Exposure to Chloramine and Chloroform in Tap Water and Adverse Perinatal Outcomes in Shanghai
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Environmental Data
2.3. Confounders
2.4. Perinatal Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Mother–Infant Pairs
3.2. Risk of Adverse Perinatal Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaves, R.S.; Guerreiro, C.S.; Cardoso, V.V.; Benoliel, M.J.; Santos, M.M. Hazard and mode of action of disinfection by-products (DBPs) in water for human consumption: Evidences and research priorities. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 223, 53–61. [Google Scholar] [CrossRef]
- Tang, Y.; Long, X.; Wu, M.; Yang, S.; Gao, N.; Xu, B.; Dutta, S. Bibliometric review of research trends on disinfection by-products in drinking water during 1975–2018. Sep. Purif. Technol. 2020, 241, 116741. [Google Scholar] [CrossRef]
- National Research Council Safe Drinking Water Council. Drinking Water and Health: Disinfectants and Disinfectant By-Products: Volume 7; National Academies Press (US): Washington, DC, USA, 1987. [Google Scholar]
- Mian, H.R.; Hu, G.; Hewage, K.; Rodriguez, M.J.; Sadiq, R. Prioritization of unregulated disinfection by-products in drinking water distribution systems for human health risk mitigation: A critical review. Water Res. 2018, 147, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Ricca, H.; Aravinthan, V.; Mahinthakumar, G. Modeling chloramine decay in full-scale drinking water supply systems. Water Environ. Res. 2019, 91, 441–454. [Google Scholar] [CrossRef]
- Williams, A.L.; Bates, C.A.; Pace, N.D.; Leonhard, M.J.; Chang, E.T.; DeSesso, J.M. Impact of chloroform exposures on reproductive and developmental outcomes: A systematic review of the scientific literature. Birth Defects Res. 2018, 110, 1267–1313. [Google Scholar] [CrossRef] [Green Version]
- Reuber, M.D. Carcinogenicity of chloroform. Environ. Health Perspect. 1979, 31, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Cheng, B.H.; Tsai, S.S.; Wu, T.N.; Lin, M.C.; Lin, K.C. Association between chlorination of drinking water and adverse pregnancy outcome in Taiwan. Environ. Health Perspect. 2000, 108, 765–768. [Google Scholar] [CrossRef]
- Lawn, J.E.; Blencowe, H.; Oza, S.; You, D.; Lee, A.C.; Waiswa, P.; Lalli, M.; Bhutta, Z.; Barros, A.J.; Christian, P.; et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef]
- Khashan, A.S.; Kenny, L.C.; Lundholm, C.; Kearney, P.M.; Gong, T.; McNamee, R.; Almqvist, C. Gestational Age and Birth Weight and the Risk of Childhood Type 1 Diabetes: A Population-Based Cohort and Sibling Design Study. Diabetes Care 2015, 38, 2308–2315. [Google Scholar] [CrossRef] [Green Version]
- Mathewson, K.J.; Chow, C.H.; Dobson, K.G.; Pope, E.I.; Schmidt, L.A.; Van Lieshout, R.J. Mental health of extremely low birth weight survivors: A systematic review and meta-analysis. Psychol. Bull. 2017, 143, 347–383. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, O.; Park, A.L.; Cohen, E.; Fell, D.B.; Ray, J.G. Premature Cardiac Disease and Death in Women Whose Infant Was Preterm and Small for Gestational Age: A Retrospective Cohort Study. JAMA Cardiol. 2018, 3, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018, 172, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.A.; Wright, J.M.; Evans, A.; Rivera-Núñez, Z.; Meyer, A.; Narotsky, M.G. Disinfection By-Product Exposures and the Risk of Musculoskeletal Birth Defects. Environ. Epidemiol. 2020, 4, e081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodds, L.; King, W.; Allen, A.C.; Armson, B.A.; Fell, D.B.; Nimrod, C. Trihalomethanes in public water supplies and risk of stillbirth. Epidemiology 2004, 15, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Singer, P.C.; Herring, A.H.; Hartmann, K.E.; Weinberg, H.S.; Makarushka, C. Exposure to drinking water disinfection by-products and pregnancy loss. Am. J. Epidemiol. 2006, 164, 1043–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iszatt, N.; Nieuwenhuijsen, M.J.; Bennett, J.E.; Toledano, M.B. Trihalomethanes in public drinking water and stillbirth and low birth weight rates: An intervention study. Environ. Int. 2014, 73, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Schwartz, J.; Dockery, D.W. The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ. Health Perspect. 2004, 112, 920–925. [Google Scholar] [CrossRef]
- Summerhayes, R.J.; Morgan, G.G.; Edwards, H.P.; Lincoln, D.; Earnest, A.; Rahman, B.; Beard, J.R. Exposure to trihalomethanes in drinking water and small-for-gestational-age births. Epidemiology 2012, 23, 15–22. [Google Scholar] [CrossRef]
- Rivera-Nunez, Z.; Wright, J.M. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J. Occup. Environ. Med. 2013, 55, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Y.X.; Liu, C.; Chen, Y.J.; Lu, W.Q.; Messerlian, C. Trimester-Specific Blood Trihalomethane and Urinary Haloacetic Acid Concentrations and Adverse Birth Outcomes: Identifying Windows of Vulnerability during Pregnancy. Environ. Health Perspect. 2020, 128, 107001. [Google Scholar] [CrossRef] [PubMed]
- The Standardization Administration of China. Standard Examination Methods for Drinking Water—Collection and Preservation of Water Samples; National Health Commission of the People’s Republic of China: Beijing, China, 2006.
- The Standardization Administration of China. Standard Examination Methods for Drinking Water—Disinfectants Parameters; National Health Commission of the People’s Republic of China: Beijing, China, 2006.
- The Standardization Administration of China. Standard Examination Methods for Drinking Water—Disinfection by-Products Parameters; National Health Commission of the People’s Republic of China: Beijing, China, 2006.
- Association, A.D. Standards of medical care in diabetes—2011. Diabetes Care 2011, 34 (Suppl. 1), S11–S61. [Google Scholar] [CrossRef] [Green Version]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.J.; He, Y.C.; Chen, L.; Dennis, C.L.; Huang, H.F.; Wu, Y.T. Effects of acute ambient pollution exposure on preterm prelabor rupture of membranes: A time-series analysis in Shanghai, China. Environ. Pollut. 2021, 276, 116756. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, J.R.; Chauan, S.; Bianchine, J.R. Controlled clinical evaluations of chlorine dioxide, chlorite and chlorate in man. Environ. Health Perspect. 1982, 46, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Fluck, S.; McKane, W.; Cairns, T.; Fairchild, V.; Lawrence, A.; Lee, J.; Murray, D.; Polpitiye, M.; Palmer, A.; Taube, D. Chloramine-induced haemolysis presenting as erythropoietin resistance. Nephrol. Dial. Transplant. 1999, 14, 1687–1691. [Google Scholar] [CrossRef]
- Carlton, B.D.; Barlett, P.; Basaran, A.; Colling, K.; Osis, I.; Smith, M.K. Reproductive effects of alternative disinfectants. Environ. Health Perspect. 1986, 69, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.S.; Berardi, M.R.; Bull, R.J. Effect of Chlorine and Monochloramine in Drinking Water on the Developing Rat Fetus. J. Appl. Toxicol. 1982, 2, 156–159. [Google Scholar] [CrossRef]
- Li, B.; Carey, M.; Workman, J.L. The Role of Chromatin during Transcription. Review 2007, 128, 707–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledano, M.B.; Nieuwenhuijsen, M.J.; Best, N.; Whitaker, H.; Hambly, P.; de Hoogh, C.; Fawell, J.; Jarup, L.; Elliott, P. Relation of trihalomethane concentrations in public water supplies to stillbirth and birth weight in three water regions in England. Environ. Health Perspect. 2005, 113, 225–232. [Google Scholar] [CrossRef]
- Cao, W.C.; Zeng, Q.; Luo, Y.; Chen, H.X.; Miao, D.Y.; Li, L.; Cheng, Y.H.; Li, M.; Wang, F.; You, L.; et al. Blood Biomarkers of Late Pregnancy Exposure to Trihalomethanes in Drinking Water and Fetal Growth Measures and Gestational Age in a Chinese Cohort. Environ. Health Perspect. 2016, 124, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Danileviciute, A.; Grazuleviciene, R.; Vencloviene, J.; Paulauskas, A.; Nieuwenhuijsen, M.J. Exposure to drinking water trihalomethanes and their association with low birth weight and small for gestational age in genetically susceptible women. Int. J. Environ. Res. Public Health 2012, 9, 4470–4485. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, C.M.; Gracia-Lavedán, E.; Ibarluzea, J.; Santa Marina, L.; Ballester, F.; Llop, S.; Tardón, A.; Fernández, M.F.; Freire, C.; Goñi, F.; et al. Exposure to trihalomethanes through different water uses and birth weight, small for gestational age, and preterm delivery in Spain. Environ. Health Perspect. 2011, 119, 1824–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazuleviciene, R.; Nieuwenhuijsen, M.J.; Vencloviene, J.; Kostopoulou-Karadanelli, M.; Krasner, S.W.; Danileviciute, A.; Balcius, G.; Kapustinskiene, V. Individual exposures to drinking water trihalomethanes, low birth weight and small for gestational age risk: A prospective Kaunas cohort study. Environ. Health 2011, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, Y.X.; Chen, Y.J.; Sun, Y.; Huang, L.L.; Cheng, Y.H.; Liu, E.N.; Lu, W.Q.; Messerlian, C. Blood and urinary biomarkers of prenatal exposure to disinfection byproducts and oxidative stress: A repeated measurement analysis. Environ. Int. 2020, 137, 105518. [Google Scholar] [CrossRef] [PubMed]
- Salas, L.A.; Baker, E.R.; Nieuwenhuijsen, M.J.; Marsit, C.J.; Christensen, B.C.; Karagas, M.R. Maternal swimming pool exposure during pregnancy in relation to birth outcomes and cord blood DNA methylation among private well users. Environ. Int. 2019, 123, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cao, W.C.; Zhou, B.; Zheng, T.Z.; Deng, Y.L.; Luo, Q.; Miao, Y.; Chen, D.; Zeng, Q.; Lu, W.Q. Urinary Biomarker of Prenatal Exposure to Disinfection Byproducts, Maternal Genetic Polymorphisms in CYP2E1 and GSTZ1, and Birth Outcomes. Environ. Sci. Technol. 2019, 53, 12026–12034. [Google Scholar] [CrossRef]
- Beddowes, E.J.; Faux, S.P.; Chipman, J.K. Chloroform, carbon tetrachloride and glutathione depletion induce secondary genotoxicity in liver cells via oxidative stress. Toxicology 2003, 187, 101–115. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, J.L.; Chen, L.C.; Zelikoff, J.T. Exposure to Ambient Particulate Matter during Specific Gestational Periods Produces Adverse Obstetric Consequences in Mice. Environ. Health Perspect. 2017, 125, 077020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Demianczuk, N.N.; Saunders, L.D.; Wang, F.L.; Fraser, W.D. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am. J. Epidemiol. 2002, 155, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victery, W.; Vander, A.J.; Shulak, J.M.; Schoeps, P.; Julius, S. Lead, hypertension, and the renin-angiotensin system in rats. J. Lab. Clin. Med. 1982, 99, 354–362. [Google Scholar]
- Jedrychowski, W.; Perera, F.; Jankowski, J.; Mrozek-Budzyn, D.; Mroz, E.; Flak, E.; Edwards, S.; Skarupa, A.; Lisowska-Miszczyk, I. Gender specific differences in neurodevelopmental effects of prenatal exposure to very low-lead levels: The prospective cohort study in three-year olds. Early Hum. Dev. 2009, 85, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioka, E.; Yokoyama, K.; Matsukawa, T.; Vigeh, M.; Hirayama, S.; Ueno, T.; Miida, T.; Makino, S.; Takeda, S. Evidence that birth weight is decreased by maternal lead levels below 5μg/dl in male newborns. Reprod. Toxicol. 2014, 47, 21–26. [Google Scholar] [CrossRef] [PubMed]
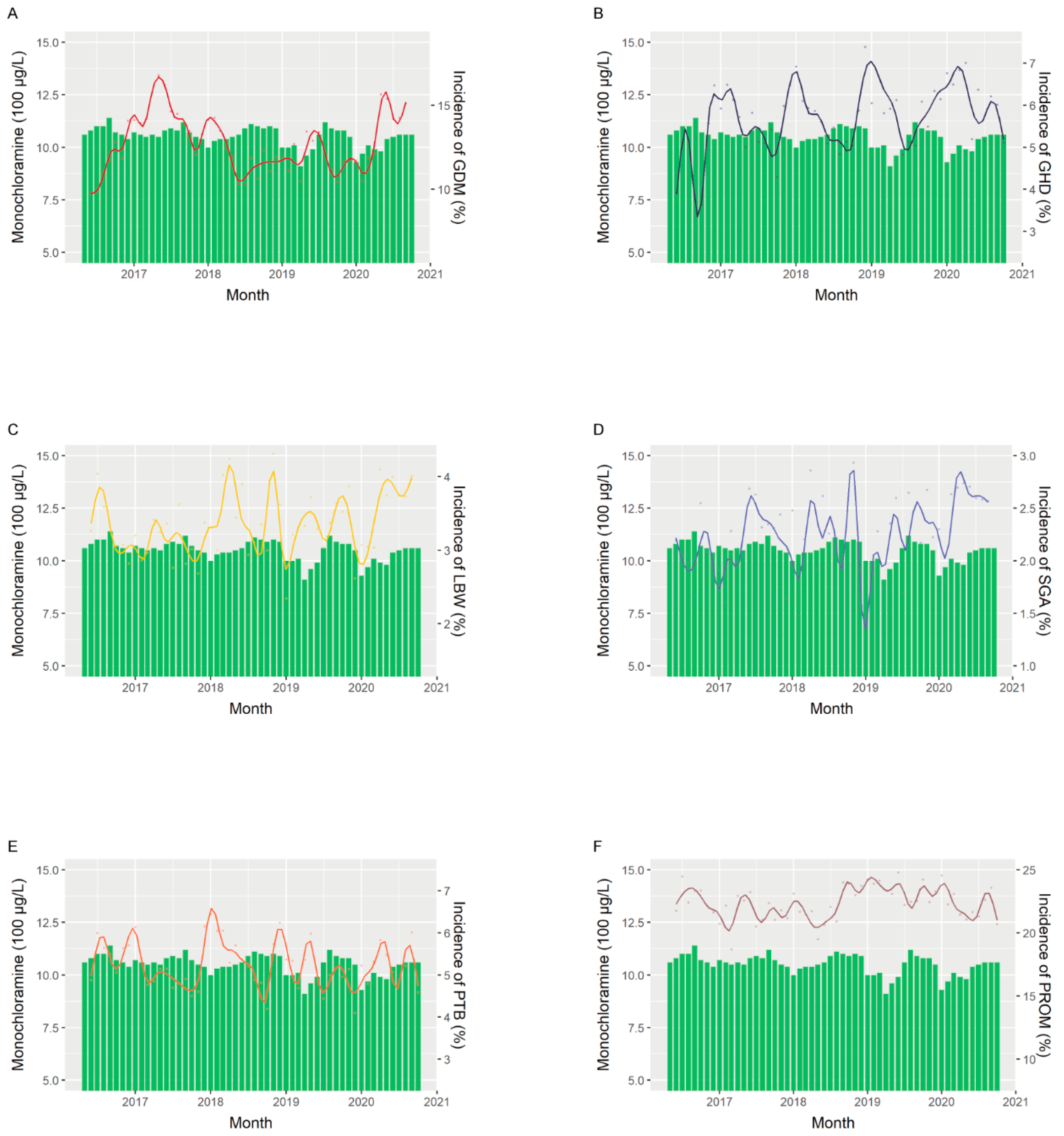
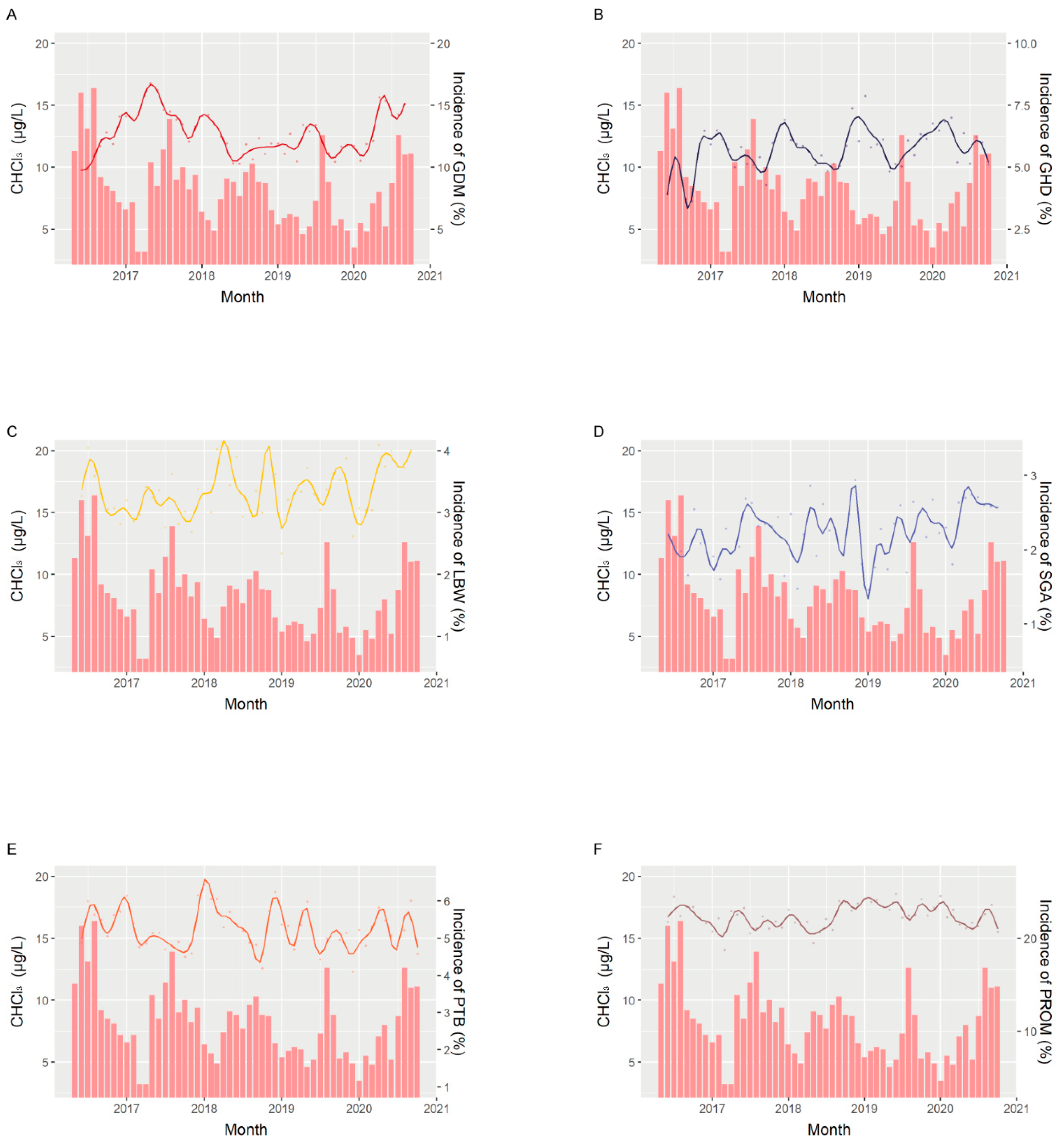
| ALL | GDM | GHD | LBW | SGA | Preterm | PROM | |
|---|---|---|---|---|---|---|---|
| (n = 109,182) | (n = 15,183) | (n = 6791) | (n = 3911) | (n = 2369) | (n = 6240) | (n = 26,618) | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Maternal sociodemographic characteristics | |||||||
| Age, mean ± SD, years | 31.01 ± 4.06 | 32.53 ± 4.26 | 31.77 ± 4.40 | 31.48 ± 4.36 | 30.58 ± 4.00 | 31.73 ± 4.37 | 30.758 ± 3.896 |
| Pre-gestational BMI, mean ± SD, kg/m2 | 21.23 ± 2.81 | 22.31 ± 3.18 | 23.06 ± 3.57 | 21.31 ± 3.14 | 20.408 ± 2.61 | 21.67 ± 3.14 | 21.191 ± 2.746 |
| Marriage | |||||||
| Married | 108,202 (99.1) | 15,010 (98.9) | 6713 (98.9) | 3883 (99.3) | 2356 (99.45) | 6198 (99.3) | 26,451 (99.4) |
| Single | 979 (0.90) | 173 (1.1) | 78 (1.2) | 28 (0.7) | 13 (0.55) | 42 (0.7) | 167 (0.6) |
| Medical insurance | |||||||
| Yes | 78,365 (71.8) | 10,814 (71.2) | 4990 (73.5) | 2830 (72.4) | 1687 (71.21) | 4478 (71.8) | 19,545 (73.4) |
| No | 30,816 (28.2) | 4369 (28.8) | 1801 (26.5) | 1081 (27.6) | 682 (28.79) | 1762 (28.2) | 7073 (26.6) |
| Occupation | |||||||
| Employed | 101,825 (93.3) | 13,971 (92.0) | 6235 (91.8) | 3565 (91.1) | 2175 (91.81) | 5726 (91.8) | 25,151 (94.5) |
| Self-employed | 3015 (2.8) | 503 (3.3) | 216 (3.2) | 138 (3.5) | 74 (3.12) | 209 (3.4) | 625 (2.4) |
| Unemployed | 4341 (4.0) | 709 (4.7) | 340 (5.0) | 208 (5.3) | 120 (5.07) | 305 (4.9) | 842 (3.2) |
| Ethnicity | |||||||
| Han | 107,694 (98.6) | 14,959 (98.5) | 6685 (98.4) | 3862 (98.8) | 2332 (98.44) | 6166 (98.8) | 26,331 (98.9) |
| Minority | 1359 (1.2) | 213 (1.4) | 92 (1.4) | 43 (1.1) | 33 (1.39) | 66 (1.1) | 258 (1.0) |
| Foreign | 128 (0.1) | 11 (0.1) | 14 (0.2) | 6 (0.2) | 4 (0.17) | 8 (0.1) | 29 (0.1) |
| Education attainment | |||||||
| High school or lower | 13,152 (15.6) | 2041 (17.4) | 1166 (23.1) | 548 (19.2) | 282 (16.25) | 869 (18.8) | 3067 (15.0) |
| College | 53,869 (63.9) | 7617 (65.1) | 3173 (62.9) | 1792 (62.8) | 1096 (63.2) | 2918 (63.2) | 12,880 (63.1) |
| Master or above | 17,229 (20.5) | 2049 (17.5) | 707 (14.0) | 513 (18.0) | 357 (20.6) | 828 (17.9) | 4463 (21.9) |
| Smoking during pregnancy | |||||||
| No | 86,167 (99.6) | 11,870 (99.5) | 5208 (99.4) | 3006 (99.7) | 1805 (99.8) | 4799 (99.6) | 20,909 (99.6) |
| Yes | 350 (0.4) | 60 (0.5) | 32 (0.6) | 10 (0.3) | 4 (0.2) | 20 (0.42) | 84 (0.4) |
| Alcohol drinking during pregnancy | |||||||
| No | 85,716 (99.1) | 11,809 (99.0) | 5199 (99.2) | 2997 (99.4) | 1799 (99.5) | 4773 (99.05) | 20,778 (99.0) |
| Yes | 802 (0.9) | 121 (1.0) | 41 (0.8) | 19 (0.6) | 10 (0.6) | 46 (1.0) | 215 (1.) |
| History of reproduction | |||||||
| parity | |||||||
| 0 | 65,812 (60.3) | 8594 (56.6) | 4432 (65.3) | 2496 (63.8) | 1646 (69.5) | 3664 (58.7) | 17,713 (66.6) |
| 1 | 36,739 (33.7) | 5506 (36.3) | 2026 (29.8) | 1198 (30.6) | 632 (26.7) | 2124 (34.0) | 7891 (29.7) |
| ≥2 | 6629 (6.1) | 1083 (7.1) | 333 (4.9) | 217 (5.6) | 91 (3.8) | 451 (7.2) | 1013 (3.8) |
| Number of previous abortions | |||||||
| 0 | 57,292 (63.5) | 7206 (57.6) | 3409 (62.5) | 2045 (63.9) | 1329 (70.1) | 3121 (61.0) | 14,246 (65.3) |
| 1–2 | 29,753 (33.0) | 4669 (37.4) | 1823 (33.4) | 1022 (31.9) | 516 (27.2) | 1749 (34.2) | 6920 (31.7) |
| ≥3 | 3192 (3.5) | 627 (5.0) | 221 (4.1) | 136 (4.3) | 52 (2.7) | 244 (4.8) | 664 (3.0) |
| Previous ectopic pregnancy | |||||||
| No | 88,270 (97.8) | 12,155 (97.2) | 5302 (97.2) | 3142 (98.1) | 1870 (98.6) | 4989 (97.6) | 21,388 (98.0) |
| Yes | 1967 (2.2) | 347 (2.8) | 151 (2.8) | 61 (1.9) | 27 (1.4) | 125 (2.4) | 442 (2.0) |
| ALL | GDM | GHD | LBW | SGA | Preterm | PROM | |
|---|---|---|---|---|---|---|---|
| (n = 109,182) | (n = 15,183) | (n = 6791) | (n = 3911) | (n = 2369) | (n = 6240) | (n = 26,618) | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Newborn gender | |||||||
| Male | 56,449 (51.7) | 7871 (51.8) | 3482 (51.3) | 1859 (47.5) | 1126 (47.5) | 3539 (56.7) | 14,018 (52.7) |
| Female | 52,732 (48.3) | 7312 (48.2) | 3309 (48.7) | 2052 (52.5) | 1243 (52.5) | 2701 (43.3) | 12,600 (47.3) |
| Gestational diabetes mellitus | |||||||
| No | 93,998 (86.1) | - | 5404 (79.6) | 3190 (81.6) | 2060 (87.0) | 5083 (81.5) | 23,162 (87.0) |
| Yes | 15,183 (13.9) | - | 1387 (20.4) | 721 (18.4) | 309 (13.0) | 1157 (18.5) | 3456 (13.0) |
| Diabetes mellitus in pregnancy | |||||||
| No | 108,544 (99.4) | - | 6658 (98.0) | 3870 (99.0) | 2358 (99.5) | 6162 (98.8) | 26,487 (99.5) |
| Yes | 637 (0.6) | - | 133 (2.0) | 41 (1.1) | 11 (0.5) | 78 (1.3) | 131 (0.5) |
| Chronic hypertension in pregnancy | |||||||
| No | 107,808 (98.7) | 14,823 (97.6) | - | 3766 (96.3) | 2321 (98.0) | 6047 (96.9) | 26,399 (99.2) |
| Yes | 1373 (1.3) | 360 (2.4) | - | 145 (3.7) | 48 (2.0) | 193 (3.1) | 219 (0.8) |
| Gestational hypertensive disorder | |||||||
| No | 102,390 (93.8) | 13,796 (90.9) | - | 3040 (77.7) | 1963 (82.9) | 5267 (84.4) | 25,472 (95.7) |
| Gestational hypertension | 3023 (2.8) | 632 (4.2) | - | 134 (3.4) | 88 (3.7) | 183 (2.9) | 644 (2.4) |
| Preeclampsia | 2395 (2.2) | 495 (3.3) | - | 191 (4.9) | 110 (4.6) | 198 (3.2) | 375 (1.4) |
| Sever preeclampsia | 1373 (1.3) | 260 (1.7) | - | 546 (14.0) | 208 (8.8) | 592 (9.5) | 127 (0.5) |
| Intrahepatic cholestasis of pregnancy | |||||||
| No | 108,345 (99.2) | 15,064 (99.2) | 6684 (98.4) | 3838 (98.1) | 2344 (98.9) | 6088 (97.6) | 26,517 (99.6) |
| Yes | 836 (0.8) | 119 (0.8) | 107 (1.6) | 73 (1.9) | 25 (1.1) | 152 (2.4) | 101 (0.4) |
| Gestational thyroid dysfunction | |||||||
| No | 101,419 (92.9) | 14,069 (92.7) | 6252 (92.1) | 3630 (92.8) | 2195 (92.7) | 5809 (93.1) | 24,790 (93.1) |
| Hyperthyroidism | 1102 (1.0) | 163 (1.1) | 96 (1.4) | 52 (1.3) | 30 (1.3) | 78 (1.3) | 283 (1.1) |
| Hypothyroidism | 6660 (6.1) | 951 (6.3) | 443 (6.5) | 229 (5.9) | 144 (6.1) | 353 (5.7) | 1545 (5.8) |
| Mode of delivery | |||||||
| Vaginal | 57,161 (52.4) | 6852 (45.1) | 2143 (31.6) | 1436 (36.7) | 1139 (48.1) | 2648 (42.4) | 17,918 (67.3) |
| Cesarean section | 47,569 (43.6) | 7785 (51.3) | 4382 (64.5) | 2398 (61.3) | 1118 (47.2) | 3463 (55.5) | 7114 (26.7) |
| Instrumental | 4451 (4.1) | 546 (3.6) | 266 (3.9) | 77 (2.0) | 112 (4.7) | 129 (2.1) | 1586 (6.0) |
| Assisted reproductive technology | |||||||
| No | 101,731 (93.2) | 13,585 (89.5) | 5966 (87.9) | 3595 (91.9) | 2207 (93.2) | 5713 (91.7) | 25,039 (94.1) |
| Yes | 7439 (6.8) | 1598 (10.5) | 823 (12.1) | 315 (8.1) | 161 (6.8) | 516 (8.3) | 1576 (5.9) |
| GDM OR (95% CI) | GHD OR (95% CI) | LBW OR (95% CI) | SGA OR (95% CI) | PTB OR (95% CI) | PROM OR (95% CI) | |
|---|---|---|---|---|---|---|
| Monochloramine (100μg/L) | ||||||
| T1 | 1.06 (1.03, 1.09) | 0.99 (0.95, 1.03) | 0.95 (0.92, 0.99) | 1.00 (0.95, 1.05) | 0.95 (0.92, 0.99) | 0.95 (0.93, 0.97) |
| T2 | 1.00 (0.98, 1.03) | 1.13 (1.09, 1.17) | 0.98 (0.94, 1.02) | 0.94 (0.89, 0.99) | 1.04 (1.00, 1.08) | 1.02 (0.99, 1.04) |
| T3 | - | - | 1.06 (1.01, 1.11) | 1.03 (0.97, 1.09) | 1.02 (0.98, 1.06) | 1.04 (1.01, 1.07) |
| All | 0.93 (0.88, 0.98) | 1.22 (1.13, 1.31) | 1.02 (0.94, 1.10) | 0.95 (0.85, 1.05) | 1.1 (1.03, 1.19) | 1.07 (1.03, 1.12) |
| CHCl3 (μg/L) | ||||||
| T1 | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.02) | 1.05 (1.03, 1.07) | 0.99 (0.96, 1.02) | 1.04 (1.02, 1.07) | 1.00 (0.99, 1.00) |
| T2 | 1.01 (0.99, 1.03) | 1.02 (0.99, 1.05) | 0.95 (0.93, 0.98) | 1.00 (0.96, 1.03) | 0.97 (0.94, 0.99) | 1.01 (1.00, 1.01) |
| T3 | - | - | 1.13 (1.09, 1.16) | 1.00 (0.96, 1.05) | 1.05 (1.01, 1.08) | 1.01 (1.00, 1.01) |
| All | 1.01 (0.98, 1.04) | 1.04 (1.00, 1.08) | 1.05 (1.02, 1.08) | 0.97 (0.92, 1.02) | 1.07 (1.03, 1.11) | 1.02 (1.01, 1.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.-M.; Li, C.; Xu, J.-J.; Zhang, H.-Q.; Su, Y.-F.; Wu, Y.-T.; Huang, H.-F. Exposure to Chloramine and Chloroform in Tap Water and Adverse Perinatal Outcomes in Shanghai. Int. J. Environ. Res. Public Health 2022, 19, 6508. https://doi.org/10.3390/ijerph19116508
Zhu S-M, Li C, Xu J-J, Zhang H-Q, Su Y-F, Wu Y-T, Huang H-F. Exposure to Chloramine and Chloroform in Tap Water and Adverse Perinatal Outcomes in Shanghai. International Journal of Environmental Research and Public Health. 2022; 19(11):6508. https://doi.org/10.3390/ijerph19116508
Chicago/Turabian StyleZhu, Si-Meng, Cheng Li, Jing-Jing Xu, Han-Qiu Zhang, Yun-Fei Su, Yan-Ting Wu, and He-Feng Huang. 2022. "Exposure to Chloramine and Chloroform in Tap Water and Adverse Perinatal Outcomes in Shanghai" International Journal of Environmental Research and Public Health 19, no. 11: 6508. https://doi.org/10.3390/ijerph19116508
APA StyleZhu, S.-M., Li, C., Xu, J.-J., Zhang, H.-Q., Su, Y.-F., Wu, Y.-T., & Huang, H.-F. (2022). Exposure to Chloramine and Chloroform in Tap Water and Adverse Perinatal Outcomes in Shanghai. International Journal of Environmental Research and Public Health, 19(11), 6508. https://doi.org/10.3390/ijerph19116508







