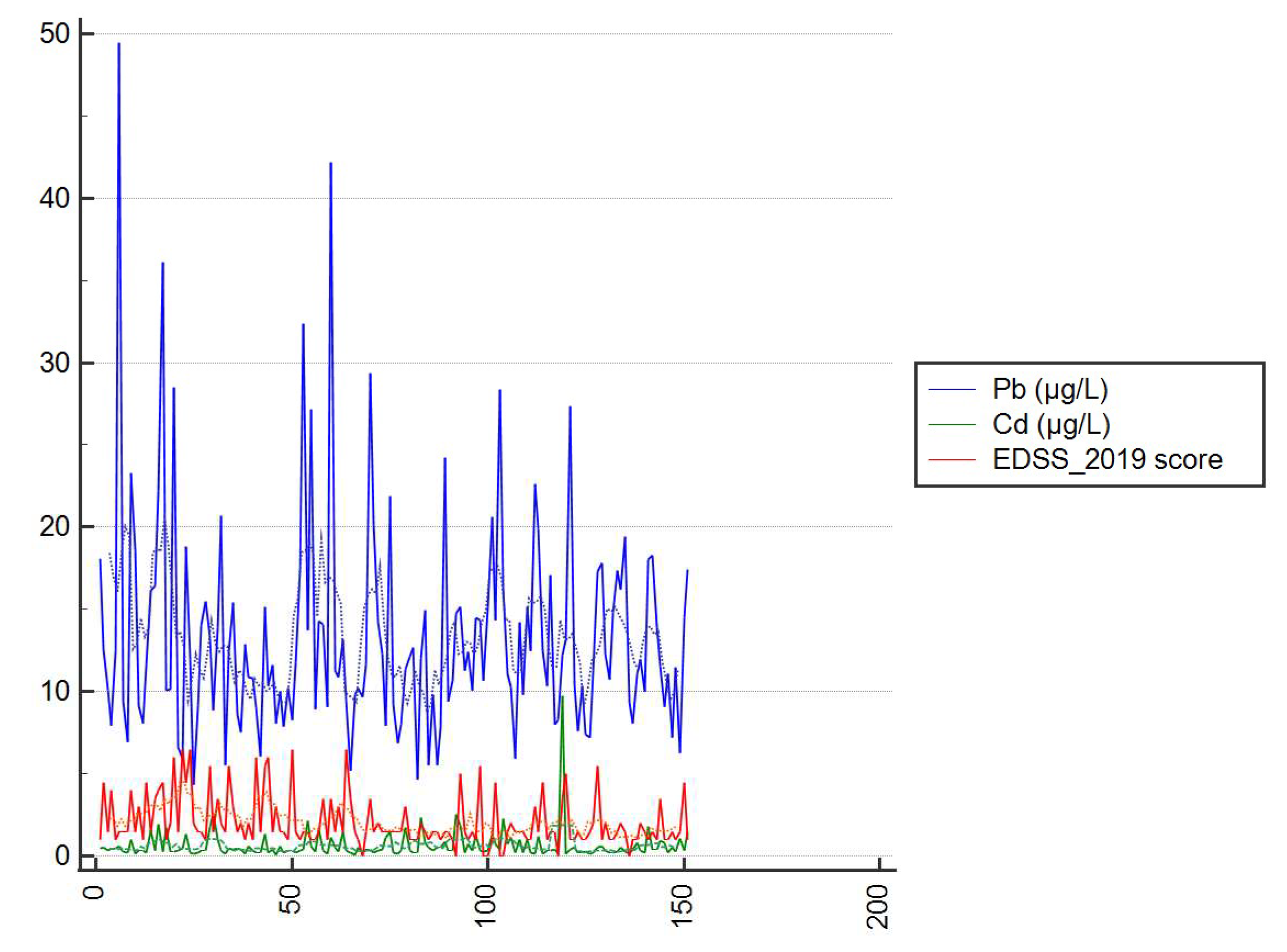
| Clinical Parameters and Sociodemographic Data | Sex | ||||||
|---|---|---|---|---|---|---|---|
| Women | Men | p | |||||
| n | Me | M | n | Me | M | ||
| EDSS 2019 (scores) | 104 | 1.5 | 1.99 | 47 | 1.5 | 2.30 | 0.58 |
| EDSS result at time of diagnosis (scores) | 104 | 1.5 | 1.72 | 47 | 1.5 | 1.95 | 0.28 |
| Age at symptom onset (years) | 104 | 29.5 | 31.11 | 47 | 27 | 29.28 | 0.15 |
| Duration of education (years) | 104 | 15 | 15.00 | 47 | 13 | 13.19 | 0.0002 |
| Clinical Parameter | Sex | Chi2 | p | ||
|---|---|---|---|---|---|
| Women | Men | ||||
| n | n | ||||
| Autoimmune diseases | No | 89 | 46 | ne | ne |
| Yes | 12 | 0 | |||
| De novo | No | 38 | 21 | 0.90 | 0.34 |
| Yes | 66 | 26 | |||
| Primary MS attack | No | 66 | 30 | 0.002 | 0.96 |
| Yes | 38 | 17 | |||
| Number of systems affected | One | 42 | 20 | 2.18 | 0.34 |
| Two | 41 | 22 | |||
| Three | 21 | 5 | |||
| Disease onset | Monofocal | 42 | 20 | 0.06 | 0.80 |
| Multifocal | 62 | 27 | |||
| Form of disease | PPMS | 0 | 1 | ne | ne |
| RRMS | 101 | 45 | |||
| SPMS | 3 | 1 | |||
| Education | Primary | 1 | 3 | 25.17 | <0.0001 |
| Vocational | 3 | 10 | |||
| Secondary | 32 | 20 | |||
| Higher | 68 | 14 | |||
| Place of residence | Village | 15 | 4 | 2.52 | 0.47 |
| City of 25,000–100,000 inhabitants | 10 | 7 | |||
| City > 100,000 inhabitants | 14 | 9 | |||
| City ≤ 25,000 inhabitants | 65 | 27 | |||
| Clinical Parameter | Element | |||
|---|---|---|---|---|
| 114Cd [µg/L] | 208Pb [µg/L] | |||
| r | p | r | p | |
| EDSS 2019 | 0.04 | 0.66 | 0.08 | 0.32 |
| EDSS score at time of diagnosis | 0.03 | 0.70 | 0.15 | 0.07 |
| Age at symptom onset | 0.19 | 0.02 | 0.18 | 0.02 |
| Duration of education [years] | −0.16 | 0.05 | −0.07 | 0.41 |
| 114Cd level [µg/L] | ne | ne | 0.34 | <0.0001 |
| 208Pb level [µg/L] | 0.34 | <0.0001 | ne | ne |
| Element | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Parameter | 114Cd | 208Pb | |||||||||||
| n | Min | Max | Me | 25–75 | p | n | Min | Max | Me | 25–75 | p | ||
| Autoimmune diseases | No | 135 | 0.06 | 9.72 | 0.35 | 0.22–0.62 | 0.38 | 135 | 4.34 | 49.49 | 11.68 | 9.41–15.16 | 0.28 |
| Yes | 12 | 0.14 | 2.59 | 0.42 | 0.33–0.85 | 12 | 5.92 | 28.38 | 10.30 | 7.91–13.63 | |||
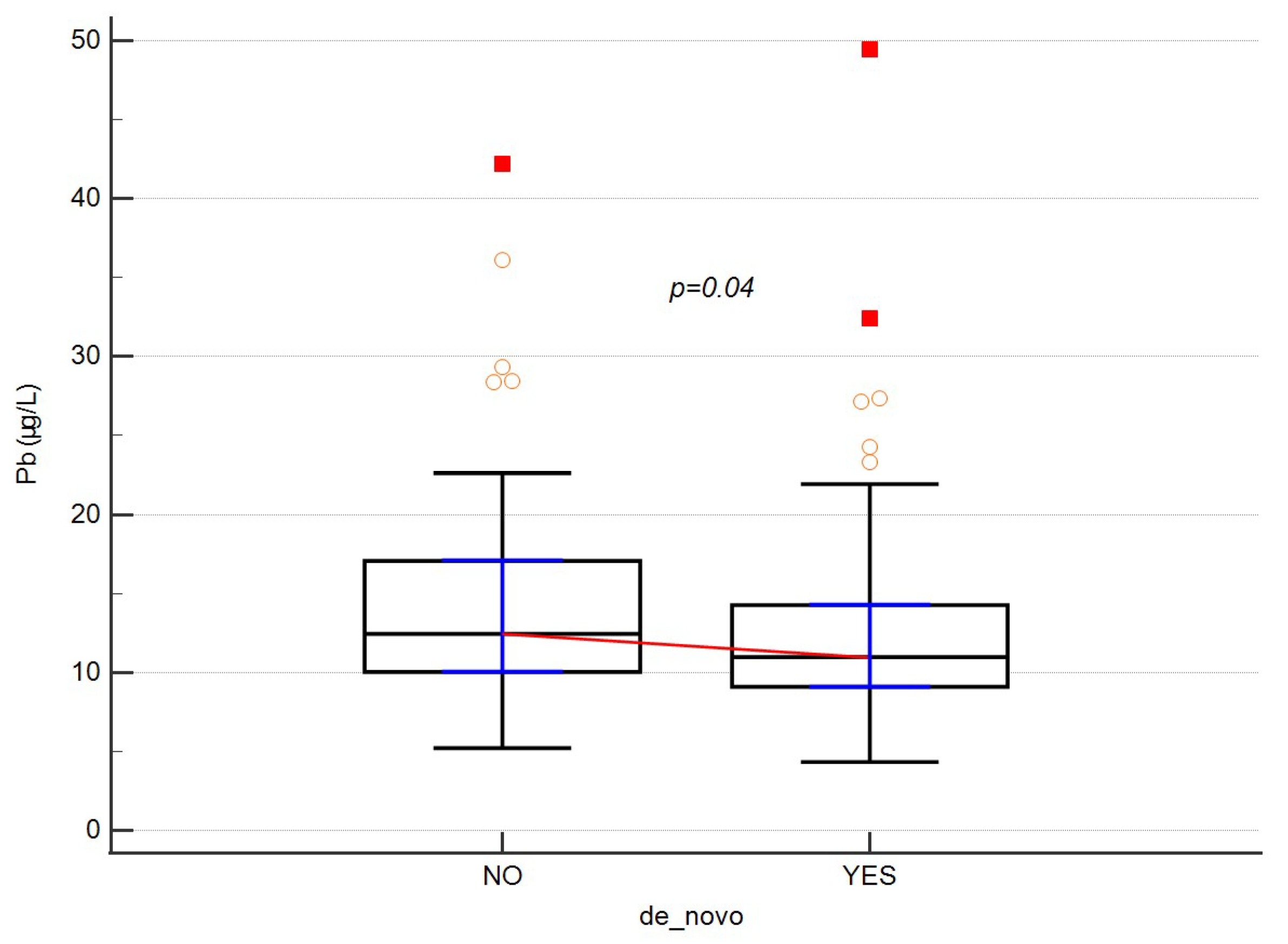
| De novo | No | 59 | 0.13 | 9.72 | 0.35 | 0.22–0.55 | 0.48 | 59 | 5.22 | 42.20 | 12.45 | 10.04–17.07 | 0.04 |
| Yes | 92 | 0.06 | 2.59 | 0.36 | 0.25–0.36 | 92 | 4.34 | 49.49 | 10.97 | 9.09–14.27 | |||
| First MS attack | No | 96 | 0.12 | 2.59 | 0.34 | 0.23–0.61 | 0.59 | 96 | 4.34 | 49.49 | 11.66 | 9.10–16.35 | 0.69 |
| Yes | 55 | 0.06 | 9.72 | 0.38 | 0.31–0.92 | 55 | 5.52 | 42.20 | 11.62 | 9.57–14.29 | |||
| Number of systems affected | One | 62 | 0.15 | 2.54 | 0.36 | 0.25–0.59 | 0.49 | 62 | 4.34 | 49.49 | 10.98 | 9.41–14.35 | 0.39 |
| Two | 63 | 0.06 | 9.72 | 0.32 | 0.21–0.55 | 63 | 5.55 | 32.41 | 12.27 | 9.14–15.16 | |||
| Three | 26 | 0.14 | 2.59 | 0.38 | 0.26–1.08 | 26 | 5.93 | 36.10 | 12.94 | 9.12–16.45 | |||
| Disease onset | Monofocal | 62 | 0.15 | 2.54 | 0.36 | 0.25–0.59 | 0.75 | 62 | 4.34 | 49.49 | 10.98 | 9.41–14.35 | 0.18 |
| Multifocal | 89 | 0.06 | 9.72 | 0.34 | 0.23–0.84 | 89 | 5.55 | 36.10 | 12.45 | 9.12–15.64 | |||
| Form of disease | PP | 1 | 0.44 | 0.44 | 0.44 | 0.44–0.44 | 0.90 | 1 | 12.45 | 12.45 | 12.45 | 12.45–12.45 | 0.89 |
| RR | 146 | 0.06 | 9720 | 0.35 | 0.23–0.35 | 146 | 4.34 | 49.49 | 11.61 | 9.35–15.15 | |||
| SP | 4 | 0.22 | 1.31 | 0.300 | 0.25–0.30 | 4 | 8.29 | 28.48 | 13.92 | 8.64–23.67 | |||
| Number of medications taken | One | 82 | 0.08 | 9.72 | 0.35 | 0.23–0.81 | 0.98 | 82 | 4.34 | 49.49 | 12.50 | 9.41–16.32 | 0.09 |
| Two | 40 | 0.14 | 2.14 | 0.36 | 0.24–0.61 | 40 | 5.22 | 32.41 | 10.85 | 9.27–12.45 | |||
| Three | 9 | 0.13 | 1.86 | 0.43 | 0.17–0.54 | 9 | 5.93 | 22.62 | 15.16 | 11.68–16.65 | |||
| Four | 13 | 0.06 | 2.59 | 0.32 | 0.20–1.21 | 13 | 4.67 | 15.15 | 10.08 | 8.64–11.981 | |||
| No data | 6 | 0.22 | 1.31 | 0.32 | 0.27–0.44 | 6 | 8.29 | 28.48 | 11.14 | 8.99–18.85 | |||
| Element | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Parameters | 114Cd | 208Pb | |||||||||||
| n | Min | Max | Me | 25–75 | p | n | Min | Max | Me | 25–75 | p | ||
| Place of residence | Village | 19 | 0.162 | 2.35 | 0.47 | 0.24–1.01 | 0.09 | 19 | 7.40 | 49.49 | 12.00 | 9.86–19.41 | 0.56 |
| City 25,000–100,000 inhabitants | 17 | 0.182 | 1.34 | 0.41 | 0.24–0.75 | 17 | 4.67 | 36.10 | 10.77 | 7.98–12.98 | |||
| City > 100,000 inhabitants | 23 | 0.06 | 2.59 | 0.59 | 0.32–1.15 | 23 | 7.83 | 42.20 | 11.34 | 9.85–14.71 | |||
| City ≤ 25,000 inhabitants | 92 | 0.08 | 9.72 | 0.33 | 0.21–0.53 | 92 | 4.34 | 32.41 | 12.19 | 9.10–15.29 | |||
| Education | Primary | 4 | 0.15 | 1.259 | 0.285 | 0.21–1.26 | 0.24 | 4 | 7.40 | 14.47 | 10.68 | 8.25–13.37 | 0.23 |
| Vocational | 13 | 0.06 | 1.95 | 0.32 | 0220–1.95 | 13 | 9.80 | 28.48 | 15.15 | 10.20–20.44 | |||
| Secondary | 52 | 0.15 | 9.72 | 0.41 | 0.31–0.97 | 52 | 4.34 | 49.49 | 11.80 | 9.42–15.86 | |||
| Higher | 82 | 0.08 | 2.59 | 0.34 | 0.21–0.55 | 82 | 5.22 | 42.20 | 11.42 | 8.58–14.51 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knyszyńska, A.; Skonieczna-Żydecka, K.; Koziarska, D.; Stachowska, L.; Kotwas, A.; Kulaszyńska, M.; Lubkowska, A.; Karakiewicz, B. Searching for the Relationship between the Concentration of Heavy Metals in the Blood and the Clinical Course of Multiple Sclerosis: A Cross-Sectional Study in Poland. Int. J. Environ. Res. Public Health 2022, 19, 6548. https://doi.org/10.3390/ijerph19116548
Knyszyńska A, Skonieczna-Żydecka K, Koziarska D, Stachowska L, Kotwas A, Kulaszyńska M, Lubkowska A, Karakiewicz B. Searching for the Relationship between the Concentration of Heavy Metals in the Blood and the Clinical Course of Multiple Sclerosis: A Cross-Sectional Study in Poland. International Journal of Environmental Research and Public Health. 2022; 19(11):6548. https://doi.org/10.3390/ijerph19116548
Chicago/Turabian StyleKnyszyńska, Anna, Karolina Skonieczna-Żydecka, Dorota Koziarska, Laura Stachowska, Artur Kotwas, Monika Kulaszyńska, Anna Lubkowska, and Beata Karakiewicz. 2022. "Searching for the Relationship between the Concentration of Heavy Metals in the Blood and the Clinical Course of Multiple Sclerosis: A Cross-Sectional Study in Poland" International Journal of Environmental Research and Public Health 19, no. 11: 6548. https://doi.org/10.3390/ijerph19116548
APA StyleKnyszyńska, A., Skonieczna-Żydecka, K., Koziarska, D., Stachowska, L., Kotwas, A., Kulaszyńska, M., Lubkowska, A., & Karakiewicz, B. (2022). Searching for the Relationship between the Concentration of Heavy Metals in the Blood and the Clinical Course of Multiple Sclerosis: A Cross-Sectional Study in Poland. International Journal of Environmental Research and Public Health, 19(11), 6548. https://doi.org/10.3390/ijerph19116548








