The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians
Abstract
:1. Introduction
2. Materials and Methods
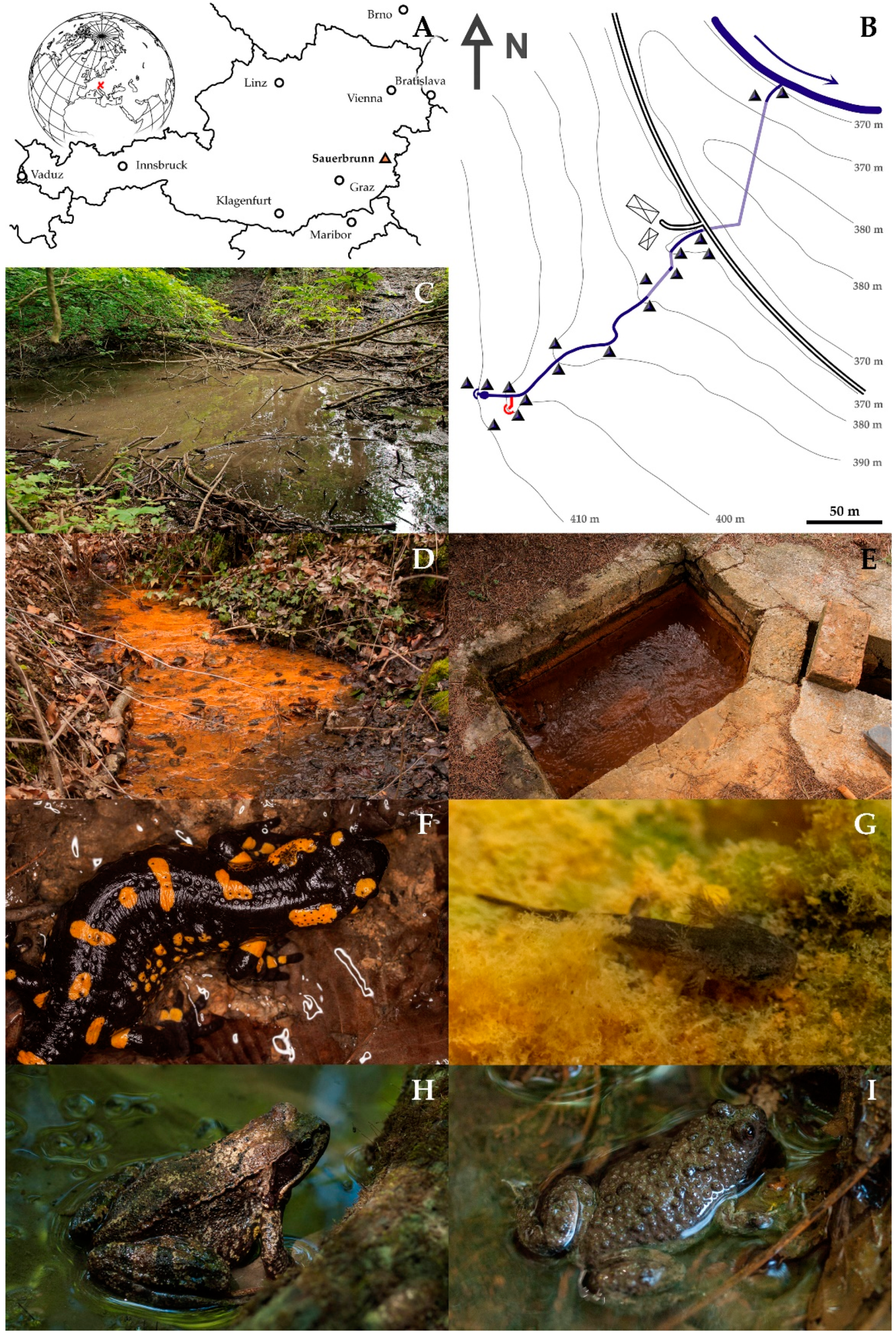
2.1. Geoglogy and Topography of the Study Site
2.2. Sampling and Observation of Animals
2.3. Water Analysis
- ○
- Dissolved Oxygen: titration by Aquamerck Oxygen Test 1.11107.0001.
- ○
- Temperature was recorded by a conventional liquid expansion thermometer. Oxygen saturation of the water was calculated by using absolute oxygen content, altitude and temperature [15].
- ○
- Carbonate Hardness: titration by Aquamerck Carbonate Hardness Test 1.08048.0001.
- ○
- Flow Speed: time of the movement of a few droplets of methylene blue was measured over a defined distance.
- ○
- pH: Voltcraft PH-100ATC electroden.
- ○
- Conductivity: Voltcraft Pure Water Tester WA-100ATC.
- ○
- Metal and Metalloid Content: samples were immediately frozen in dry ice in order to prevent precipitation of metals, and stored at <−20 °C until analysis.
2.4. Soil Analysis
- ○
- ○
- Soil pH: 10 g soil were extracted overnight in 100 mL 1 M NH4NO3 under constant shaking (1 Hz). pH was measured electronically (Schott Lab 850) in the supernatant.
- ○
- Bioavailable Metals and Metalloids were determined by sequential extraction, following [20]. 1 g soil was extracted with 90 mL simulated gastric juice for 4 h in order to simulate metal release in the stomach and then centrifuged (4000 rpm, 20 min). In a second step, the compounds of artificial bile solution were added in order to simulate conditions in the upper intestines; after 20 h, the sample was centrifuged again. Table 1 specifies the composition of the extractants. Digestion took place at room temperature. After each centrifugation, a sample was taken, filtered through 0.2 µm SFCA non-sterile membrane filters (Sartorius stedim Biotech, Göttingen, Germany), frozen until further analyses.
- ○
- Total Metalloids and Metals: Approximately 0.5 g of oven-dried, sieved sediment samples were weighed using an analytical balance into glass tubes, and 9 mL of 34% HNO3 (TraceSELECT® Fluka) and 1 mL of 30% H2O2 were added. Covered with air coolers, samples were processed for 2 h at 130 °C in a heating block. The leached samples were transferred to 20 mL volumetric flasks and topped up to volume using Milli-Q® water. Before analyses, samples were filtered through 0.2 μm PTFE membrane syringe filters (VWR, Radnor, PA, USA). Reference samples comprising 0.2 g (dry weight) of marine sediment PACS-2 obtained from the National Research Council Canada (NRCC, Ottawa, ON, Canada) were digested and diluted in the same manner as described above.
2.5. StatisticsD
3. Results
3.1. Amphibians
- ○
- S. salamandra: each spring, larvae were abundant in the well and the little pond below (total 67). 9 larvae were found in the well of the point source, where they were hiding between limonite precipitations and filamentous bacteria (Figure 1D,G). 14 individuals were registered in the wooded gorge, in small patches without noticeable current, 2 individuals close to entrance to the second underground passage, also in a section virtually without current. No larvae were found in the floodplain. All larvae were very small (<3 cm). No growth was observed, and their number decreased during spring. Only three fully grown terrestrial adult individuals were observed in March 2016 and May 2021, all pregnant females (Figure 1F).
- ○
- R. temporaria: both subadult and adult individuals were observed (Figure 1H), but no tadpoles. The greatest abundance (59 individuals) was found in the wooded gorge. 6 individuals were at the well of the main creek and in the pond just below, 2 individuals roamed around the creek between the two underground passages. Remarkably, 4 individuals were swimming in the point source, in the most contaminated water. No R. temporaria was observed in the floodplain.
- ○
- B. variegata: Three adult specimens of B. variegata were found, each time in the water (Figure 1I), one in the pond below the well, one in the wooded gorge and one between the two underground passages. Furthermore, B. variegata was common in the ponds of the flood plain, where also subadult individuals and tadpoles were found. These ponds exhibited slightly increased As concentrations (23.7 µg/L) and no detectable Sb.
- ○
- Other herpetological observations in the region included Pelophylax spp. in fishponds next to the Tauchenbach River, but never close to the As-Sb creek. Bufo bufo and Ichthyosaura alpestris were not observed, though both species occur in the region. One subadult specimen of Natrix natrix was found directly in the point source in May 2016, where it was preying on frogs which were frequent at this part of the creek at that time.
- ○
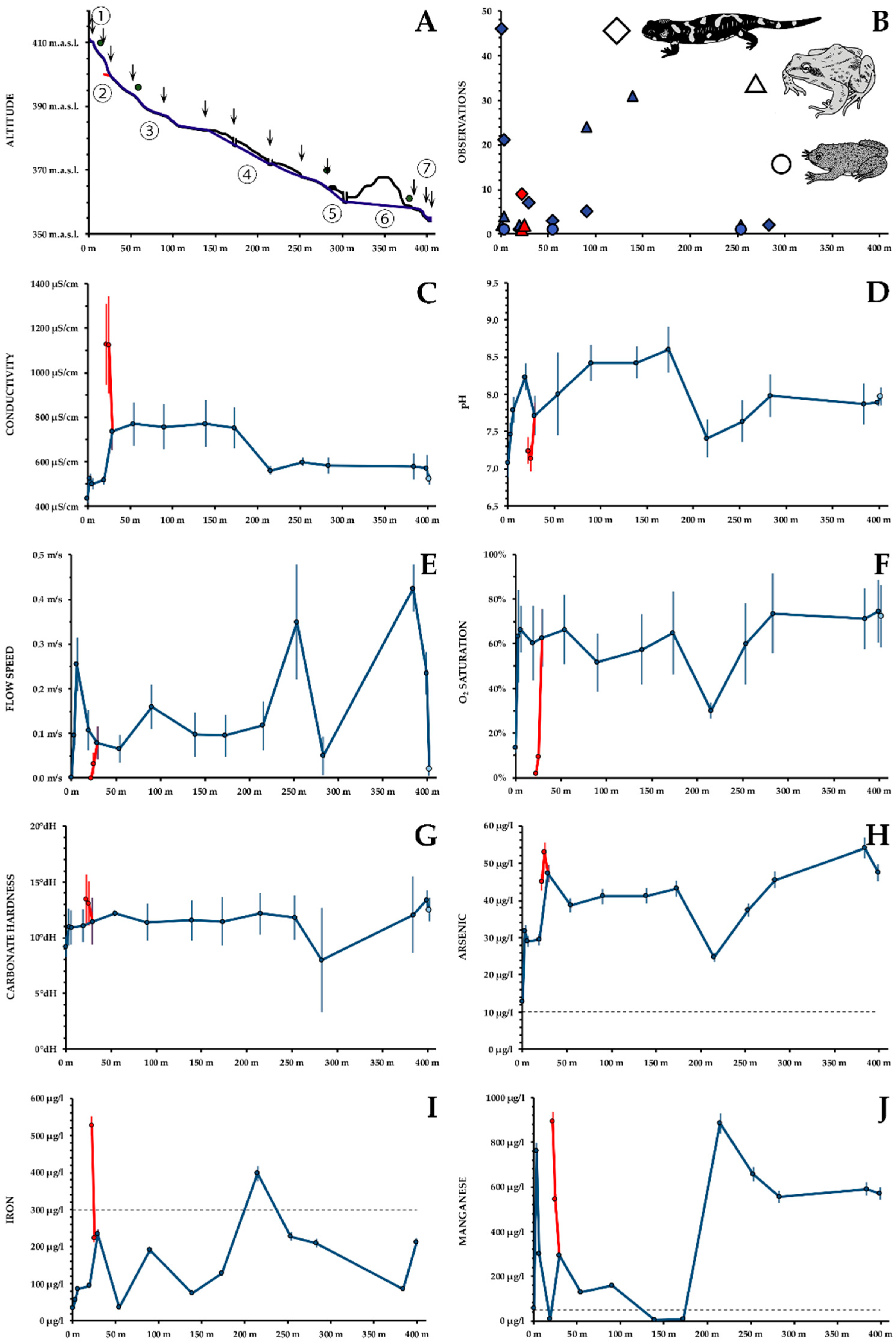
- Figure 2B shows the total number of observations for each section of the creek.
3.2. Water Chemistry
- ○
- Conductivity: with 437 ± 22 μS/cm, conductivity is not excessively high at the main well. Until the influx of the point source, conductivity increases slightly, due to minor influx of mine drainage water. The point source shows a conductivity of 1127 ± 123 μS/cm. After mixing of the creek and the drainage water, a conductivity of 736 ± 85 μS/cm is found. This value remains quite constant until the first underground passage of the water, where an influx of uncontaminated water may occur. The resulting, lower conductivity of about 600 μS/cm remains subsequently constant, until the creek drains into the Tauchenbach with a conductivity of 522 ± 22 μS/cm (Figure 2C).
- ○
- pH: Throughout the creek, neutral or slightly alkaline pH values are found. At the well, pH is 7.1 ± 0.2 and increases subsequently to 8.2 ± 0.2. The water of the point source is neutral as well (7.2 ± 0.1), resulting in 7.7 ± 0.3 after the conflux. The pH rises again to 8.6 ± 0.3. In the first underground passage pH decreases to 7.4 ± 0.3 and rises again to 7.9 ± 0.1, similar to the pH of the Tauchenbach River (Figure 2D).
- ○
- Flow Speed changes throughout the creek according to the inclination. At the wells of the main creek and the point source, virtually no flow can be observed. At three small cascades, flow speed exceeds 25 cm/s (Figure 2E).
- ○
- Dissolved O2 content shows pronounced changes. Both at the well of the main creek (concentration 2.0 ± 0.4 mg/L; saturation 14 ± 3%) and at the point source (0.3 ± 0.3 mg/L; 2 ± 2%), O2 is very low. Throughout the creek values of 7–9 mg/L; 60–75% are found, with the underground passage as notable exception (4.1 ± 0.5 mg/L; 30 ± 4%; Figure 2F).
- ○
- Hardness: Carbonate hardness at the well is 9 ± 1°dH and increases subsequently to 12 ± 0°dH. At the point source 13 ± 2°dH are found. Within the underground passage, carbonate hardness decreases to 8 ± 5°dH, and rises subsequently to 13 ± 1°dH, similar to the Tauchenbach River (Figure 2G). Ca shows similar but more pronounced (data not shown).
- ○
- Dissolved As is the main metalloid in the creek. From the well to the Tauchenbach River, the recommended concentration of 10 µg/L (WHO 2011) was consistently exceeded. The lowest concentrations was found at the main well (12.8 ± 0.6 μg/L). A few meters downstream As has risen to 31.7 ± 1.6 μg/L, obviously due to the aforementioned minor influxes of mine drainage water. At the point source, As concentrations up to 52.8 ± 2.6 μg/L was measured. After the conflux approximately 40 μg/L were found until the first underground passage. Here, As decreases to 24.7 ± 1.2 μg/L. Subsequently, As reaches 54.0 ± 2.7 μg/L, after the second underground passage, indicating a highly complex behaviour of As (Figure 2H).
- ○
- Dissolved Sb is below the detection limit throughout the main creek.
- ○
- Dissolved Fe is rare in the well (34 ± 2 μg/L), but occurrs in higher concentrations in the water of the pond (55 ± 3 μg/L). In the point source, an Fe concentration of 525 ± 26 μg/L is found, which decreases to 223 ± 11 μg/L over a few meters. Subsequently, Fe shows pronounced fluctuations, achieving 397 ± 20 μg/L at the first underground passage (Figure 2I).
- ○
- Dissolved Mn is low at the well of the main creek (59 ± 3 μg/L), but peaks in the pond below (761 ± 38 μg/L) and in the point source (893 ± 45 μg/L). At the lower end of the wooded gorge, before the first underground passage, Mn concentrations are below the detection limit. Within the passage, Mn reaches 884 ± 20 μg/L, with values about 600 μg/L until the Tauchenbach River. Throughout most of the creek, Mn concentrations exceed the threshold of 50 μg/L (Council of the European Union 1998; Figure 2J).
- ○
- Dissolved Ni is rare at the well of the main creek (6.0 ± 0.3 μg/L). At the point source 84 ± 4 μg/L are found, resulting in 35 ± 1.8 μg/L after the conflux. Ni concentrations are occasionally above the threshold of 70 μg/L (WHO 2011).
- ○
- Other metals such as Cu, Pb and Zn were found in concentrations below threshold values for toxicity, or even below the detection limits.
3.3. Creek Sediment
3.4. Alluvial Soil
- ○
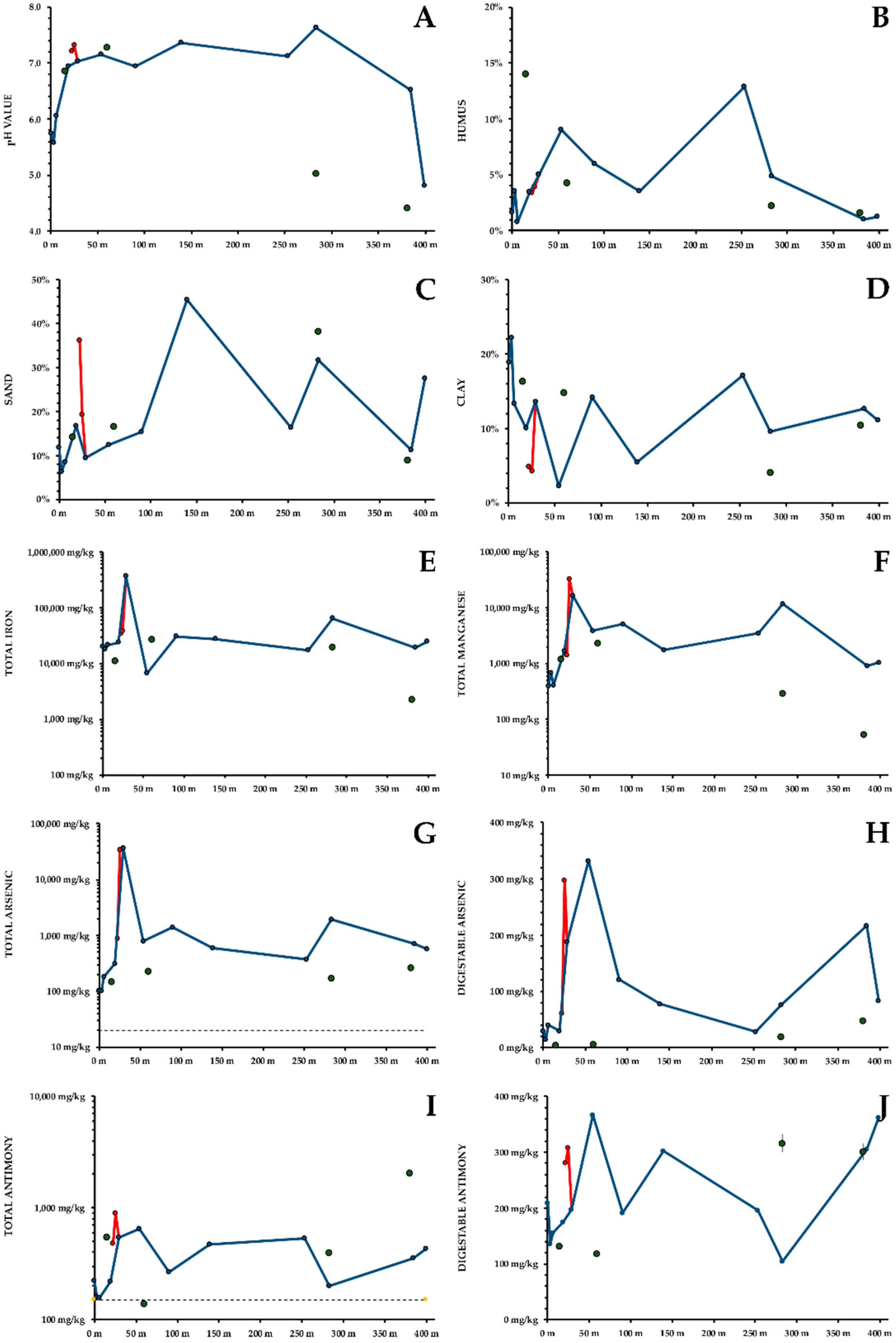
- Soil pH was mildly acidic to circumneutral (6–7) throughout the course of the creek (Figure 3A). An additional sampling point next to the second underground passage in a conifer stand, and the flood plain of the Tauchenbach showed a more acidic pH (5).
- ○
- Humus content of the soil fluctuates significantly between 1% and 14% (Figure 3B). The humus content in the forested upper part of the creek, especially under coniferous trees, was higher than in the flood plain.
- ○
- Grain Size Distribution: sand content (0.63–2 mm, Figure 3C) was high at the point source (36%), in the wooded gorge (45%), and in the flood plain (32%). Coarse silt (20–63 μm) oscillated between 30% and 45%, with higher concentrations in the flood plain and the upper part of the creek. Medium and fine silt (2–20 μm) were highest next to the pond, below the conflux and at the end of the second underground passage. Clay (<2 μm, Figure 3D) varied widely, no general trend was obvious, though the clay content was lower next to the point source.
- ○
- Soil Fe is not necessarily a toxic contaminant. From the well to the floodplain, including the additional sampling points, Fe concentrations were usually between 20,000 and 30,000 mg/kg, with no obvious trend. At the point source, an Fe concentration of 361,576 ± 18,079 mg/kg was found, corresponding to a limonite content of at least 57.5%. High Fe concentrations (64,589 ± 3229 mg/kg) are also found at a tiny floodplain close to a second underground passage (Figure 3E). This coincides with an As peak for this sampling spot.
- ○
- Soil Mn concentrations were comparatively low above the point source (400–700 mg/kg) but rose to 32,855 ± 1643 mg/kg at the point source. Subsequently, Mn concentrations remained high (1700–5000 mg/kg, >10,000 mg/kg at two sampling sites, Figure 3F).
- ○
- Soil As was significantly increased; the recommended threshold of 20 mg/kg [23] was exceeded throughout the habitat (Figure 3G). Around the well and at the sampling points in some distance to the creek, As concentrations of 100–300 mg/kg were found. Below the point source and next to the conflux, As concentration was about 35,000 mg/kg. Below the conflux, As content was typically 400–800 mg/kg, though higher values were found twice. Both gastric and intestinal available As concentration was low above the point source, and at the sampling sites in some distance to the creek. Concentrations of gastric and intestinal available As peaked at the point source (317 ± 16 mg/kg and 298 ± 15 mg/kg), though the relative availabilities were extremely low (0.9% for both). A second peak was found 25 m downstream (286 ± 15 mg/kg and 333 ± 17 mg/kg, respectively). In the wooded gorge and below, gastric As availability was relatively constant (approximately 60 mg/kg). Intestinal availability went down to 28 ± 1 mg/kg but increased during the second underground passage and reached 216 ± 11 mg/kg in the floodplain (Figure 3H shows total digestible As). Maximum As digestibility is found where the clay content of the soil reaches its minimum.
- ○
- Soil Sb concentrations were above the recommended threshold of 150 mg/kg [24] virtually throughout the habitat. Total Sb showed a similar distribution as As, but was less mobile (Figure 3I). Just below the point source, Sb concentration peaked with 902 ± 45 mg/kg. Subsequently, most Sb concentrations oscillated between 300 and 500 mg/kg. At three additional sampling points, Sb concentrations were comparable to the bank of the creek. Only little Sb was mobilised by simulated gastric solution. Gastric availability of Sb was 17.4–28.7% next to the point source, and <0.1% everywhere else. Intestinal Sb availability was >50% at most sampling points. At the well intestinal available Sb was rather low (210 ± 11 mg/kg), peaks were found up to 25 m below (309–367 mg/kg), followed by decreasing concentrations. In the floodplain concentrations of intestinal available Sb were higher (363 ± 18 mg/kg). Figure 3J shows total digestible Sb.
- ○
- Other metals were only moderately increased. Soil Ni concentrations were just below the threshold of 75 mg/kg [25] at the well of the main creek and at the floodplain, but peaked below the point source (3253 ± 163 mg/kg) and again between the two underground passages (637 ± 32 mg/kg). Soil Pb concentration were well below the threshold of 300 mg/kg [25], except for the point source (242 ± 12 mg/kg). Soil Zn concentrations were predominantly close to the threshold of 300 mg/kg [25] but exceeded it only at the point source (1919 ± 96 mg/kg) and at the little floodplain close to the second underground passage (322 ± 16 mg/kg). Soil Cu oscillated between 10–279 mg/kg but showed no obvious correlation with the point source.
4. Discussion
4.1. Availability of Potentially Toxic Elements
4.2. Ecology of the Sauerbrunn Area
4.3. Amphibians of the Sauerbrunn Area
- The abundance of suitable prey animals is very low throughout the creek. Death of the larvae might be caused by starvation. Cannibalism might account for occasional successful metamorphosis sufficient to maintain the population.
- Metal and metalloid content of the water exceeds applicable thresholds throughout the creek. Reduced growth of S. salamandra larvae have been observed in waters contaminated by As and multiple metals [37], the same seems to apply to other Urodela [38]. Thus, fatal concentrations of metalloids might be absorbed via the permeable larval skin. However, larval mortality was also high above the point source.
- Ingestion of limonite particles seems unavoidable during suction-snapping. The particles are considerably smaller than potential prey and are easily dispersed. They contain high concentration of metalloids which become bioavailable under intestinal conditions. Limonite accumulates especially under those low-current conditions preferred by the larvae (Figure 1G). Furthermore, trace element concentrations in the tissue of S. salamandra larvae correlate poorly with concentrations in water [37], indicating that uptake from sediment might be more relevant than from water. Thus, it is suggested that ingestion of As-Sb-rich limonite is a major stressor for S. salamandra larvae in the creek, though they do not seem to avoid metalloid-rich microhabitats and may not be able to sense increased metalloid concentrations.
5. Conclusions
- High concentrations of As-Sb found in the creek, which precipitate together with limonite wherever Fe2+ is oxidised under aerobic conditions. Flooding deposits metalloids also at the banks.
- Metalloids become readily bioavailable under simulated intestinal conditions, making uptake of potentially toxic metalloids very likely.
- B. variegata and R. temporaria are frequent in the mining area but seem to avoid the most metal rich parts of the creek, at least to some degree.
- Larvae of S. salamandra exhibit a high mortality in the creek. Ingestion of As-Sb-rich limonite may be a major factor, besides absorption through the skin and starvation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, D. Amphibian conservation: Are we on the right track? Biol. Conserv. 2008, 141, 2912–2917. [Google Scholar] [CrossRef]
- Gollmann, G. Rote Liste der in Österreich gefährdeten Lurche (Amphibia) und Kriechtiere (Reptilia). In Rote Listen Gefährdeter Tiere Österreichs. Teil 2: Kriechtiere, Lurche, Fische, Nachtfalter, Weichtiere; Bundesministerium für Land und Forstwirtschaft, Ed.; Böhlau Verlag: Wien, Austria, 2007. [Google Scholar]
- Bundesgesetzblatt (Federal Law Announcement). Übereinkommen über die Erhaltung der Europäischen Wildlebenden Pflanzen und Tiere und ihrer Natürlichen Lebensräume. Bundesgesetzbl. Rep. Österr. 1983, 148, 1766–1794. [Google Scholar]
- Adlassnig, W.; Wernitznig, S.; Lichtscheidl, I.K. Historical copper spoil heaps in Salzburg/Austria. Geology, mining history, contamination and vegetation. In Bio-Geo Interactions in Metal Contaminated Soils; Kothe, E., Varma, A., Eds.; Springer: Frankfurt, Germany, 2011; pp. 201–231. [Google Scholar]
- Blaustein, A.R.; Romansic, J.M.; Kiesecker, J.M.; Hatch, A.C. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 2003, 9, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-H.; Jackson, A.G.; Karasov, W.H. Chronic exposure to pentavalent arsenic of larval leopard frogs (Rana pipiens): Bioaccumulation and reduced swimming. Ecotoxicology 2009, 18, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Adlassnig, W.; Sassmann, S.; Grawunder, A.; Puschenreiter, M.; Horvath, A.; Koller-Peroutka, M. Amphibians in metal-contaminated habitats. Salamandra 2013, 49, 149–158. [Google Scholar]
- Pollak, A. Neuere Untersuchungen auf der Antimonerzlagerstätte Schlaining. Berg-Hüttenmänn. Monatsh. 1955, 100, 137–145. [Google Scholar]
- Cerny, I. Geochemische Untersuchung von Karbonatgesteinen im Antimonbergbau Schlaining (Burgenland). Berg-Hüttenmänn. Monatsh. 1981, 126, 524–527. [Google Scholar]
- Nordstrom, D.K. Mine waters: Acidic to circumneutral. Elements 2011, 7, 393–398. [Google Scholar] [CrossRef]
- Steinhauser, G.; Adlassnig, W.; Lendl, T.; Peroutka, M.; Weidinger, M.; Lichtscheidl, I.K.; Bichler, M. Metalloid contaminated microhabitats and their biodiversity at a former antimony mining site in Schlaining, Austria. Open Env. Sci. 2009, 3, 20–35. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. Source and behaviour of arsenic in natural waters. In United Nations Synthesis Report on Arsenic in Drinking Water; World Health Organisation: Geneva, Switzerland, 2001; Chapter 1; pp. 2–61. [Google Scholar]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Roméo, M. Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: Effects of pH, concentration and reversibility. Desalination 2011, 281, 93–99. [Google Scholar] [CrossRef]
- O’Reilly, S.E.; Strawn, D.G.; Sparks, D.L. Residence Time Effects on Arsenate Adsorption/Desorption Mechanisms on Goethite. Soil Sci. Soc. Am. J. 2001, 65, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Oehme, F.; Schuler, P. Gelöst-Sauerstoff-Messung; A. Hüthig-Verlag: Heidelberg, Germany, 1983; p. 146. [Google Scholar]
- Storch, V.; Welsch, U. Kükenthal Zoologisches Praktikum; Springer: Berlin, Germany, 2014; p. 552. [Google Scholar]
- Durbin, R.P. Anion requirements for gastric acid secretion. J. Gen. Physiol. 1964, 47, 735–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Une, M.; Matsumoto, N.; Kihira, K.; Yasuhara, M.; Kuramoto, T.; Hoshita, T. Bile salts of frogs: A new higher bile acid, 3 alpha, 7 alpha, 12 alpha, 26-tetrahydroxy-5 beta-cholestanoic acid from the bile Rana plancyi. J. Lipid Res. 1980, 21, 269–276. [Google Scholar] [CrossRef]
- Feder, M.E.; Burggreen, W.W. Environmental Physiology of the Amphibians; The University of Chicago Press: Chicago, IL, USA, 1992; p. 472. [Google Scholar]
- Intawongse, M.; Dean, J.R. Use of the physiologically-based extraction test to assess the oral bioaccessiblity of metals in vegetable plants grown in contaminated soil. Environ. Poll. 2008, 152, 60–72. [Google Scholar] [CrossRef]
- Kandeler, E. Humusbestimmung durch Naßoxidation. In Bodenbiologische Arbeitsmethoden, 2nd ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin, Germany, 1993; pp. 356–357. [Google Scholar]
- Öhlinger, R. Bestimmung der Korngrößenverteilung. In Bodenbiologische Arbeitsmethoden, 2nd ed.; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin, Germany, 1993; pp. 347–350. [Google Scholar]
- Umweltbundesamt. Zulässige Grenzwerte (Richtwerte) für Schadstoffe in Klärschlamm und Boden; Umweltbundesamt der Republik Österreich: Vienna, Austria, 2003. [Google Scholar]
- Mergenthaler, B.; Richner, T. Mobilität und geochemisches Verhalten von Antimon im Boden von Schiessanlagen. Master’ Thesis, Eidgenössische Technische Hochschule, Zürich, Switzerland, 2002. [Google Scholar]
- Hein, H.; Klaus, S.; Meyer, A.; Schwedt, G. Richt- und Grenzwerte. Teil A: Übersichten zu den Deutschen und Europäischen Richtlinien; Springer VDI-Verlag: Düsseldorf, Deutschland, 2016. [Google Scholar]
- Ackermann, J.; Vetterlein, D.; Kuehn, T.; Kaiser, K.; Jahn, R. Minerals controlling arsenic distribution in floodplain soils. Europ. J. Soil. Sci. 2010, 61, 588–598. [Google Scholar] [CrossRef]
- Gál, J.; Hursthouse, A.; Cuthbert, S. Bioavailability of arsenic and antimony in soils from an abandoned mining area, Glendinning (SW Scotland). J. Environ. Sci. Health 2007, 42, 1263–1274. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisions to arsenic: A critical review. Environ. Poll. 2010, 158, 1169–1181. [Google Scholar] [CrossRef]
- Mierzwa, J.; Mumbi, R.; Avedananda, R.; Rakshit, S.E.; Michael, E.; Sarkar, D. Antimony (V) Adsorption at the Hematite–Water Interface: A Macroscopic and In Situ ATR-FTIR Study. Soil Syst. 2021, 5, 20. [Google Scholar] [CrossRef]
- Thiesmeier, B. Der Feuersalamander; Laurenti-Verlag: Bielefeld, Germany, 2004; p. 192. [Google Scholar]
- Gollmann, B.; Gollmann, G. Die Gelbbauchunke. Von der Suhle zur Radspur; Laurenti Verlag: Bielefeld, Germany, 2012; p. 176. [Google Scholar]
- Seidel, B. Streifzug durch die Verhaltens- und Populationsbiologie von Gelbbauchunken, Bombina variegata (L., 1758) (Anura: Bombinatoridae), in einem Habitat mit temporären Gewässern. In Naturschutzreport. Verbreitung, Ökologie und Schutz der Gelbbauchunke; Nöllert, A., Ed.; Thüringer Landesanstalt für Umwelt: Jena, Germany, 1996; Volume 11, pp. 16–31. [Google Scholar]
- Nöllert, A.; Nöllert, C. Die Amphibien Europas. Bestimmung-Gefährdung-Schutz; Kosmos Verlag GmbH: Stuttgart, Germany, 1992; p. 192. [Google Scholar]
- Moriarty, M.M.; Koch, I.; Reimer, J. Arsenic species and uptake in amphibians (Rana clamitans and Bufo americanus). Environ. Sci. Processes Impacts 2013, 15, 1520–1528. [Google Scholar] [CrossRef]
- Dovick, M.A.; Arkle, R.S.; Kulp, T.R.; Pilliod, D.S. Extreme arsenic and antimony uptake and tolerance in toad tadpoles duringdevelopment in highly contaminated wetlands. Environ. Sci. Technol. 2020, 54, 7983–7991. [Google Scholar] [CrossRef]
- Langdon, C.J.; Piearce, T.G.; Meharg, A.A.; Semple, K.T. Interactions between earthworms and arsenic in the soil environment: A review. Environ. Poll. 2003, 124, 361–373. [Google Scholar] [CrossRef]
- Pavlović, S.; Krizmanić, I.; Borković-Mitić, S.; Stojsavljević, A.; Mitić, B. A first record of the antioxidant defense and selected trace elements in Salamandra salamandra larvae on Mt. Avala and Mt. Vršački Breg (Serbia). Arch. Biol. Sci. 2020, 72, 491–501. [Google Scholar] [CrossRef]
- Chang, J.-S.; Man, G.; Kyoung-Woong, K. Effect of arsenic on p53 mutation and occurrence of teratogenic salamanders: Their potential as ecological indicators for arsenic contamination. Chemosphere 2009, 75, 948–954. [Google Scholar] [CrossRef] [PubMed]
| Gastric Solution | Bile Solution | ||
|---|---|---|---|
| Pepsin | 1.25 g/L | Pankreatin | 45 mg/L |
| Sodium Malate | 0.5 g/L | Bile Salt | 155 mg/L |
| Sodium Citrate | 0.5 g/L | NaHCO3 | pH 7.0 |
| Lactic Acid | 420 µL/L | ||
| Acetic Acid | 500 µL/L | ||
| HCl | pH 2.5 | ||
| Element | Method | Reference Value (mg/kg) | Measured Mean ± SD (mg/kg) | Recovery Rate | LOD (mg/kg) |
|---|---|---|---|---|---|
| Mn | TXRF | 440 ± 19 | 350 ± 26 | 79.5% | 2.0 |
| Fe | TXRF | 40,900 ± 600 | 36,600 ± 420 | 89.8% | 10.0 |
| Ni | TXRF | 39.5 ± 2.3 | 35.6 ± 1.7 | 90.0% | 0.4 |
| Cu | TXRF | 310 ± 12 | 319 ± 21 | 102.9% | 0.8 |
| Zotn | TXRF | 364 ± 23 | 107.2 ± 5.5 | 101.8% | 2.0 |
| As | TXRF | 26.2 ± 1.5 | 26.8 ± 2.0 | 102.3% | 2.0 |
| As | GF-AAS | 26.2 ± 1.5 | 27.5 ± 5.1 | 104.9% | 0.008 |
| Pb | TXRF | 183 ± 8 | 182 ± 6 | 99.2% | 2.0 |
| Element | Method | LOD (µg/L) |
|---|---|---|
| Mn | TXRF | 30 |
| Fe | TXRF | 50 |
| Ni | TXRF | 25 |
| Cu | TXRF | 20 |
| Zn | TXRF | 10 |
| As | GF-AAS | 0.2 |
| Sb | Flame-AAS | 1000 |
| Pb | TXRF | 15 |
| Spearman’s ρ | p-Value | ||
|---|---|---|---|
| Total As | Total Fe | 0.562 | 0.024 |
| Total As | Total Mn | 0.700 | 0.019 |
| Available As (mg/kg) | Total As | 0.833 | <0.001 |
| Available As (mg/kg) | Total Mn | 0.606 | 0.008 |
| Available As (mg/kg) | Clay | −0.515 | 0.029 |
| Availability As (%) | Total Mn | −0.556 | 0.017 |
| Availability As (%) | Total As | −0.538 | 0.021 |
| Available Sb (mg/kg) | Total Sb | 0.486 | 0.041 |
| Available Sb (mg/kg) | Clay | −0.682 | 0.002 |
| Availability Sb (%) | Total Sb | −0.829 | <0.001 |
| Availability Sb (%) | Humus | −0.538 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adlassnig, W.; Schmidt, B.; Jirsa, F.; Gradwohl, A.; Ivesic, C.; Koller-Peroutka, M. The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians. Int. J. Environ. Res. Public Health 2022, 19, 6010. https://doi.org/10.3390/ijerph19106010
Adlassnig W, Schmidt B, Jirsa F, Gradwohl A, Ivesic C, Koller-Peroutka M. The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians. International Journal of Environmental Research and Public Health. 2022; 19(10):6010. https://doi.org/10.3390/ijerph19106010
Chicago/Turabian StyleAdlassnig, Wolfram, Brigitte Schmidt, Franz Jirsa, Andreas Gradwohl, Caroline Ivesic, and Marianne Koller-Peroutka. 2022. "The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians" International Journal of Environmental Research and Public Health 19, no. 10: 6010. https://doi.org/10.3390/ijerph19106010
APA StyleAdlassnig, W., Schmidt, B., Jirsa, F., Gradwohl, A., Ivesic, C., & Koller-Peroutka, M. (2022). The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians. International Journal of Environmental Research and Public Health, 19(10), 6010. https://doi.org/10.3390/ijerph19106010







