Analysis of Patients with Alcohol Dependence Treated in Silesian Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Secombe, R.J.; Stewart, P.C. The impact of alcohol-related admissions on resource use in critically ill patients from 2009 to 2015: An observational study. Anaesth. Intensive Care 2018, 46, 58–66. [Google Scholar] [CrossRef] [PubMed]
- WHO_2015 World Health Organization. Alcohol Fact Sheet. Geneva: World Health Organization. 2015. Available online: http://www.who.int/mediacentre/factsheets/fs349/en/ (accessed on 16 February 2022).
- Mehta, A.J. Alcoholism and critical illness: A review. World J. Crit. Care Med. 2016, 5, 27–35. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.O., Jr.; Lu, B.; Ali, N.A.; Martin, G.S.; Aberegg, S.K.; Marsh, C.B.; Lemeshow, S.; Douglas, I.S. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit. Care Med. 2007, 35, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Bucher, B.; Moore, F.A.; Moore, E.E.; Parsons, P.E. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996, 275, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.; Tønnesen, H.; Andreasson, S.; Helander, A.; Conigrave, K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin. Exp. Res. 2001, 25 (Suppl. S5), 164S–170S. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, N.; Bell, S.; Shen, C.; Nguyen, V.; La, J.; Dubreuil, M.; Strymish, J.; Brophy, M.; Mehta, M.; Wu, H.; et al. Disulfiram use is associated with lower risk of COVID-19: A retrospective cohort study Observational Study. PLoS ONE 2021, 16, e0259061. [Google Scholar] [CrossRef]
- McPeake, J.M.; Shaw, M.; O’Neill, A.; Forrest, E.; Puxty, A.; Quasim, T.; Kinsella, J. Do alcohol use disorders impact on long term outcomes from intensive care? Crit. Care 2015, 19, 185. [Google Scholar] [CrossRef]
- Cervellione, K.L.; Shah, A.; Patel, M.C.; Duran, L.C.; Ullah, T.; Thurm, C. Alcohol and Drug Abuse Resource Utilization in the ICU. Subst. Abuse. 2019, 13, 1178221819869327. [Google Scholar] [CrossRef]
- McPeake, J.; Forrest, E.; Quasim, T.; Kinsella, J.; O’Neill, A. Health and social consequences of an alcohol-related admission to critical care: A qualitative study. BMJ Open 2016, 6, e009944. [Google Scholar] [CrossRef][Green Version]
- Uusaro, A.; Parviainen, I.; Tenhunen, J.J.; Ruokonen, E. The proportion of intensive care unit admissions related to alcohol use: A prospective cohort study. Acta Anaesthesiol. Scand. 2005, 49, 1236–1240. [Google Scholar] [CrossRef]
- Krzych, Ł.J.; Czempik, P.F.; Kucewicz-Czech, E.; Knapik, P. Silesian Registry of Intensive Care Units. Anaesthesiol. Intensive Ther. 2017, 49, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Weigl, W.; Adamski, J.; Gorynski, P.; Kanski, A.; Hultström, M. Mortality rate is higher in Polish intensive care units than in other European countries. Intensive Care Med. 2017, 43, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Knapik, P.; Krzych, Ł.J.; Weigl, W.; Adamski, J.; Hultstöm, M. Mortality rate in Polish intensive care units is lower than predicted according to the APACHE II scoring system. Intensive Care Med. 2017, 43, 1745–1746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weigl, W.; Adamski, J.; Gorynski, P.; Kanski, A.; Hultström, M. ICU mortality and variables associated with ICU survival in Poland: A nationwide database study. Eur. J. Anaesthesiol. 2018, 35, 949–954. [Google Scholar] [CrossRef]
- Rehm, J.; Anderson, P. Prevalence of and potential influencing factors for alcohol dependence in Europe. Eur. Addict. Res. 2015, 21, 6–18. [Google Scholar] [CrossRef]
- Geary, T.T.; O’Brien, P.; Ramsay, S.; Cook, B. Scottish Intensive Care Trainees’ Audit Share Group A national service evaluation of the impact of alcohol on admissions to Scottish intensive care units. Anaesthesia 2012, 67, 1132–1137. [Google Scholar] [CrossRef]
- Paul Secombe, P.; Campbell, L.; Brown, A.; Bailey, M.; Pilcher, D. Alcohol misuse and critical care admissions in the Northern Territory. Intern. Med. J. 2021, 51, 1433–1440. [Google Scholar] [CrossRef]
- Riuttanen, A.; Jäntti, S.J.; Mattila, V.M. Alcohol use in severely injured trauma patients. Sci. Rep. 2020, 10, 17891. [Google Scholar] [CrossRef]
- Wojtyniak, B.; Goryński, P. Sytuacja zdrowotna ludnosci Polski i jej uwarunkowania. Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny. Available online: https://kampania17celow.pl/wp-content/uploads/2017/06/sytuacja-zdrowotna-ludnosci-w-polsce-2016-s.pdf (accessed on 16 February 2022).
- Wit, M.; Best, A.M.; Gennings, C.; Burnham, E.L.; Moss, M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin. Exp. Res. 2007, 31, 1224–1230. [Google Scholar] [CrossRef]
- Lyubinets, O.; Kachmarska, M.; Sygit, K.M.; Cipora, E.; Grshybowskyj, J. Mortality and Alcohol as Its Cause-Comparative Characteristics of the Two Neighboring Countries: Ukraine and Poland. Int. J. Environ. Res. Public Health 2021, 18, 10810. [Google Scholar] [CrossRef]
- Lieber, C.S. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res. Health 2003, 27, 220–231. [Google Scholar] [PubMed]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’s Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef] [PubMed]
- Weil, Z.M.; Corrigan, J.D.; Karelina, K. Alcohol Use Disorder and Traumatic Brain Injury. Alcohol Res. 2018, 39, 171–180. [Google Scholar] [PubMed]
- McHugo, G.J.; Krassenbaum, S.; Donley, S.; Corrigan, J.D.; Bogner, J.; Drake, R.E. The Prevalence of Traumatic Brain Injury Among People with Co-Occurring Mental Health and Substance Use Disorders. J. Head Trauma Rehabil. 2017, 32, E65–E74. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Schmidlin, A.; Klinzing, S.; Schüpbach, R.; Unseld, S.; Pagnamenta, A. Delayed prophylaxis with unfractionated heparin increases the risk of venous thromboembolic events in patients with moderate to severe traumatic brain injury: A retrospective analysis. Anest. Int. Ter. 2020, 52, 28–33. [Google Scholar] [CrossRef]
- Montravers, P.; Kantor, E.; Constantin, J.M.; Lefrant, J.Y.; Lescot, T.; Nesseler, N.; Paugam, C.; Jabaudon, M.; Dupont, H. Epidemiology and prognosis of anti-infective therapy in the ICU setting during acute pancreatitis: A cohort study. Crit. Care 2019, 23, 393. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef]
- Soran, A.; Chelluri, L.; Lee, K.K.; Tisherman, S.A. Outcome and quality of life of patients with acute pancreatitis requiring intensive care. J. Surg. Res. 2000, 91, 89–94. [Google Scholar] [CrossRef]
- Fernandez, A.C.; Gicquelais, R.E.; Jannausch, M.; Bohnert, A.S.B. The Role of Drugs in Alcohol Poisoning and Blackout Events: A Latent Class Analysis of a Residential Treatment Sample. Alcohol Clin. Exp. Res. 2019, 43, 2431–2437. [Google Scholar] [CrossRef]
- Long, B.; Lentz, S.; Gottlieb, M. Alcoholic Ketoacidosis: Etiologies, Evaluation, and Management. J. Emerg. Med. 2021, 61, 658–665. [Google Scholar] [CrossRef]
- Day, E.; Rudd, J.H.F. Alcohol use disorders and the heart. Addiction 2019, 114, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Stoyle, G.; Fawcett, P.; Malagon, I. The use of VV-ECMO in patients with drug dependencies. J. Artif. Organs 2018, 21, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Gacouin, A.; Painvin, B.; Coirier, V.; Quelven, Q.; Delange, B.; Joussellin, V.; Belicard, F.; L’her, F.; Maamar, A.; Tulzo, Y.L.; et al. Impact on ICU mortality of moderate alcohol consumption in patients admitted with infection. J. Crit. Care 2020, 57, 91–96. [Google Scholar] [CrossRef] [PubMed]

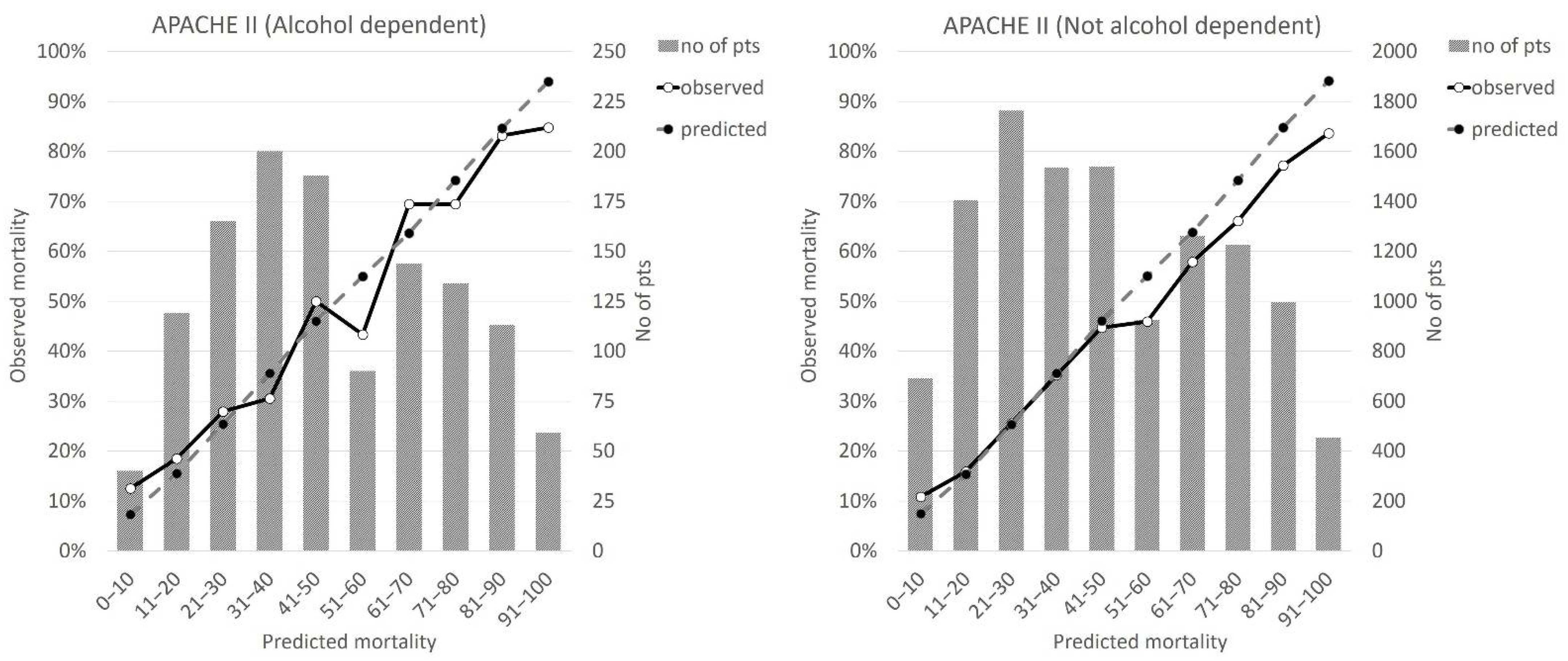

| Alcohol Dependent | Not Alcohol Dependent | p | ||||
|---|---|---|---|---|---|---|
| (n = 2285) | (n = 23,131) | |||||
| Admission | First | 2189 | (95.8%) | 21,781 | (94.2%) | 0.002 |
| Second | 92 | (4.0%) | 1176 | (5.1%) | 0.030 | |
| Another | 4 | (0.2%) | 174 | (0.8%) | 0.002 | |
| Co-morbidities | Coronary artery disease | 355 | (15.5%) | 10,137 | (43.8%) | <0.001 |
| Heart failure | 344 | (15.1%) | 8500 | (36.7%) | <0.001 | |
| Arterial hypertension | 626 | (27.4%) | 12,622 | (54.6%) | <0.001 | |
| Disseminated atherosclerosis | 405 | (17.7%) | 8116 | (35.1%) | <0.001 | |
| Chronic respiratory failure | 147 | (6.4%) | 3013 | (13.0%) | <0.001 | |
| Home oxygen therapy | 3 | (0.1%) | 378 | (1.6%) | <0.001 | |
| Extreme obesity | 39 | (1.7%) | 1417 | (6.1%) | <0.001 | |
| Cachexia | 253 | (11.1%) | 648 | (2.8%) | <0.001 | |
| Diabetes | 270 | (11.8%) | 6120 | (26.5%) | <0.001 | |
| Chronic renal failure | 120 | (5.3%) | 3629 | (15.7%) | <0.001 | |
| Dialysis dependency | 11 | (0.5%) | 310 | (1.3%) | 0.001 | |
| Previous cerebral stroke | 78 | (3.4%) | 1824 | (7.9%) | <0.001 | |
| Chronic neurological disorders | 219 | (9.6%) | 1736 | (7.5%) | <0.001 | |
| Systemic autoimmune diseases | 4 | (0.2%) | 304 | (1.3%) | <0.001 | |
| Post-transplant | 1 | (0.0%) | 73 | (0.3%) | 0.036 | |
| Cancer | 27 | (1.2%) | 2095 | (9.1%) | <0.001 | |
| Pregnancy | 0 | (0.0%) | 41 | (0.2%) | 0.082 | |
| None | 0 | (0.0%) | 2413 | (10.4%) | <0.001 | |
| Variables | Alcohol Dependent | Not Alcohol Dependent | p | ||
|---|---|---|---|---|---|
| (n = 2285) | (n = 23,131) | ||||
| Acute respiratory failure | 1722 | (75.4%) | 17,259 | (74.6%) | 0.448 |
| Exacerbation of resp. failure | 82 | (3.6%) | 1975 | (8.5%) | <0.001 |
| Exacerbation of circulatory failure | 857 | (37.5%) | 11,053 | (47.8%) | <0.001 |
| Multiorgan failure | 373 | (16.3%) | 2929 | (12.7%) | <0.001 |
| Shock | 676 | (29.6%) | 6961 | (30.1%) | 0.629 |
| Cardiac arrest | 717 | (31.4%) | 5401 | (23.3%) | <0.001 |
| Disorders of consciousness | 1185 | (51.9%) | 8866 | (38.3%) | <0.001 |
| Postoperative | 285 | (12.5%) | 7516 | (32.5%) | <0.001 |
| Multiple trauma | 117 | (5.1%) | 819 | (3.5%) | <0.001 |
| Craniocerebral trauma | 287 | (12.6%) | 896 | (3.9%) | <0.001 |
| Acute pancreatitis | 94 | (4.1%) | 293 | (1.3%) | <0.001 |
| Obstetric complications | 0 | (0.0%) | 87 | (0.4%) | 0.006 |
| Acute neurological disorders | 197 | (8.6%) | 1729 | (7.5%) | 0.053 |
| Intoxication | 148 | (6.5%) | 225 | (1.0%) | <0.001 |
| Severe metabolic disorders | 299 | (13.1%) | 1095 | (4.7%) | <0.001 |
| Bacterial infection | 414 | (18.1%) | 4412 | (19.1%) | 0.279 |
| Sepsis | 174 | (7.6%) | 1632 | (7.1%) | 0.342 |
| Viral infection | 6 | (0.3%) | 115 | (0.5%) | 0.163 |
| Variables | Alcohol Dependent | Not Alcohol Dependent | p | ||
|---|---|---|---|---|---|
| (n = 2285) | (n = 23,131) | ||||
| Use of catecholamines | 1684 | (73.7%) | 16,894 | (73.0%) | 0.512 |
| Intubation | 1522 | (66.6%) | 14,942 | (64.6%) | 0.058 |
| Tracheostomy | 356 | (15.6%) | 3826 | (16.5%) | 0.249 |
| Invasive ventilation | 1991 | (87.1%) | 18,991 | (82.1%) | <0.001 |
| Non-invasive ventilation | 62 | (2.7%) | 1132 | (4.9%) | <0.001 |
| Renal replacement therapy | 249 | (10.9%) | 2472 | (10.7%) | 0.784 |
| Operation while in the ICU | 191 | (8.4%) | 2092 | (9.0%) | 0.292 |
| Therapeutic hypothermia | 31 | (1.4%) | 200 | (0.9%) | 0.025 |
| Intra-aortic balloon pump | 13 | (0.6%) | 638 | (2.8%) | <0.001 |
| Extracorporeal membrane oxygenation | 4 | (0.2%) | 113 | (0.5%) | 0.051 |
| Non-Survivors | Survivors | p | ||||
|---|---|---|---|---|---|---|
| (n = 1092) | (n = 1193) | |||||
| Demographic data | Female sex | 241 | (22.1%) | 202 | (16.9%) | 0.002 |
| Age >65 years | 164 | (15.0%) | 155 | (13.0%) | 0.182 | |
| Admission | First | 1056 | (96.7%) | 1133 | (95.0%) | 0.050 |
| Second | 34 | (3.1%) | 58 | (4.9%) | 0.044 | |
| Another | 2 | (0.2%) | 2 | (0.2%) | 0.680 | |
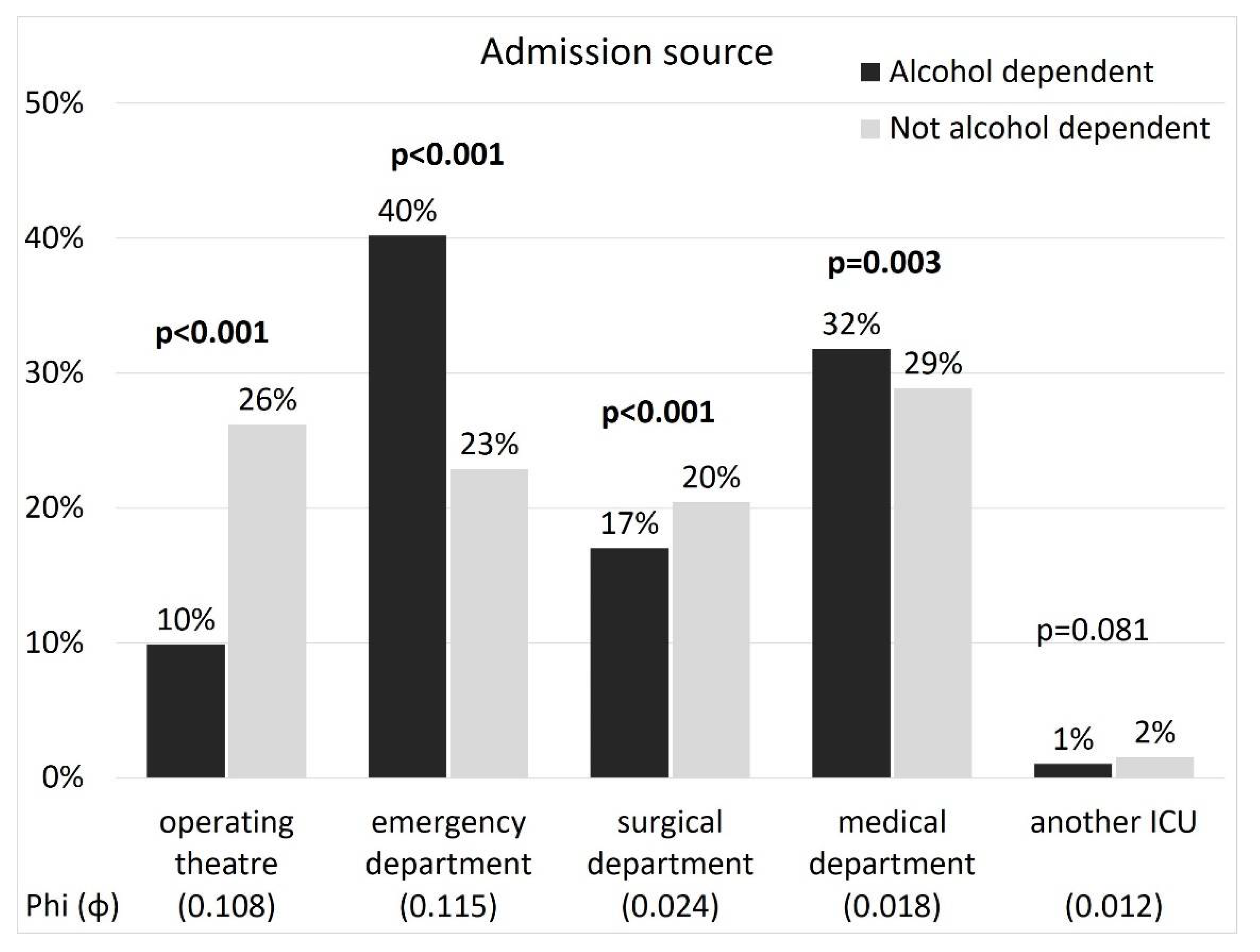
| Admission source | Operating theatre | 89 | (8.2%) | 137 | (11.5%) | 0.009 |
| Emergency department | 436 | (39.9%) | 483 | (40.5%) | 0.818 | |
| Surgical department | 192 | (17.6%) | 197 | (16.5%) | 0.533 | |
| Medical department | 363 | (33.2%) | 364 | (30.5%) | 0.175 | |
| Another ICU | 12 | (1.1%) | 12 | (1.0%) | 0.990 | |
| Comorbidities | Coronary artery disease | 171 | (15.7%) | 184 | (15.4%) | 0.922 |
| Heart failure | 189 | (17.3%) | 155 | (13.0%) | 0.005 | |
| Arterial hypertension | 292 | (26.7%) | 334 | (28.0%) | 0.531 | |
| Disseminated atherosclerosis | 234 | (21.4%) | 171 | (14.3%) | <0.001 | |
| Chronic respiratory failure | 71 | (6.5%) | 76 | (6.4%) | 0.966 | |
| Home oxygen therapy | 2 | (0.2%) | 1 | (0.1%) | 0.939 | |
| Extreme obesity | 19 | (1.7%) | 20 | (1.7%) | 0.964 | |
| Cachexia | 152 | (13.9%) | 101 | (8.5%) | <0.001 | |
| Diabetes | 137 | (12.5%) | 133 | (11.1%) | 0.333 | |
| Chronic renal failure | 79 | (7.2%) | 41 | (3.4%) | <0.001 | |
| Dialysis dependency | 7 | (0.6%) | 4 | (0.3%) | 0.452 | |
| Previous cerebral stroke | 39 | (3.6%) | 39 | (3.3%) | 0.778 | |
| Chronic neurological disorders | 107 | (9.8%) | 112 | (9.4%) | 0.793 | |
| Systemic autoimmune diseases | 2 | (0.2%) | 2 | (0.2%) | 0.680 | |
| Post-transplant | 1 | (0.1%) | 0 | (0.0%) | 0.965 | |
| Cancer | 14 | (1.3%) | 13 | (1.1%) | 0.817 | |
| Pregnancy | 0 | (0.0%) | 0 | (0.0%) | - | |
| Other | 205 | (18.8%) | 188 | (15.8%) | 0.064 | |
| Variables | Non-Survivors | Survivors | p | ||
|---|---|---|---|---|---|
| (n = 1092) | (n = 1193) | ||||
| Acute respiratory failure | 858 | (78.6%) | 864 | (72.4%) | 0.001 |
| Exacerbation of respiratory failure | 36 | (3.3%) | 46 | (3.9%) | 0.545 |
| Exacerbation of circulatory failure | 537 | (49.2%) | 320 | (26.8%) | <0.001 |
| Multiorgan failure | 281 | (25.7%) | 92 | (7.7%) | <0.001 |
| Shock | 460 | (42.1%) | 216 | (18.1%) | <0.001 |
| Cardiac arrest | 480 | (44.0%) | 237 | (19.9%) | <0.001 |
| Disorders of consciousness | 582 | (53.3%) | 603 | (50.5%) | 0.203 |
| Postoperative | 118 | (10.8%) | 167 | (14.0%) | 0.025 |
| Multiple trauma | 39 | (3.6%) | 78 | (6.5%) | 0.002 |
| Craniocerebral trauma | 115 | (10.5%) | 172 | (14.4%) | 0.006 |
| Acute pancreatitis | 53 | (4.9%) | 41 | (3.4%) | 0.090 |
| Obstetric complications | 0 | (0.0%) | 0 | (0.0%) | - |
| Acute neurological disorders | 85 | (7.8%) | 112 | (9.4%) | 0.197 |
| Intoxication | 49 | (4.5%) | 99 | (8.3%) | <0.001 |
| Severe metabolic disorders | 201 | (18.4%) | 98 | (8.2%) | <0.001 |
| Bacterial infection | 207 | (19.0%) | 207 | (17.4%) | 0.347 |
| Sepsis | 94 | (8.6%) | 80 | (6.7%) | 0.092 |
| Viral infection | 3 | (0.3%) | 3 | (0.3%) | 0.764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łowicka-Smolarek, M.; Kokoszka-Bargieł, I.; Knapik, M.; Śmietanka, K.; Dyrda, P.; Możdżeń, M.; Kurczab, M.; Borkowski, J.; Knapik, P. Analysis of Patients with Alcohol Dependence Treated in Silesian Intensive Care Units. Int. J. Environ. Res. Public Health 2022, 19, 5914. https://doi.org/10.3390/ijerph19105914
Łowicka-Smolarek M, Kokoszka-Bargieł I, Knapik M, Śmietanka K, Dyrda P, Możdżeń M, Kurczab M, Borkowski J, Knapik P. Analysis of Patients with Alcohol Dependence Treated in Silesian Intensive Care Units. International Journal of Environmental Research and Public Health. 2022; 19(10):5914. https://doi.org/10.3390/ijerph19105914
Chicago/Turabian StyleŁowicka-Smolarek, Małgorzata, Izabela Kokoszka-Bargieł, Małgorzata Knapik, Konstanty Śmietanka, Piotr Dyrda, Mateusz Możdżeń, Magdalena Kurczab, Jarosław Borkowski, and Piotr Knapik. 2022. "Analysis of Patients with Alcohol Dependence Treated in Silesian Intensive Care Units" International Journal of Environmental Research and Public Health 19, no. 10: 5914. https://doi.org/10.3390/ijerph19105914
APA StyleŁowicka-Smolarek, M., Kokoszka-Bargieł, I., Knapik, M., Śmietanka, K., Dyrda, P., Możdżeń, M., Kurczab, M., Borkowski, J., & Knapik, P. (2022). Analysis of Patients with Alcohol Dependence Treated in Silesian Intensive Care Units. International Journal of Environmental Research and Public Health, 19(10), 5914. https://doi.org/10.3390/ijerph19105914






