Acute, Sublethal, and Developmental Toxicity of Kratom (Mitragyna speciosa Korth.) Leaf Preparations on Caenorhabditis elegans as an Invertebrate Model for Human Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Kratom Powder
2.3. Analysis of Kratom Extracts
2.4. C. elegans Strain and Culture Conditions
2.5. Preparation of Kratom Extracts
2.6. Analysis of Developmental and Reproductive Toxicity in C. elegans
2.7. Thrashing Analysis
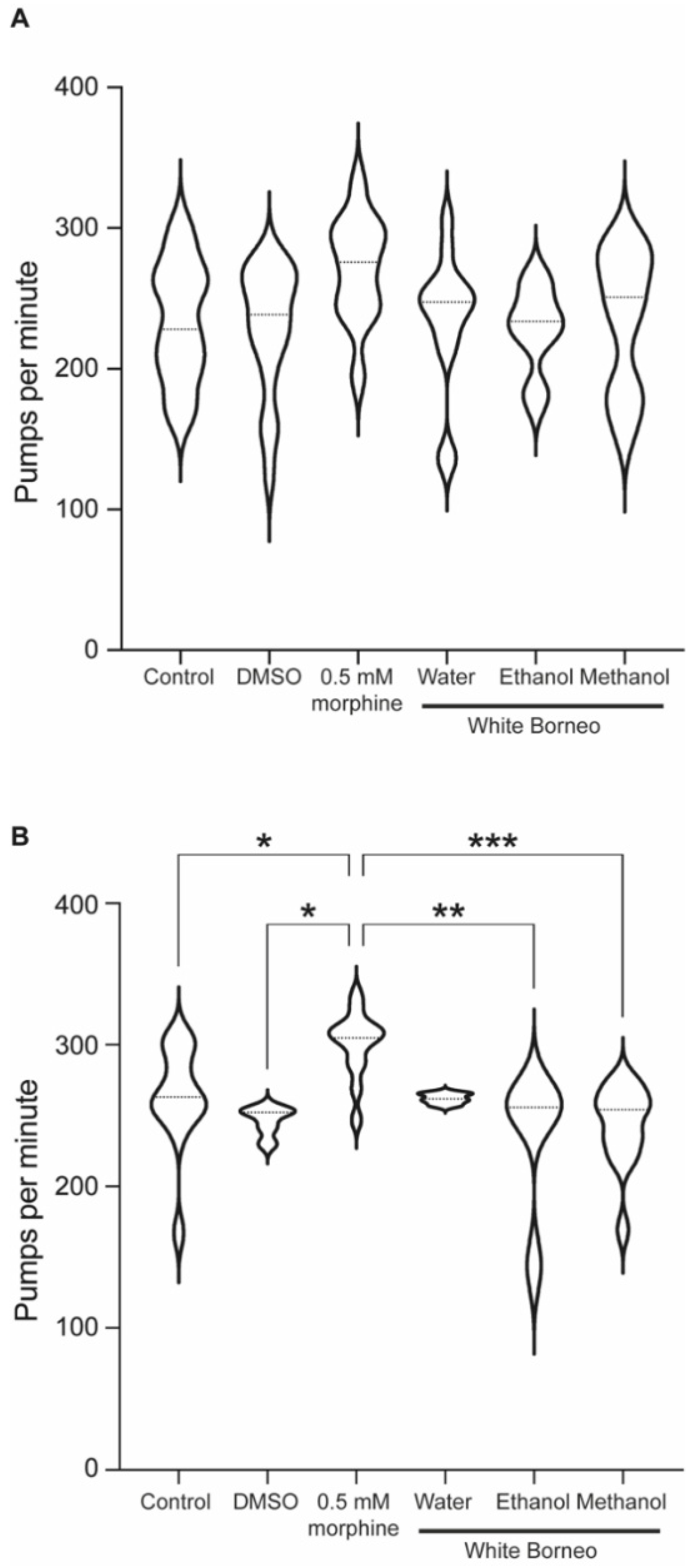
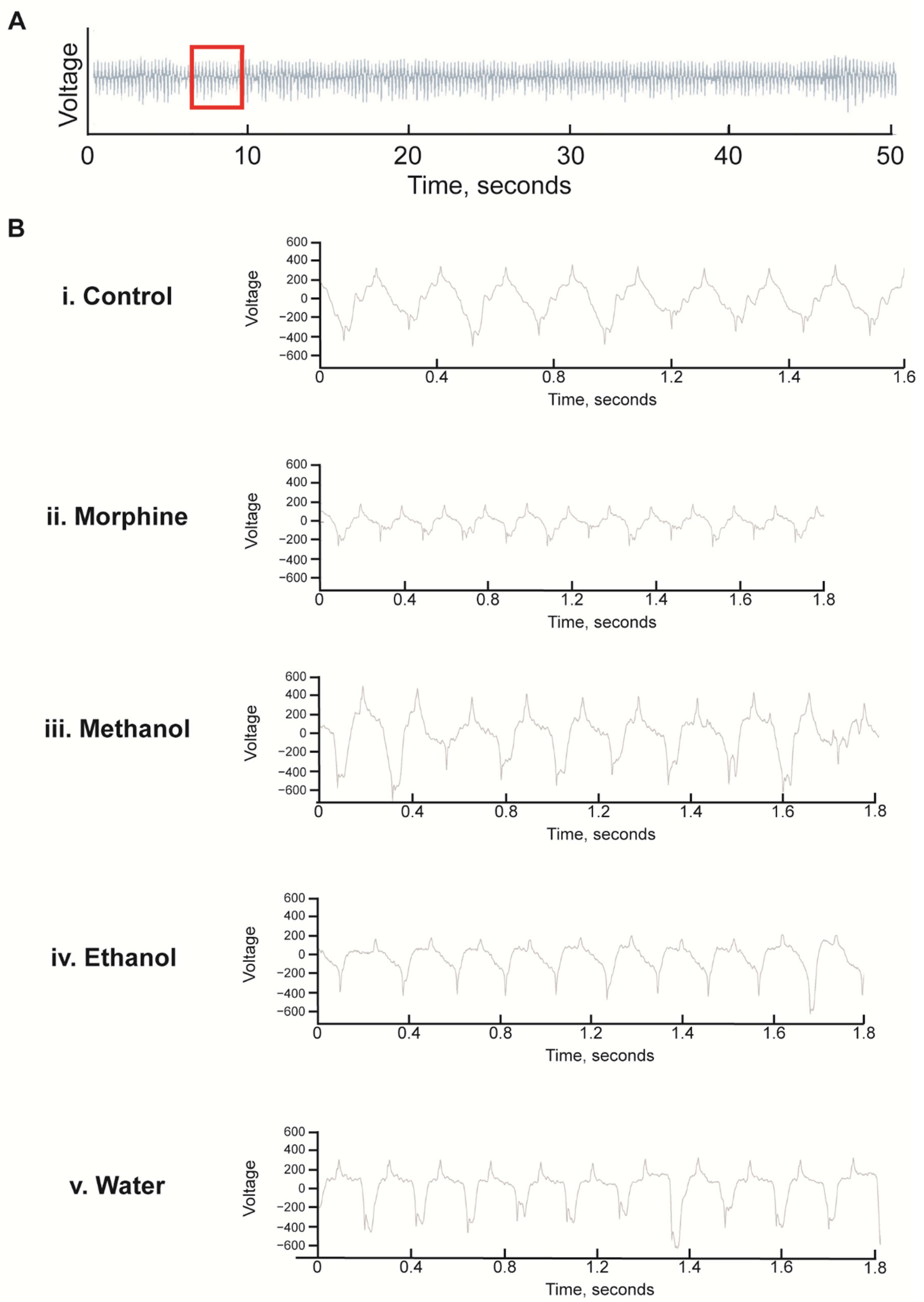
2.8. Pharyngeal Pumping
2.9. Data and Statistical Analysis
3. Results
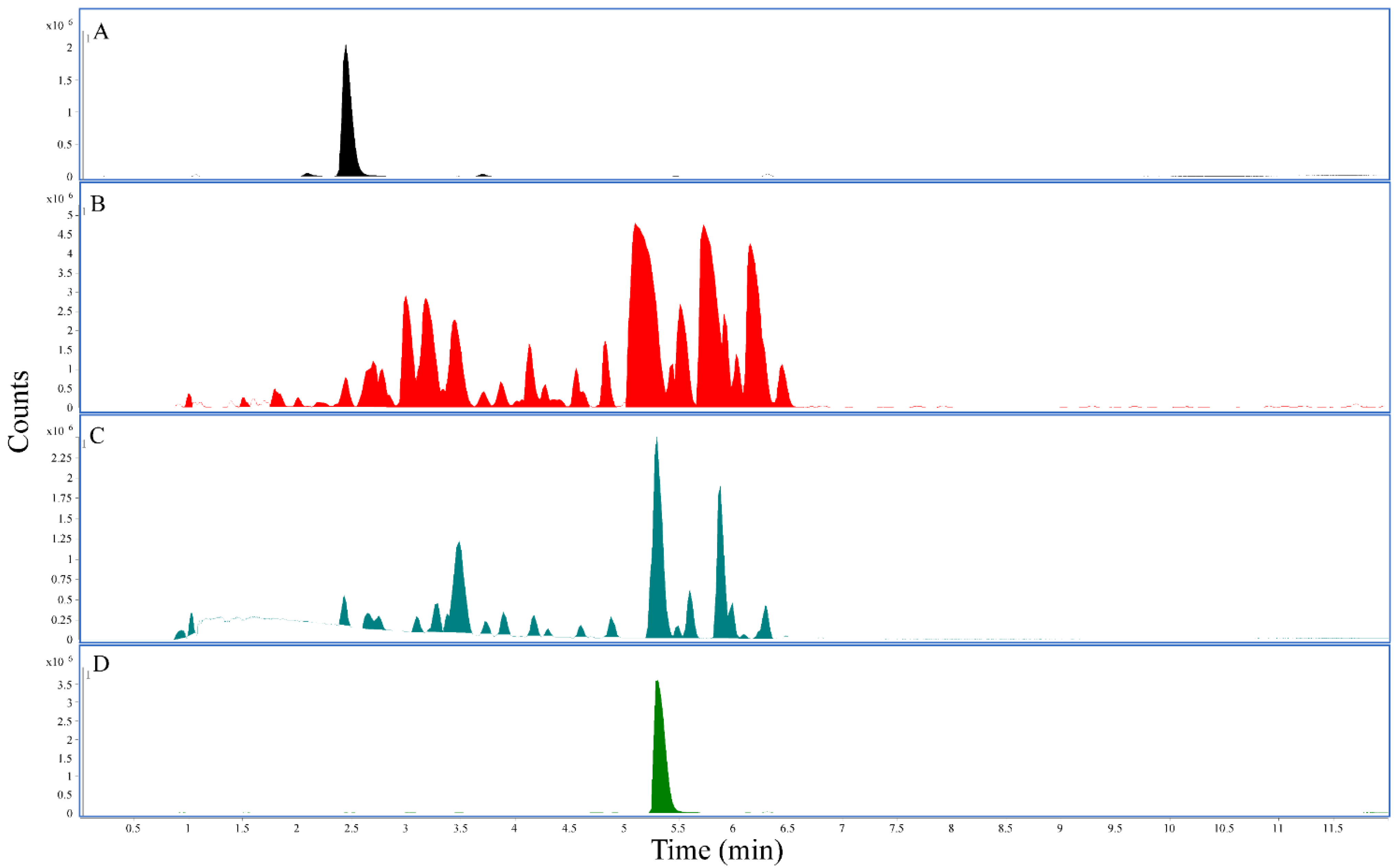
3.1. Mitragynine and 7-Hydroxymitragynine Content in Kratom Samples Depended on Extraction Solvent
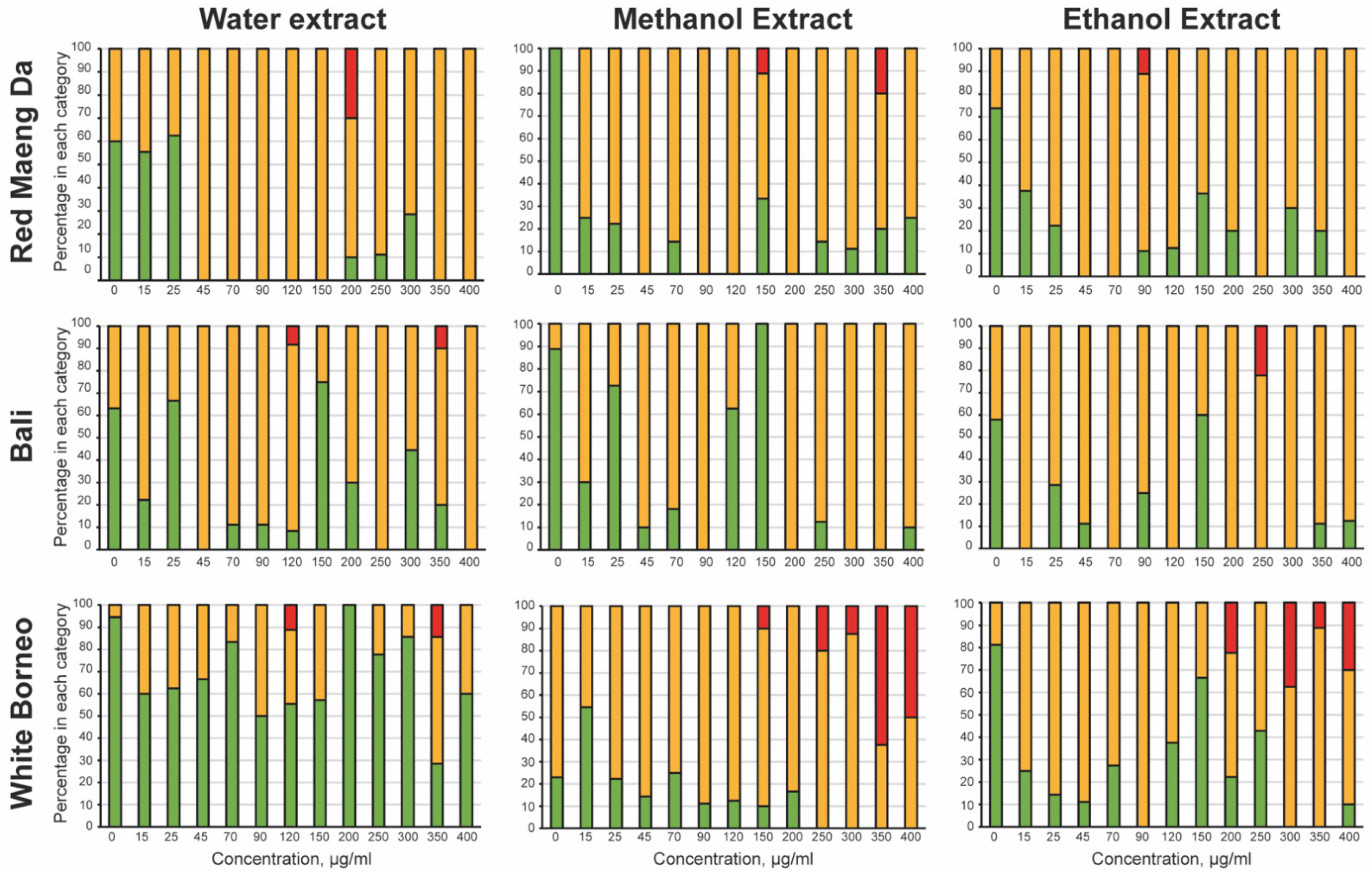
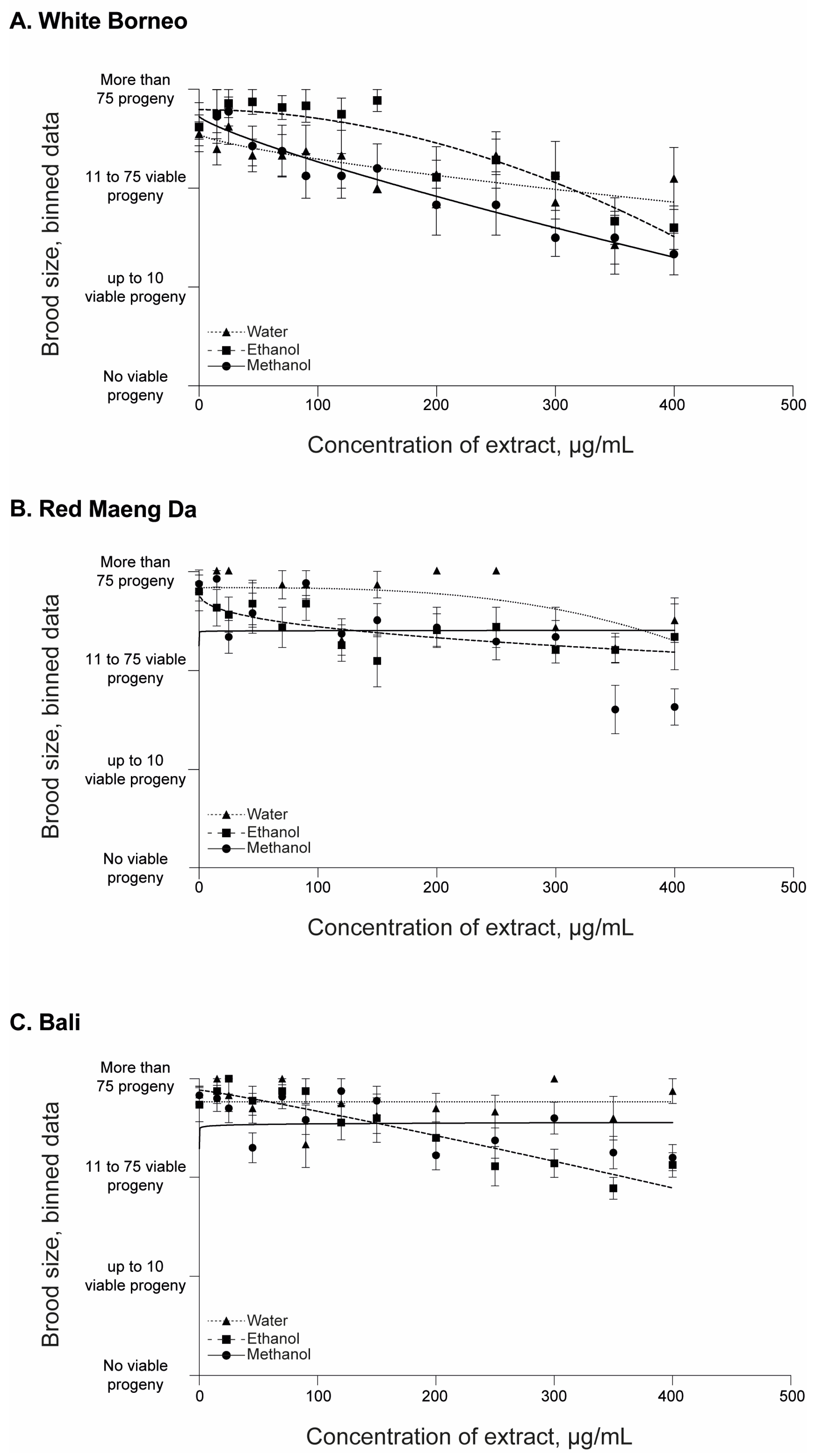
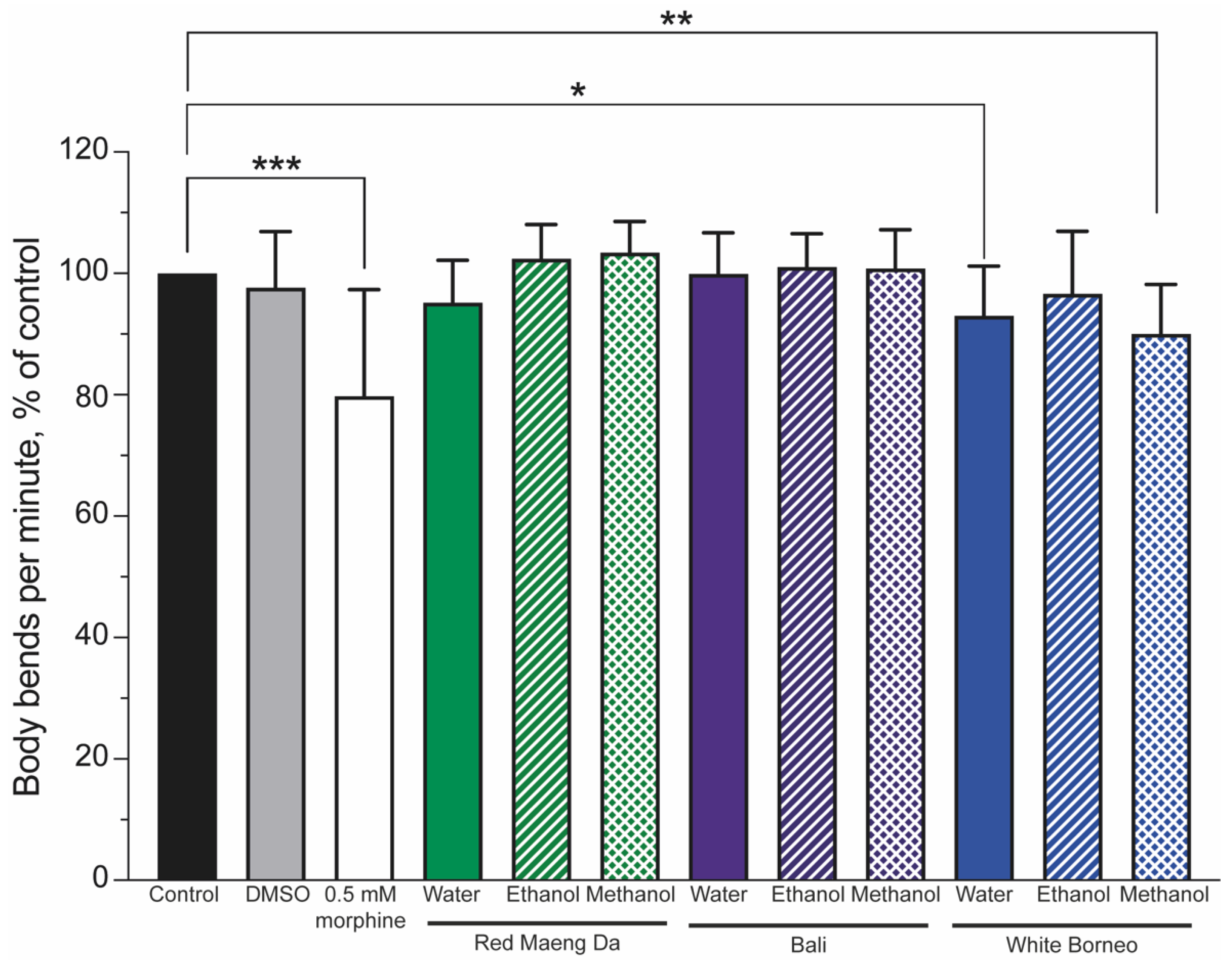
3.2. Kratom Dose-Dependently Reduced Brood Size and Health of Parents and Progeny
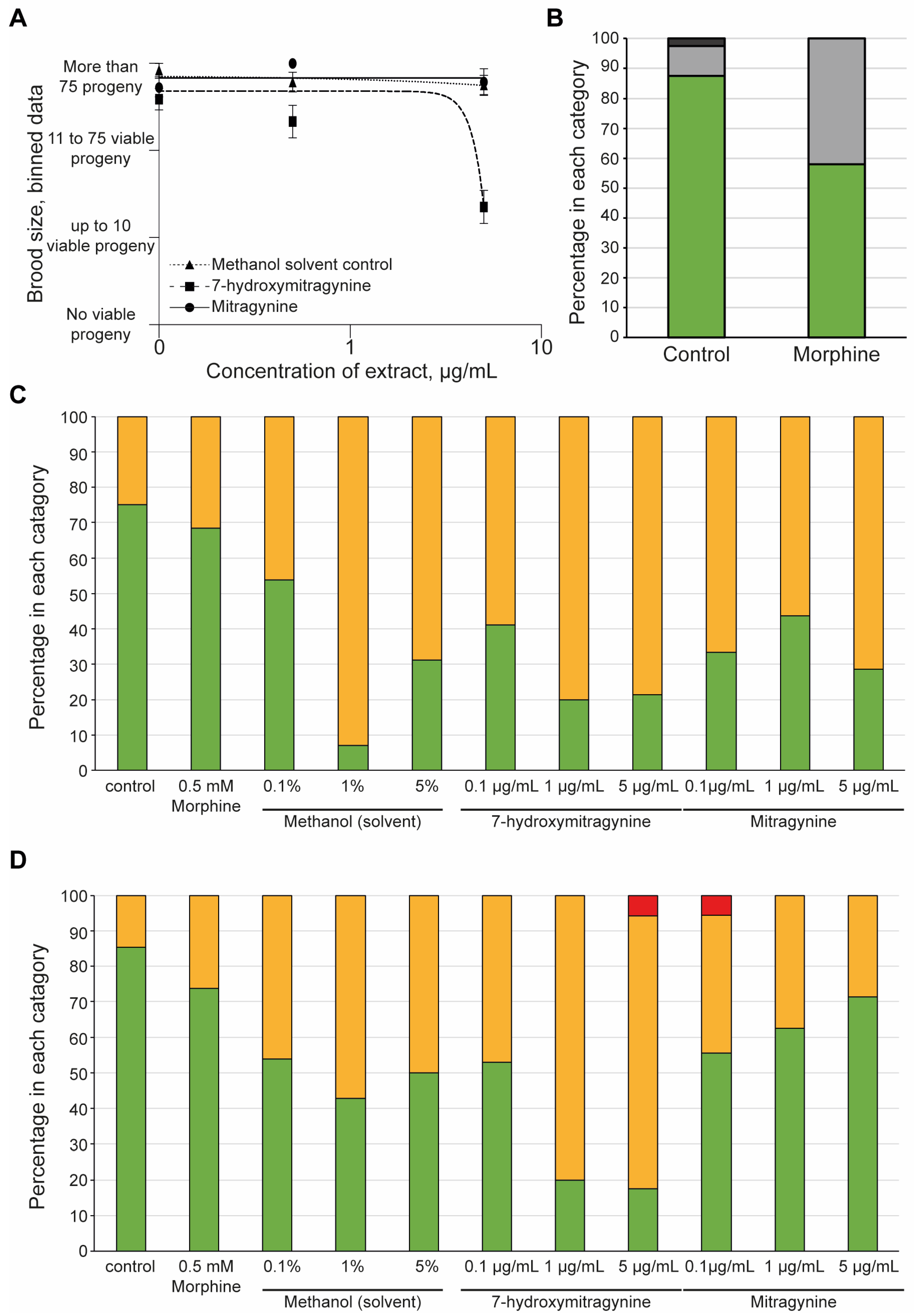
3.3. 7-Hydroxymitragynine Did Present with Toxic and Developmental Effects
3.4. Morphine but Not Kratom Displayed Toxic Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veltri, C.; Grundmann, O. Current perspectives on the impact of Kratom use. Subst. Abus. Rehabil. 2019, 10, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Ku Md Saad, S.; Soelar, S.A.; Mohamed Yusof, Z.; Warijo, O. Exploring adolescents’ practice and perspective on the use and misuse of kratom in northwest Malaysia. J. Ethn. Subst. Abus. 2021. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Cornett, E.M.; Kaye, A.D. Kratom-Pharmacology, clinical implications and outlook: A comprehensive review. Pain Ther. 2020, 9, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Cinosi, E.; Martinotti, G.; Simanato, P.; Singh, D.; Demetrovics, Z.; Roman-Urrestarazu, A.; Saverio Bersani, F.; Vicknasingam, B.; Piazzon, G.; Li, J.-H.; et al. Following “the Roots” of Kratom (Mitragyna speciosa): The Evolution of an Enhancer from a Traditional Use to Increase Work and Productivity in Southeast Asia to a Recreational Psychoactive Drug in Western Countries. Biomed. Res. Int. 2015, 2015, 968786. [Google Scholar] [CrossRef] [PubMed]
- Fowble, K.; Musah, R.A. A validated method for the quantification of mitragynine in sixteen commercially available Kratom (Mitragyna speciosa) products. Forensic Sci. Int. 2019, 299, 195–202. [Google Scholar] [CrossRef]
- Kruegel, A.C.; Gassaway, M.M.; Kapoor, A.; Varadi, A.; Majumdar, S.; Filzola, M.; Javutch, J.A.; Sames, D. Synthetic and receptor signalling explorations of the mitragyna alkaloids: Mitragynine and an atypical molecular framewrok for opioid receptor modulators. J. Am. Chem. Soc. 2016, 138, 6754–6764. [Google Scholar] [CrossRef]
- Kruegel, A.C.; Grundmann, O. The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 2018, 134, 108–120. [Google Scholar] [CrossRef]
- Karunakaran, T.; Ngew, K.Z.; Zailan, A.A.D.; Jong, V.Y.M.; Baker, M.H.A. The Chemical and Pharmacological Properties of Mitragynine and Its Diastereomers: An Insight Review. Front. Pharmacol. 2022, 13, 805986. [Google Scholar] [CrossRef]
- Takayama, H.; Ishikawa, H.; Kurihara, M.; Kitajima, M.; Aimi, N.; Ponglux, D.; Koyama, F.; Matsumoto, K.; Moriyama, T.; Yamamoto, L.T.; et al. Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: Discovery of opioid agonists structurally different from other opioid ligands. J. Med. Chem. 2002, 45, 1949–1956. [Google Scholar] [CrossRef]
- Zhou, Y.; Ramsey, S.; Provasi, D.; El Daibani, A.; Appourchaux, K.; Chakraborty, S.; Kapoor, A.; Che, T.; Majumdar, S.; Filizola, M. Predicted Mode of Binding to and Allosteric Modulation of the μ-Opioid Receptor by Kratom’s Alkaloids with Reported Antinociception. Biochemistry 2021, 60, 1420–1429. [Google Scholar] [CrossRef]
- Obeng, S.; Wilkerson, J.L.; Leon, F.; Reeves, M.E.; Restrepo, L.F.; Gamez-Jimenez, L.R.; Patel, A.; Pennington, A.E.; Taylor, V.A.; Ho, N.P.; et al. Pharmacological Comparison of Mitragynine and 7-Hydroxymitragynine: In Vitro Affinity and Efficacy for μ-Opioid Receptor and Opioid-like Behavioral Effects in Rats. J. Pharmacol. Exp. Ther. 2021, 376, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Horie, S.; Ishikawa, H.; Takayama, H.; Aimi, N.; Ponglux, D.; Wantanabe, K. Antinociceptive Effect of 7-hydroxymitragynine in Mice: Discovery of an Orally Active Opioid Analgesic from the Thai Medicinal Herb Mitragyna Speciosa. Life Sci. 2004, 74, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O. Patterns of Kratom use and health impact in the US-Results from an online survey. Drug Alcohol Depend. 2017, 176, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romeu, A.; Cox, D.J.; Smith, K.E.; Dunn, K.E.; Griffiths, R.R. Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020, 208, 107849. [Google Scholar] [CrossRef]
- Covvey, J.R.; Vogel, S.M.; Peckham, A.M.; Evoy, K.E. Prevalence and characteristics of self-reported kratom use in a representative US general population sample. J. Addict. Dis. 2020, 38, 506–513. [Google Scholar] [CrossRef]
- Abdulla, M.F.I.L.B.; Singh, D. The Adverse Cardiovascular Effects and Cardiotoxicity of Kratom (Mitragyna speciosa Korth.): A Comprehensive Review. Front. Pharmacol. 2021, 12, 726003. [Google Scholar] [CrossRef]
- Botejue, M.; Walia, G.; Shahin, O.; Sharma, J.; Zackria, R. Kratom-Induced Liver Injury: A Case Series and Clinical Implications. Cureus 2021, 13, e14679. [Google Scholar] [CrossRef]
- Burke, D.J.; Mahonski, S.G.; van Cott, A.C. Breakthrough Seizure Associated with Kratom Use in Patients with Epilepsy. Neurol. Clin. Pract. 2021, 11, 78–84. [Google Scholar] [CrossRef]
- Abdulla, M.F.I.L.B.; Tan, K.L.; Narayanan, S.; Yuvashnee, N.; Chear, N.J.Y.; Singh, D.; Grundmann, O.; Henningfield, J.E. Is kratom (Mitragyna speciosa Korth.) use associated with ECG abnormalities? Electrocardiogram comparisons between regular kratom users and controls. Clin. Toxicol. 2021, 59, 400–408. [Google Scholar] [CrossRef]
- Damodaram, T.; Chear, N.J.Y.; Murugaiyah, V.; Mordi, m.N.; Ramanathan, S. Comparative Toxicity Assessment of Kratom Decoction, Mitragynine and Speciociliatine Versus Morphine on Zebrafish (Danio rerio) Embryos. Front. Pharmacol. 2021, 12, 714918. [Google Scholar] [CrossRef]
- Hughes, S.; Sturzenabum, S.R. Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on life history traits. Environ. Pollut. 2007, 145, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Pears, C.; Woollard, A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017, 7, 9839. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, W.; Zeng, Y.; Jing, H.; Zhang, N.; Flavel, M.; Jois, M.; Han, J.-D.J.; Xian, B.; Li, G. Classification and prediction of toxicity of chemicals using an automated phenotypic profiling of Caenorhabditis elegans. BMC Pharmacol. Toxicol. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.A.; Smith, M.V.; Freedman, J.H. Caenorhabditis elegans as a model in developmental toxicology. Methods Mol. Biol. 2012, 889, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.A.; Smith, M.V.; Co, C.A.; Pirone, J.R.; Rice, J.R.; Shockley, K.R.; Freedman, J.H. Developmental Effects of the ToxCast™ Phase I and Phase II Chemicals in Caenorhabditis elegans and Corresponding Responses in Zebrafish, Rats, and Rabbits. Environ. Health Perspect. 2016, 124, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Harlow, P.H.; Perry, S.J.; Widdison, S.; Daniels, S.; Bondo, E.; Lamberth, C.; Currie, R.A.; Flemming, A.J. The nematode Caenorhabditis elegans as a tool to predict chemical activity on mammalian development and identify mechanims influencing toxicological outcome. Sci. Rep. 2016, 6, 22965. [Google Scholar] [CrossRef]
- Byerly, L.; Cassada, R.C.; Russell, R.L. The life cycle of the nematode Caenorhaditis elegans I: Wild type growth and reproduction. Dev. Biol. 1976, 51, 23–33. [Google Scholar] [CrossRef]
- Lai, C.H.; Chou, C.Y.; Ch’ang, L.Y.; Liu, C.S.; Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef]
- Hillier, L.W.; Coulson, A.; Murray, J.I.; Bao, Z.; Sulston, J.E.; Waterson, R.H. Genomics in C. elegans: So many genes, such a little worm. Genome Res. 2005, 15, 1651–1660. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philisophical Trans. R. Soc. Lond. B 1986, 314, 1–340. [Google Scholar]
- Cook, S.J.; Jarrell, T.A.; Brittin, C.A.; Wang, Y.; Bloniarz, A.E.; Yakovlev, M.A.; Nguyen, K.C.Q.; Tang, T.-H.; Bayer, E.A.; Duerr, J.S.; et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 2019, 571, 63–71. [Google Scholar] [CrossRef]
- Hughes, S.; Celikel, T. Prominent Inhibitory Projections Guide Sensorimotor Computation: An Invertebrate Perspective. BioEssays 2019, 41, 1900088. [Google Scholar] [CrossRef] [PubMed]
- Katner, S.N.; Neal-Beliveau, B.; Engelman, E.A. Embryonic Methamphetamine Exposure Inhibits Methamphetamine Cue Conditioning and Reduces Dopamine Concentrations in Adult N2 Caenorhabditis elegans. Dev. Neurosci. 2016, 38, 139–149. [Google Scholar] [CrossRef]
- Engleman, E.A.; Steagall, K.B.; Bredhold, K.E.; Breach, M.; Kline, H.L.; Bell, R.L.; Katner, S.N.; Neal-Beliveau, B.S. Caenorhabditis elegans show preference for stimulants and potential as a model organism for medications screening. Front. Physiol. 2018, 9, 1200. [Google Scholar] [CrossRef]
- Musselman, H.N.; Neal-Beliveau, B.; Nass, R.; Engelman, E.A. Chemosensory cue conditioning with stimulants in a Caenorhabditis elegans animal model of addiction. Behav. Neurosci. 2012, 126, 445–456. [Google Scholar] [CrossRef]
- Mills, H.; Ortega, A.; Law, W.; Hapiak, V.; Summers, P.; Clark, T.; Komunicki, R. Opiates modulate noxious chemical nociception thorugh a complex monoaminergic/pepidergic cascade. J. Neurosci. 2016, 36, 5498–5508. [Google Scholar] [CrossRef]
- Nieto-Fernandez, F.; Andrieux, S.; Idrees, S.; Bagnall, C.; Pryor, S.C.; Sood, R. The effects of opioids and their antagonists on the nocifensive response of Caenorhabditis elegans to noxious thermal stimuli. Invertebarte Neurosci. 2009, 9, 195–200. [Google Scholar] [CrossRef][Green Version]
- Cheong, M.C.; Artyukkin, A.B.; You, Y.J.; Avery, L. An opiod-like system regulating feeding behaviour in C. elegans. eLife 2015, 4, e06683. [Google Scholar] [CrossRef]
- Sulston, J.E.; Horvitz, H.R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977, 56, 110–156. [Google Scholar] [CrossRef]
- Lehner, B.; Crombie, B.; Tischler, J.; Fortunato, A.; Fraser, A.G. Systematic mapping of genetic interactions in Caenrohabditis elegans identified common modifiers of diverse signalling pathways. Nat. Genet. 2006, 38, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Shaye, D.D.; Greenwald, I. OrthoList: A compendium of C. elegans genes with human orthalogs. PLoS ONE 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Artal-Sanz, M.; de Jong, L.; Tavernarakis, N. Caernorhabditis elegans: A versitle platform for drug discovery. Biotechnol. J. 2006, 1, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans; OUP Oxford: Oxford, UK, 2006. [Google Scholar]
- Heshof, R.; Visscher, B.; Promel, S.; Hughes, S. Large-scale cultivation of Caenorhabditis elegans in a bioreactor using a labor-friendly fed-batch approach. BioTechniques 2019, 67, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Domnic, G.; Chear, N.J.-Y.; Rahman, S.F.A.; Ramanathan, S.; Lo, K.-W.; Singh, D.; Mohana-Kumaran, N. Combinations of indole based alkaloids from Mitragyna speciosa (Kratom) and cisplatin inhibit cell proliferation and migration of nasopharyngeal carcinoma cell lines. J. Ethnopharmacol. 2021, 279, 114391. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Hughes, S.; van Dop, M.; Kolsters, N.; van de Klashorst, D.; Pogosova, A.; Rijs, A.M. Using a Caenorhabditis elegans Parkinson’s Disease Model to Assess Disease Progression and Therapy Efficiency. Pharmaceuticals 2022, 15, 512. [Google Scholar] [CrossRef]
- Ellwood, R.A.; Hewitt, J.E.; Torregrossa, R.; Phillip, A.M.; Hardee, J.P.; Hughes, S.; van de Klashorst, D.; Gharahdaghi, N.; Anupom, T.; Slade, L.; et al. Mitochondrial hydrogen sulphide supplementation improves health in the C. elegans Duchenne Muscular Dystrophy model. Proc. Natl. Acad. Sci. USA 2021, 118, e2019342118. [Google Scholar] [CrossRef]
- van de Klashorst, D.; van den Elzen, A.; Weeteling, J.; Roberts, M.; Desai, T.; Bottoms, L.; Hughes, S. Montmorency tart cherry (Prunus cerasus L.) acts as a calories restriction mimetic that increases intestinal fat and lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103890. [Google Scholar] [CrossRef]
- Katiki, L.M.; Ferreira, J.F.S.; Zajac, A.M.; Masler, C.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F.T. Caenorhabditis elegans as a model to screen plant extracts and compounds as natural anthelmintics for veterinary use. Vetinary Parasitol. 2011, 182, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Spensley, M.; Del Borrello, S.; Pajkic, D.; Fraser, A.G. Acute Effects of Drugs on Caenorhabditis elegans Movement Reveal Complex Responses and Plasticity. G3 Genes Genomes Genet. 2018, 8, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Kolsters, N.; van de Klashorst, D.; Kreuter, E.; Berger Buter, K. An extract of Rosaceae, Solanaceae and Zingiberaceae increases health span and mobility in Caenorhabditis elegans. BMC Nutr. 2022, 8, 5. [Google Scholar] [CrossRef]
- Anderson, G.L.; Cole, R.D.; Williams, P.L. Assessing behavioral toxicity with Caenorhabditis elegans. Environ. Toxicol. Chem. 2004, 23, 1235–1240. [Google Scholar] [CrossRef]
- Wellenberg, A.; Weides, L.; Kurzke, J.; Hennecke, T.; Bornhorst, J.; Crone, B.; Karst, U.; Brinckmann, V.; Fritz, G.; Honnen, S. The use of C. elegans as a 3R-compliant in vivo model for the chemoprovention of cisplatin-induced neurotoxicity. Exp. Neurol. 2021, 341, 113705. [Google Scholar] [CrossRef]
- Ju, J.; Saul, N.; Kochan, C.; Putschew, A.; Pu, Y.; Yin, L.; Steinberg, C.E.W. Cyanobacterial Xenobiotics as Evaluated by a Caenorhabditis elegans Neurotoxicity Screening Test. Int. J. Environ. Res. Public Health 2014, 115, 4589–4606. [Google Scholar] [CrossRef]
- Boyd, W.A.; McBride, S.J.; Freedman, J.H. Effects of Genetic Mutations and Chemical Exposures on Caenorhabditis elegans Feeding: Evaluation of a Novel, High-Throughput Screening Assay. PLoS ONE 2007, 2, e1259. [Google Scholar] [CrossRef]
- Trojanowski, N.F.; Raizen, D.M.; Fang-Yen, C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci. Rep. 2016, 6, 2940. [Google Scholar] [CrossRef]
- Manwill, P.K.; Flores-Bocanegra, L.; Khin, M.; Raja, H.A.; Cech, N.B.; Oberlies, N.H.; Todd, D.A. Kratom (Mitragyna speciosa) Validation: Quantitative Analysis of Indole and Oxindole Alkaloids Reveals Chemotypes of Plants and Products. Planta Med. 2022. [Google Scholar] [CrossRef]
- Chear, N.J.-Y.; Leon, F.; Sharma, A.; Kanumuri, S.R.R.; Zwolinski, G.; Abboud, K.A.; Singh, D.; Restrepo, L.F.; Patel, A.; Hiranita, T.; et al. Exploring the Chemistry of Alkaloids from Malaysian Mitragyna speciosa (Kratom) and the Role of Oxindoles on Human Opioid Receptors. J. Nat. Prod. 2021, 84, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Leon, F.; Obeng, S.; Mottinelli, M.; Chen, Y.; King, T.I.; Berthold, E.C.; Kamble, S.H.; Restrepo, L.F.; Patel, A.; Gamez-Jimenez, L.R.; et al. Activity of Mitragyna speciosa (“Kratom”) Alkaloids at Serotonin Receptors. J. Med. Chem. 2021, 64, 13510–13523. [Google Scholar] [CrossRef] [PubMed]
- Patti, C.L.; Frussa-Filha, R.; Silva, R.H.; Carvalho, R.C.; Kameda, S.R.; Takatsu-Coleman, A.L.; Cunha, J.L.S.; Abilio, V.C. Behavioural characterisation of morphine effects on motor activity in mice. Pharmacol. Biochem. Behav. 2005, 81, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, N.; Gulec, G.; Ozluk, K. Effects of intracerebroventricularly-injected morphine on anxiety, memory retrieval and locomotor activity in rats: Involvement of vasopressinergic system and nitric oxide pathway. Pharmacol. Biochem. Behav. 2006, 85, 859–867. [Google Scholar] [CrossRef]
- Imeh-Nathaniel, A.; Rincon, N.; Orfanakos, V.B.; Brechtel, L.; Wormack, L.; Richardson, E.; Huber, R.; Nathaniel, T.I. Effects of chronic cocaine, morphine and methamphetamine on the mobility, immobility and stereotyped behaviors in crayfish. Behav. Brain Res. 2017, 332, 120–125. [Google Scholar] [CrossRef]
- Kavaliers, M.; Hirst, M.; Teskey, G.C. A functional role for an opiate system in snail thermal behavior. Science 1983, 220, 99–101. [Google Scholar] [CrossRef]
- Wang, D.; Stoveken, H.M.; Zucca, S.; Dao, M.; Orlandi, C.; Song, C.; Masuho, I.; Johnston, C.; Opperman, K.J.; Giles, A.C.; et al. Genetic behavioral screen identifies an orphan anti-opioid system. Science 2019, 365, 1267–1273. [Google Scholar] [CrossRef]
- Martin, W.R.; Wikler, A.; Eades, C.G.; Pescor, F.T. Tolerance to and physical dependence on morphine in rats. Psychopharmaologica 1963, 4, 247–260. [Google Scholar] [CrossRef]
- Calahorro, F.; Holden-Dye, L.; O’Connor, V. Impact of drug solvents on C. elegans pharyngeal pumping. Toxicol. Rep. 2021, 8, 1240–1247. [Google Scholar] [CrossRef]
| Concentration | %CV Mitragynine | % CV 7-Hydroxymitragynine | ||
|---|---|---|---|---|
| Intraday | Interday | Intraday | Interday | |
| 100 μg/mL | 5.66% | 12.93% | 3.32% | 6.85% |
| 10 μg/mL | 2.07% | 5.28% | 3.49% | 9.59% |
| 1 μg/mL | 6.61% | 12.44% | 5.20% | 11.91% |
| 100 ng/mL | 15.69% | 16.60% | 5.29% | 8.58% |
| 10 ng/mL | ND | 10.29% | ND | 14.85% |
| Sample | Solvent | Mitragynine, μg/mg (%) | 7-Hydroxymitragynine, ng/mg (%) |
|---|---|---|---|
| White Borneo | ethanol | 36.44 (3.6%) | 1.90 (0.002%) |
| water | 8.14 (0.8%) | 0.0 (0.000%) | |
| methanol | 37.01 (3.7%) | 12.05 (0.012%) | |
| Red Maeng Da | ethanol | 36.48 (3.6%) | 4.70 (0.005%) |
| water | 4.71 (0.5%) | 10.16 (0.010%) | |
| methanol | 34.28 (3.4%) | 52.15 (0.052%) | |
| Bali | ethanol | 20.53 (2.1%) | 18.28 (0.018%) |
| water | 1.37 (0.1%) | 14.32 (0.014%) | |
| methanol | 27.71 (2.8%) | 11.11 (0.011%) |
| Number | Pumps Per Minute | Pump Duration (Milliseconds) | Interpump Interval (Milliseconds) | |
|---|---|---|---|---|
| Negative control | 21 | 229 | 109 | 273 |
| Solvent control (0.6% DMSO) | 17 | 229 | 105 | 276 |
| Morphine 0.5 mM | 12 | 271 * | 103 | 226 * |
| White Borneo Water extract | 14 | 235 | 112 | 270 |
| White Borneo Ethanol Extract | 14 | 228 | 115 | 268 |
| White Borneo Methanol Extract | 15 | 235 | 109 | 268 |
| Number | Pumps Per Minute | Pump Duration (Milliseconds) | Interpump Interval (Milliseconds) | |
|---|---|---|---|---|
| Negative control | 13 | 263 | 263 | 233 |
| Solvent control (0.6% DMSO) | 5 | 246 | 125 * | 244 |
| Morphine 0.5 mM | 13 | 301 ** | 98 | 201 * |
| White Borneo Water extract | 5 | 262 * | 114 * | 230 * |
| White Borneo Ethanol Extract | 5 | 235 | 119 * | 269 |
| White Borneo Methanol Extract | 13 | 243 | 124 | 252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, S.; van de Klashorst, D.; Veltri, C.A.; Grundmann, O. Acute, Sublethal, and Developmental Toxicity of Kratom (Mitragyna speciosa Korth.) Leaf Preparations on Caenorhabditis elegans as an Invertebrate Model for Human Exposure. Int. J. Environ. Res. Public Health 2022, 19, 6294. https://doi.org/10.3390/ijerph19106294
Hughes S, van de Klashorst D, Veltri CA, Grundmann O. Acute, Sublethal, and Developmental Toxicity of Kratom (Mitragyna speciosa Korth.) Leaf Preparations on Caenorhabditis elegans as an Invertebrate Model for Human Exposure. International Journal of Environmental Research and Public Health. 2022; 19(10):6294. https://doi.org/10.3390/ijerph19106294
Chicago/Turabian StyleHughes, Samantha, David van de Klashorst, Charles A. Veltri, and Oliver Grundmann. 2022. "Acute, Sublethal, and Developmental Toxicity of Kratom (Mitragyna speciosa Korth.) Leaf Preparations on Caenorhabditis elegans as an Invertebrate Model for Human Exposure" International Journal of Environmental Research and Public Health 19, no. 10: 6294. https://doi.org/10.3390/ijerph19106294
APA StyleHughes, S., van de Klashorst, D., Veltri, C. A., & Grundmann, O. (2022). Acute, Sublethal, and Developmental Toxicity of Kratom (Mitragyna speciosa Korth.) Leaf Preparations on Caenorhabditis elegans as an Invertebrate Model for Human Exposure. International Journal of Environmental Research and Public Health, 19(10), 6294. https://doi.org/10.3390/ijerph19106294








