Rural–Urban Differences in Non-Local Primary Care Utilization among People with Osteoarthritis: The Role of Area-Level Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prevalent OA Cohort in 2013
2.2. Standard Geographic Areas
2.3. Non-Local PCP Utilization at the LGA Level
2.4. Independent Variables at LGA Level
2.4.1. Predisposing Factors
2.4.2. Enabling Factors
2.4.3. Need Factors
2.5. Statistical Analysis
2.5.1. Exploratory Data Analysis
2.5.2. Multivariate Linear Regression
2.5.3. Geographically Weighted Regression
3. Results
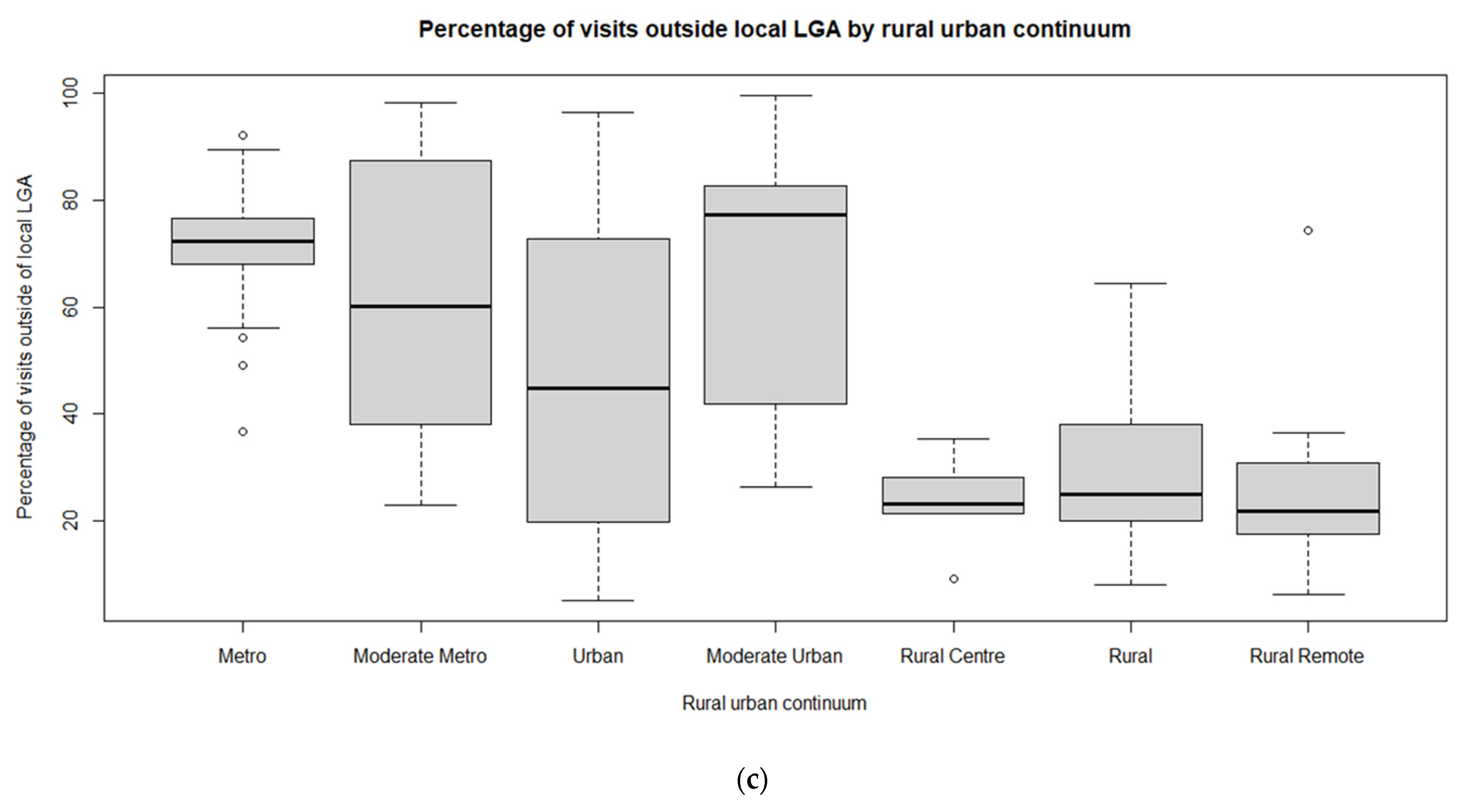
3.1. Exploratory Spatial Data Analysis
3.2. Multivariate Linear Regression Models
3.3. Geographically Weighted Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Mitura, V.; Bollman, R.D.; Canada, S. The Health of Rural Canadians: A Rural-Urban Comparison of Health Indicators. 2003, Volume 4. Available online: http://www.statcan.ca/cgi-bin/downpub/freepub.cgi (accessed on 19 June 2019).
- Marshall, D.A.; Liu, X.; Shahid, R.; Bertazzon, S.; Seidel, J.E.; Patel, A.B.; Nasr, M.; Barber, C.E.H.; McDonald, T.; Sharma, R.; et al. Geographic variation in osteoarthritis prevalence in Alberta: A spatial analysis approach. Appl. Geogr. 2019, 103, 112–121. [Google Scholar] [CrossRef]
- Liu, X.; Shahid, R.; Patel, A.B.; McDonald, T.; Bertazzon, S.; Waters, N.; Seidel, J.E.; Marshall, D.A. Geospatial patterns of comorbidity prevalence among people with osteoarthritis in Alberta Canada. BMC Public Health 2020, 20, 1551. [Google Scholar] [CrossRef]
- Leite, A.A.; Costa, A.J.G.; de Lima, B.d.A.M.; Padilha, A.V.L.; de Albuquerque, E.C.; Marques, C.D.L. Comorbidities in patients with osteoarthritis: Frequency and impact on pain and physical function. Rev. Bras. Reumatol. 2011, 51, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Birtwhistle, R.; Morkem, R.; Peat, G.; Williamson, T.; Green, M.E.; Khan, S.; Jordan, K.P. Prevalence and management of osteoarthritis in primary care: An epidemiologic cohort study from the Canadian Primary Care Sentinel Surveillance Network. CMAJ Open 2015, 3, E270–E275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopec, J.A.; Rahman, M.M.; Berthelot, J.-M.; Le Petit, C.; Aghajanian, J.; Sayre, E.C.; Cibere, J.; Anis, A.H.; Badley, E.M. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J. Rheumatol. 2007, 34, 386–393. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17183616 (accessed on 3 April 2018).
- Marshall, D.A.; Vanderby, S.; Barnabe, C.; MacDonald, K.V.; Maxwell, C.; Mosher, D.; Wasylak, T.; Lix, L.; Enns, E.; Frank, C.; et al. Estimating the Burden of Osteoarthritis to Plan for the Future. Arthritis Care Res. 2015, 67, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Hart, D.A.; Werle, J.; Robert, J.; Kania-Richmond, A. Long wait times for knee and hip total joint replacement in Canada: An isolated health system problem, or a symptom of a larger problem? Osteoarthr. Cartil. Open 2021, 3, 100141. [Google Scholar] [CrossRef]
- Aggarwal, M.; Hutchison, B. Towards a Primary Care Strategy for Canada; Canada Canadian Foundation for Healthcare Improvement: Ottawa, ON, Canada, 2012. Available online: http://www.cfhi-fcass.ca (accessed on 10 March 2020).
- Starfield, B.; Shi, L.; Macinko, J. Contribution of primary care to health systems and health. Milbank Q. 2005, 83, 457–502. [Google Scholar] [CrossRef]
- Alberta Health. Alberta Health Primary Health Care—Community Profiles Community Profile: Cold Lake Health Data and Summary; Government of Alberta: Edmonton, AB, Canada, 2015.
- Pong, R.W.; DesMeules, M.; Heng, D.; Lagacé, C.; Guernsey, J.R.; Kazanjian, A.; Manuel, D.; Pitblado, J.R.; Bollman, R.; Koren, I.; et al. Patterns of health services utilization in rural Canada. Chronic Dis. Inj. Can. 2011, 31 (Suppl. 1), 1–36. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22047772 (accessed on 11 April 2018). [CrossRef]
- Wilson, M.M.; Devasahayam, A.J.; Pollock, N.J.; Dubrowski, A.; Renouf, T. Rural family physician perspectives on communication with urban specialists: A qualitative study. BMJ Open 2021, 11, e043470. [Google Scholar] [CrossRef]
- Andersen, R.M. Revisiting the Behavioral Model and Access to Medical Care: Does It Matter? J. Health Soc. Behav. 1995, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barnabe, C.; Hemmelgarn, B.; Jones, C.A.; Peschken, C.A.; Voaklander, D.; Joseph, L.; Bernatsky, S.; Esdaile, J.M.; Marshall, D.A. Imbalance of prevalence and specialty care for osteoarthritis for First Nations people in Alberta, Canada. J. Rheumatol. 2015, 42, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lagace, C.; O’Donnell, S.; McRae, L.; Badley, E.; MacKay, C. Life with Arthritis in Canada: A Personal and Public Health Challenge; Public Health Agency of Canada: Ottawa, ON, Ontario, 2010.
- Aday, L.A.; Anderson, R. A framework for the study of access to medical care. Health Serv. Res. Res. 1974, 9, 208–220. [Google Scholar] [CrossRef]
- Khan, A.A.; Bhardwaj, S.M. Access to health care: A conceptual framework and its relevance to health care planning. Eval. Health Prof. 1994, 17, 60–67. [Google Scholar] [CrossRef]
- Khan, A.A. An Integrated Approach to Measuring Potential Spatial Access to Health Care Services. Plann. Sci 1992, 26, 215–281. [Google Scholar] [CrossRef]
- Guagliardo, M.F. Spatial accessibility of primary care: Concepts, methods and challenges. Int. J. Health Geogr. 2004, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Mudd, A.E.; Michael, Y.L.; Diez-Roux, A.V.; Maltenfort, M.; Moore, K.; Melly, S.; Lê-Scherban, F.; Forrest, C.B. Primary Care Accessibility Effects on Health Care Utilization Among Urban Children. Acad. Pediatr. 2020, 20, 871–878. [Google Scholar] [CrossRef]
- Fishman, J.; McLafferty, S.; Galanter, W. Does Spatial Access to Primary Care Affect Emergency Department Utilization for Nonemergent Conditions? Health Serv. Res. 2018, 53, 489–508. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Hulme, C.; Farragher, T.; Clarke, G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open 2016, 6, e013059. [Google Scholar] [CrossRef]
- Reyes, C.; Garcia-Gil, M.; Elorza, J.M.; Mendez-Boo, L.; Hermosilla, E.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K.; Bolibar, B.; et al. Socio-economic status and the risk of developing hand, hip or knee osteoarthritis: A region-wide ecological study. Osteoarthr. Cartil. 2015, 23, 1323–1329. [Google Scholar] [CrossRef] [Green Version]
- Cañizares, M.; Power, J.D.; Perruccio, A.V.; Badley, E.M. Association of regional racial/cultural context and socioeconomic status with arthritis in the population: A multilevel analysis. Arthritis Care Res. 2008, 59, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.M.; Sauerzapf, V.; Haynes, R.; Zhao, H.; Forman, D.; Jones, A.P. Social and geographical factors affecting access to treatment of lung cancer. Br. J. Cancer 2009, 101, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Dejardin, O.; Jones, A.P.; Rachet, B.; Morris, E.; Bouvier, V.; Jooste, V.; Coombes, E.; Forman, D.; Bouvier, A.M.; Launoy, G. The influence of geographical access to health care and material deprivation on colorectal cancer survival: Evidence from France and England. Health Place 2014, 30, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-C.; Shoff, C.; Noah, A.J. Spatializing health research: What we know and where we are heading. Geospat. Health 2013, 7, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, M. Health geography II. Prog. Hum. Geogr. 2016, 40, 546–554. [Google Scholar] [CrossRef]
- Photis, Y.N. Disease and Health Care Geographies: Mapping Trends and Patterns in a GIS. Health Sci. J. 2016, 10, 1–8. Available online: http://journals.imedpub.com (accessed on 11 April 2018).
- Alberta Aboriginal Relations. Aboriginal Peoples of Alberta: Yesterday, Today and Tomorrow; Government of Alberta: Edmonton, AB, Canada, 2013; ISBN 978-1-4601-13080.
- Canadian Medical Association. CMA Position Statement: Ensuring Equitable Access to Care: Strategies for Governments, Health System Planners, and the Medical Profession; Canadian Medical Association: Ottawa, ON, Canada, 2013.
- Liu, X.; Seidel, J.E.; McDonald, T.; Waters, N.; Patel, A.B.; Bertazzon, S.; Shahid, R.; Marshall, D.A. Rural-urban disparities in realized access to general practitioners, orthopedic surgeons, and physiotherapists among people with osteoarthritis. Appl. Geogr. 2022, submitted.
- Widdifield, J.; Jaakkimainen, R.L.; Gatley, J.M.; Hawker, G.A.; Lix, L.M.; Bernatsky, S.; Ravi, B.; Wasserstein, D.; Yu, B.; Tu, K. Validation of canadian health administrative data algorithms for estimating trends in the incidence and prevalence of osteoarthritis. Osteoarthr. Cartil. Open 2020, 2, 100115. [Google Scholar] [CrossRef]
- Alberta Health Services and Alberta Health. Official Standard Geographic Areas. 2017. Available online: https://open.alberta.ca/dataset/a14b50c9-94b2-4024-8ee5-c13fb70abb4a/resource/70fd0f2c-5a7c-45a3-bdaa-e1b4f4c5d9a4/download/Official-Standard-Geographic-Area-Document.pdf (accessed on 27 May 2018).
- Alberta Health. Postal Code Translator File (PCTF); Alberta Health Services: Edmonton, AB, Canada, 2013.
- Alberta Health Servcies Applied Research and Evaluation Services Primary Health Care. Alberta Facilities Distance/Time Look Up Table; Alberta Health Services: Edmonton, AB, Canada, 2016; pp. 1–12.
- Hodgson, K.; Deeny, S.R.; Steventon, A. Ambulatory care-sensitive conditions: Their potential uses and limitations. BMJ Qual. Saf. 2019, 28, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Sibley, L.M.; Weiner, J.P. An evaluation of access to health care services along the rural-urban continuum in Canada. BMC Health Serv. Res. 2011, 11, 20. [Google Scholar] [CrossRef]
- Clark, K.; John, P.S.; Menec, V.; Cloutier, D.; Newall, N.; O’Connell, M.; Tate, R. Healthcare utilisation among Canadian adults in rural and urban areas—The Canadian Longitudinal Study on Aging. Can. J. Rural Med. 2021, 26, 69. [Google Scholar] [CrossRef]
- Cliff, A.D.; Ord, J.K. Spatial Autocorrelation; Pion: London, UK, 1973. [Google Scholar]
- Ord, J.K.; Getis, A. Testing for Local Spatial Autocorrelation in the Presence of Global Autocorrelation. J. Reg. Sci. 2001, 41, 411–432. [Google Scholar] [CrossRef]
- Anselin, L. Local Indicators of Spatial Association-LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Seber, G.A.; Lee, A.J. Linear Regression Analysis; John Wiley & Sone: Hoboken, NJ, USA, 2003; ISBN 0-471-41540-5. [Google Scholar]
- Christensen, R. Plane Answers to Complex Questions. The Theory of Linear Models, 4th ed.; Springer-Verlag: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Bertazzon, S.; Hara, P.D.O.; Barrett, O.; Serra-sogas, N. Geospatial analysis of oil discharges observed by the National Aerial Surveillance Program in the Canadian Paci fi c Ocean. Appl. Geogr. 2014, 52, 78–89. [Google Scholar] [CrossRef]
- Anselin, L. Lagrange Multiplier Test Diagnostics for Spatial Dependence and Spatial Heterogeneity. Geogr. Anal. 1988, 20, 1–17. [Google Scholar] [CrossRef]
- Chi, G.; Zhu, J. Spatial regression models for demographic analysis. Popul. Res. Policy Rev. 2008, 27, 17–42. [Google Scholar] [CrossRef]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; Wiley: Hoboken, NJ, USA, 2002; Available online: https://books.google.ca/books?id=9DZgV1vXOuMC&pg=PA256&lpg=PA256&dq=Some+notes+on+parametric+significance+tests+for+geographically+weighted+regression%27&source=bl&ots=65zJPdwfDJ&sig=u45BiX7wML7V4c4XxyySwGMK6RM&hl=en&sa=X&ved=0ahUKEwj6zdvh6qXXAhUB02MKHXzU (accessed on 4 November 2017).
- Fotheringham, A.S.; Charlton, M.E.; Brunsdon, C. Geographically weighted regression: A natural evolution of the expansion method for spatial data analysis. Environ. Plan. A 1998, 30, 1905–1927. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.137.4544&rep=rep1&type=pdf (accessed on 4 November 2017). [CrossRef]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M. Spatial nonstationarity and autoregressive models. Environ. Plan. A 1998, 30, 957–973. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.455.8881&rep=rep1&type=pdf (accessed on 4 November 2017). [CrossRef]
- Waters, N. Motivations and Methods for Replication in Geography: Working with Data Streams. Ann. Am. Assoc. Geogr. 2021, 111, 1291–1299. [Google Scholar] [CrossRef]
- Anselin, L. Spatial Regression. In The SAGE Handbook of Spatial Analysis; SAGE Publications, Ltd.: New York, NY, USA, 2009; pp. 254–275. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification BT—Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 215–222. [Google Scholar] [CrossRef]
- Ford, M.M.; Highfield, L.D. Exploring the Spatial Association between Social Deprivation and Cardiovascular Disease Mortality at the Neighborhood Level. PLoS ONE 2016, 11, e0146085. [Google Scholar] [CrossRef] [Green Version]
- Marshall, D.; Liu, X.; Bertazzon, S.; Patel, A.; Mosher, D.; Homik, J.; Kata, S.; Smith, C.; Robert, J.; Barber, C. Geographic Variation in the Prevalence of Rheumatoid Arthritis in Alberta: A Spatial Analysis. In Proceedings of the Canadian Rheumatology Association Annual Scientific Meeting, Victoria, BC, Canada, 26–29 February 2020. [Google Scholar]
- Primary Care Access to Orthopaedics. Available online: https://www.specialistlink.ca/clinical-pathways-and-specialty-access (accessed on 18 May 2022).
- Cleveland, R.J.; Schwartz, T.A.; Prizer, L.P.; Randolph, R.; Schoster, B.; Renner, J.B.; Jordan, J.M.; Callahan, L.F. Associations of educational attainment, occupation, and community poverty with hip osteoarthritis. Arthritis Care Res. 2013, 65, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, L.F.; Cleveland, R.J.; Shreffler, J.; Schwartz, T.A.; Schoster, B.; Randolph, R.; Renner, J.B.; Jordan, J.M. Associations of educational attainment, occupation and community poverty with knee osteoarthritis in the Johnston County (North Carolina) osteoarthritis project. Arthritis Res. Ther. 2011, 13, R169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleveland, R.J.; Luong, M.-L.N.; Knight, J.B.; Schoster, B.; Renner, J.B.; Jordan, J.M.; Callahan, L.F. Independent associations of socioeconomic factors with disability and pain in adults with knee osteoarthritis. BMC Musculoskelet. Disord. 2013, 14, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, N.; Ben-Shlomo, Y. From the surgery to the surgeon: Does deprivation influence consultation and operation rates? Br. J. Gen. Pract. 1995, 45, 127–131. [Google Scholar]
- Brennan, S.L.; Lane, S.E.; Lorimer, M.; Buchbinder, R.; Wluka, A.E.; Page, R.S.; Osborne, R.H.; Pasco, J.A.; Sanders, K.M.; Cashman, K.; et al. Associations between socioeconomic status and primary total knee joint replacements performed for osteoarthritis across Australia 2003–10: Data from the Australian Orthopaedic Association National Joint Replacement Registry. BMC Musculoskelet. Disord. 2014, 15, 356. [Google Scholar] [CrossRef] [Green Version]
- Lemstra, M.; Mackenbach, J.; Neudorf, C.; Nannapaneni, U. High health care utilization and costs associated with lower socio-economic status: Results from a linked dataset. Can. J. Public Health 2009, 100, 180–183. [Google Scholar] [CrossRef]
- Benefits of a Patient’s Medical Home. Available online: http://www.topalbertadoctors.org/file/top--evidence-summary--benefits-of-pmh.pdf (accessed on 19 June 2019).
- Bahler, B.; Aasman, E.; Bhella, V.; Hilner, J.; Lee, S.; Myhr, S.; Potter, T.; La, R.J.; Warren, V. The Integrated Health Neighbourhood of the Future: White Paper on Transforming Primary and Community-Based Care; Primary Care Alliance: Mt Dora, FL, USA, 2020. [Google Scholar]
- McCauley, L.; Phillips, R.L.; Meisnere, M.; Robinson, S.K. Implementing High-Quality Primary Care: Rebuilding the Foundation of Health Care. In Implementing High-Quality Primary Care; The National Academies Press: Washington, DC, USA, 2021; pp. 1–428. [Google Scholar] [CrossRef]
- Swift, A.; Liu, L.; Uber, J. Reducing MAUP bias of correlation statistics between water quality and GI illness. Comput. Environ. Urban Syst. 2008, 32, 134–148. [Google Scholar] [CrossRef]








| Variables | Definition | Sources | |
|---|---|---|---|
| Response variable | Non-local PCP utilization | Percentage of PCP visits outside patients’ local LGA (outside visits/total visits of LGA*100) | Alberta Health (AH) Physician Claims |
| Predisposing factors | MedAge2013 | Median age of OA patients visiting PCP in 2012/2013 | AH Population Registry and AH Physician Claims |
| Per65Lone | Percentage of 65 years of age and older who live alone | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| PerFemale | Percentage of female patients among total OA patients visiting PCP (number of females/total patients visiting PCP*100) | AH Population Registry and AH Physician Claims | |
| PerLoneFemale | Percentage of female lone-parent families | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| PerImmig | Percentage of immigrants who arrived in the last five years | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| PerAborig | Percentage of First Nations with treaty status and Inuit | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| Enabling factors | MedTime | Median travel time of PCP visits | AH Population Registry and AH Physician Claims |
| ACSC | Ambulatory care sensitive conditions, age-standardized separation rate per 100,000 population | Alberta Health Primary Health Care—Community Profiles 2013. Data source is ambulatory care data. | |
| DoC | Discontinuity of care index. Percentage of patients that have chronic conditions but do not have a PCP visits within a three-year timeframe over the total general population | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Physician Claims and Alberta Health Population Registry. | |
| PerUniDeg | Percentage of population with university certificate, diploma, or degree | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| PerLICO | Percentage of families with after-tax low-income | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| AvgFIncome | Average census family income | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Statistics Canada Federal Census and Alberta Health Population Registry. | |
| Rurban_2cat | Broad rural vs. broad urban | Alberta Standard Geographic Areas | |
| Needs | CruRateOA | Crude rate of people with OA among general population (Registry) (per 100 population) | AH Population Registry and AH Physician Claims |
| CmbOver3Rate | Age-standardized rate of people with three and more comorbidities among general population (per 100 population) | Alberta Health Primary Health Care—Community Profiles 2013. Data sources are Population Registry and Physician Claims. | |
| Non-Local PCP | MedTime | MedAge2013 | PerFemale | PerAborig | PerLoneFemale | Per65Lone | PerLICO | AvgFIncome | PerImmig | PerUniDeg | ACSC | CmbOver3Rate | DoC | CruRateOA | Rurban_2cat | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-local PCP | 1 | 0.55 | −0.10 | 0.12 | −0.15 | 0.13 | −0.14 | 0.26 | 0.30 | 0.38 | 0.46 | −0.44 | −0.23 | −0.36 | −0.29 | 0.70 |
| MedTime | 0.55 | 1 | −0.15 | −0.19 | 0.06 | −0.19 | −0.12 | −0.16 | 0.17 | −0.04 | 0.10 | −0.15 | −0.08 | −0.10 | 0.06 | 0.16 |
| MedAge2013 | −0.10 | −0.15 | 1 | 0.40 | −0.45 | −0.16 | 0.32 | 0.11 | −0.14 | −0.02 | 0.15 | −0.20 | −0.25 | 0.06 | 0.30 | −0.09 |
| PerFemale | 0.12 | −0.19 | 0.40 | 1 | −0.16 | 0.24 | −0.05 | 0.37 | 0.05 | 0.39 | 0.28 | −0.11 | −0.05 | −0.19 | −0.15 | 0.24 |
| PerAborig | −0.15 | 0.06 | −0.45 | −0.16 | 1 | 0.37 | −0.14 | −0.03 | −0.24 | −0.22 | −0.22 | 0.70 | 0.60 | 0.20 | 0.19 | −0.30 |
| PerLoneFemale | 0.13 | −0.19 | −0.16 | 0.24 | 0.37 | 1 | 0.04 | 0.60 | −0.29 | 0.24 | 0.05 | 0.26 | 0.45 | −0.01 | −0.08 | 0.19 |
| Per65Lone | −0.14 | −0.12 | 0.32 | −0.05 | −0.14 | 0.04 | 1 | 0.05 | −0.19 | 0.02 | 0.08 | −0.02 | −0.08 | 0.12 | 0.16 | −0.08 |
| PerLICO | 0.26 | −0.16 | 0.11 | 0.37 | −0.03 | 0.60 | 0.05 | 1 | −0.27 | 0.52 | 0.17 | −0.01 | 0.19 | −0.05 | −0.10 | 0.27 |
| AvgFIncome | 0.30 | 0.17 | −0.14 | 0.05 | −0.24 | −0.29 | −0.19 | −0.27 | 1 | 0.19 | 0.63 | −0.46 | −0.48 | −0.41 | −0.54 | 0.44 |
| PerImmig | 0.38 | −0.04 | −0.02 | 0.39 | −0.22 | 0.24 | 0.02 | 0.52 | 0.19 | 1 | 0.59 | −0.39 | −0.26 | −0.33 | −0.45 | 0.49 |
| PerUniDeg | 0.46 | 0.10 | 0.15 | 0.28 | −0.22 | 0.05 | 0.08 | 0.17 | 0.63 | 0.59 | 1 | −0.56 | −0.56 | −0.41 | −0.44 | 0.55 |
| ACSC | −0.44 | −0.15 | −0.20 | −0.11 | 0.70 | 0.26 | −0.02 | −0.01 | −0.46 | −0.39 | −0.56 | 1 | 0.79 | 0.35 | 0.39 | −0.61 |
| CmbOver3Rate | −0.23 | −0.08 | −0.25 | −0.05 | 0.60 | 0.45 | −0.08 | 0.19 | −0.48 | −0.26 | −0.56 | 0.79 | 1 | 0.38 | 0.33 | −0.35 |
| DoC | −0.36 | −0.10 | 0.06 | −0.19 | 0.20 | −0.01 | 0.12 | −0.05 | −0.41 | −0.33 | −0.41 | 0.35 | 0.38 | 1 | 0.33 | −0.53 |
| CruRateOA | −0.29 | 0.06 | 0.30 | −0.15 | 0.19 | −0.08 | 0.16 | −0.10 | −0.54 | −0.45 | −0.44 | 0.39 | 0.33 | 0.33 | 1 | −0.43 |
| Rurban_2cat | 0.70 | 0.16 | −0.09 | 0.24 | −0.30 | 0.19 | −0.08 | 0.27 | 0.44 | 0.49 | 0.55 | −0.61 | −0.35 | −0.53 | −0.43 | 1 |
| Variables | Linear Regression (Alberta, 132 LGAs) | Linear Regression (Broad Rural, 71 LGAs) | Linear Regression (Broad Urban, 61 LGAs) | GWR Model | Global Linear Regression (No Rural–Urban) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | t Value | Beta | t Value | Beta | t Value | Beta | t Value | Beta | t Value | ||
| Predisposing factors | MedTime | 1.15 | 10.21 | 0.90 | 13.17 | 2.20 | 7.02 | 1.23 | 8.15 | 1.24 | 9.30 |
| ACSC | −0.02 | −3.45 | −0.02 | −3.02 | |||||||
| DoC | −1.14 | −2.59 | −0.93 | −2.35 | |||||||
| PerLICO | 1.40 | 3.79 | 1.01 | 3.07 | 2.40 | 3.95 | 2.19 | 4.74 | 2.28 | 5.28 | |
| PerUniDeg | 0.27 | 1.62 | 0.39 | 2.48 | |||||||
| Rurban_2cat (Urban) | 29.46 | 11.46 | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Model diagnostic | R2 | 0.72 | 0.72 | 0.47 | 0.63 | 0.60 | |||||
| Adj. R2 | 0.71 | 0.72 | 0.45 | 0.59 | |||||||
| AIC | 1066.27 | 487.09 | 522.72 | 1110.03 | 1115.30 | ||||||
| Res.SE | 13.85 | 7.58 | 16.87 | 16.41 | 16.58 | ||||||
| Shapiro–Wilk test | 0.97, p = 0.006 | 0.98, p = 0.68 | 0.99, p = 0.99 | 0.98, p= 0.15 | 0.98, p = 0.052 | ||||||
| Breusch–Pagan test | 24.63, df = 3, p = 0 | 3.02, df = 2, p = 0.22 | 3.33, df = 2, p = 0.19 | N/A | 15.95, df = 5, p = 0.007 | ||||||
| Moran’s I test | −0.03, p = 0.69 | N/A | N/A | 0.02, p = 0.56 | −0.007, p = 0.45 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Seidel, J.E.; McDonald, T.; Waters, N.; Patel, A.B.; Shahid, R.; Bertazzon, S.; Marshall, D.A. Rural–Urban Differences in Non-Local Primary Care Utilization among People with Osteoarthritis: The Role of Area-Level Factors. Int. J. Environ. Res. Public Health 2022, 19, 6392. https://doi.org/10.3390/ijerph19116392
Liu X, Seidel JE, McDonald T, Waters N, Patel AB, Shahid R, Bertazzon S, Marshall DA. Rural–Urban Differences in Non-Local Primary Care Utilization among People with Osteoarthritis: The Role of Area-Level Factors. International Journal of Environmental Research and Public Health. 2022; 19(11):6392. https://doi.org/10.3390/ijerph19116392
Chicago/Turabian StyleLiu, Xiaoxiao, Judy E. Seidel, Terrence McDonald, Nigel Waters, Alka B. Patel, Rizwan Shahid, Stefania Bertazzon, and Deborah A. Marshall. 2022. "Rural–Urban Differences in Non-Local Primary Care Utilization among People with Osteoarthritis: The Role of Area-Level Factors" International Journal of Environmental Research and Public Health 19, no. 11: 6392. https://doi.org/10.3390/ijerph19116392
APA StyleLiu, X., Seidel, J. E., McDonald, T., Waters, N., Patel, A. B., Shahid, R., Bertazzon, S., & Marshall, D. A. (2022). Rural–Urban Differences in Non-Local Primary Care Utilization among People with Osteoarthritis: The Role of Area-Level Factors. International Journal of Environmental Research and Public Health, 19(11), 6392. https://doi.org/10.3390/ijerph19116392






