The Optical Characterization and Distribution of Dissolved Organic Matter in Water Regimes of Qilian Mountains Watershed
Abstract
:1. Introduction
2. Materials and Methods
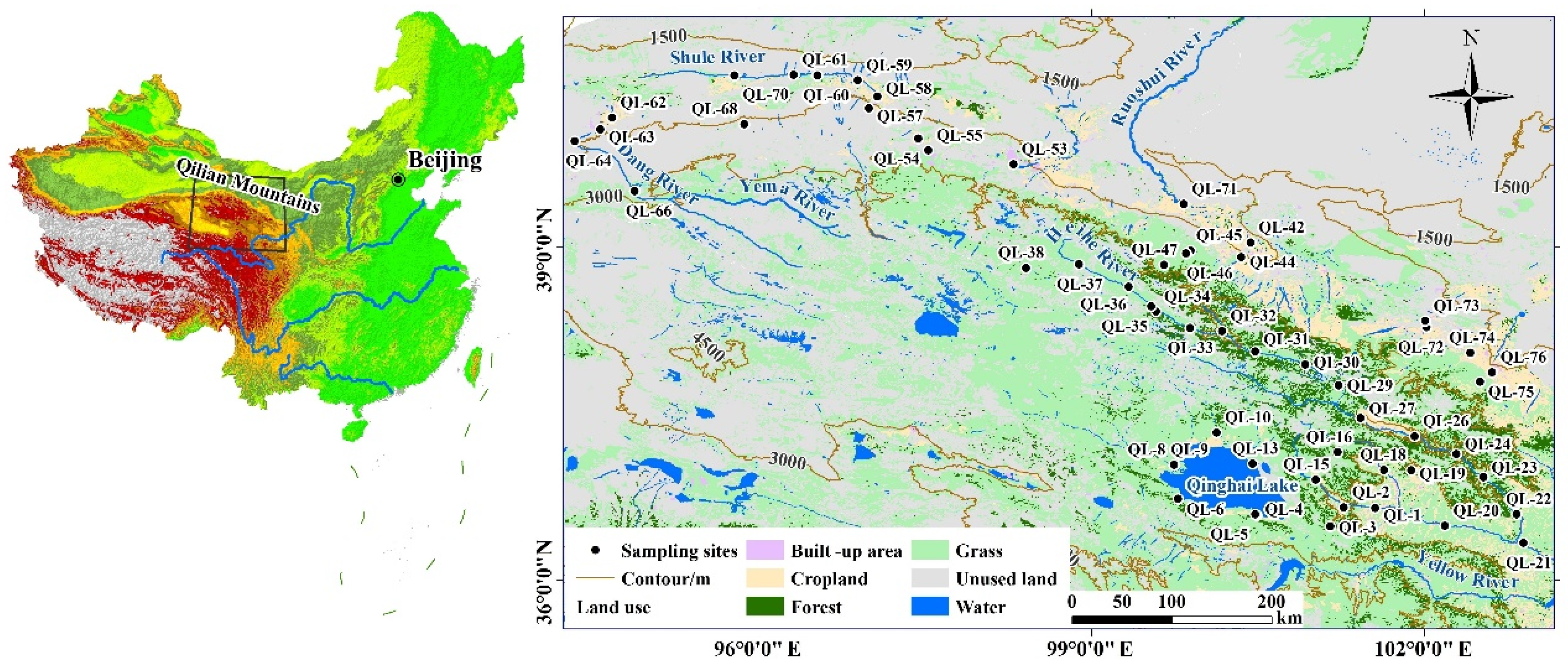
2.1. Description of the Study Sites
2.2. Analytical Studies
2.3. Absorbance and Fluorescence Spectroscopy
2.4. Data Management and Statistical Analysis
3. Results
3.1. Hydro-Chemical Characteristics of the River and Lake Water Samples
3.1.1. Inorganic Parameters
3.1.2. Organic Parameters
4. Discussion
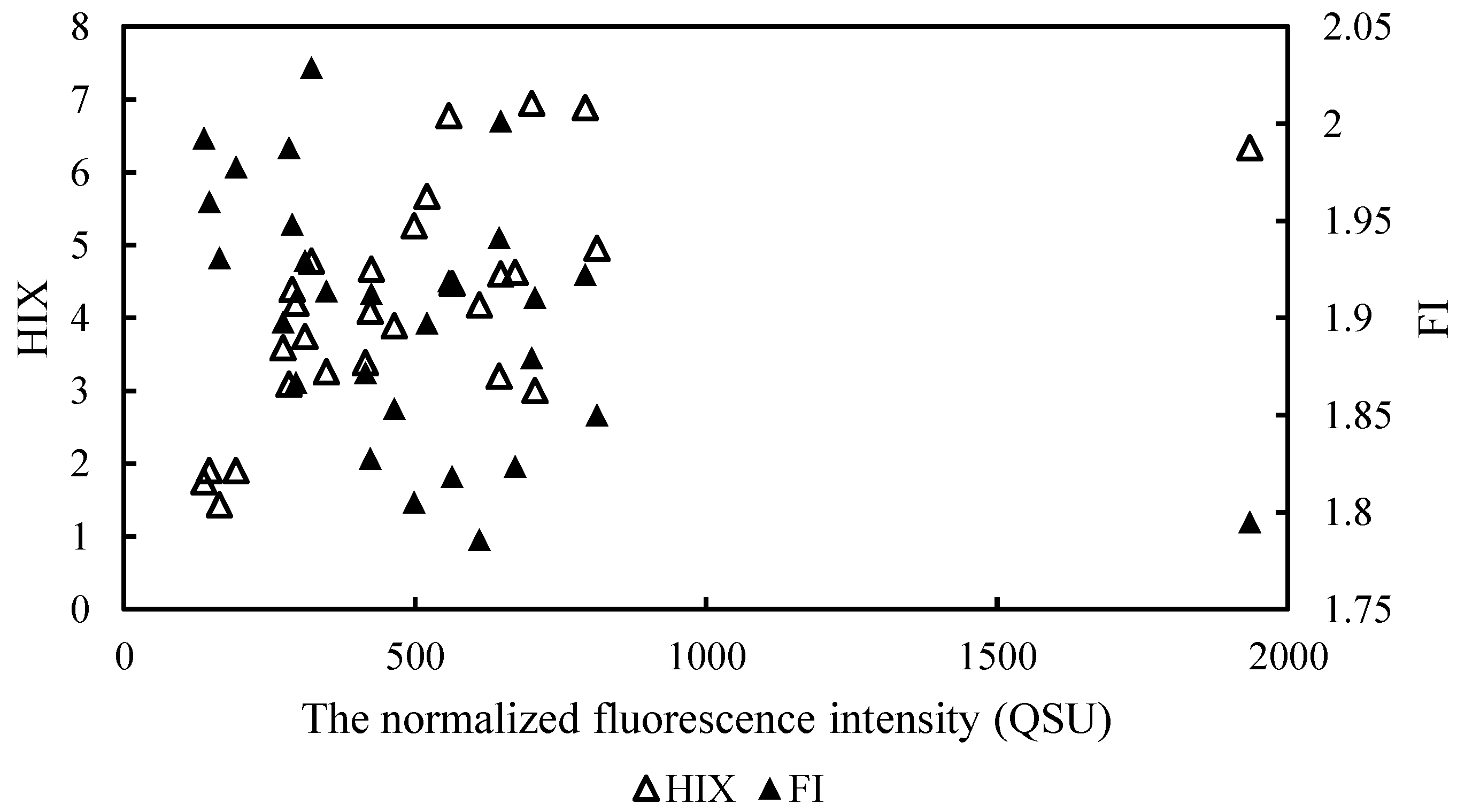
4.1. Fluorescent Indices
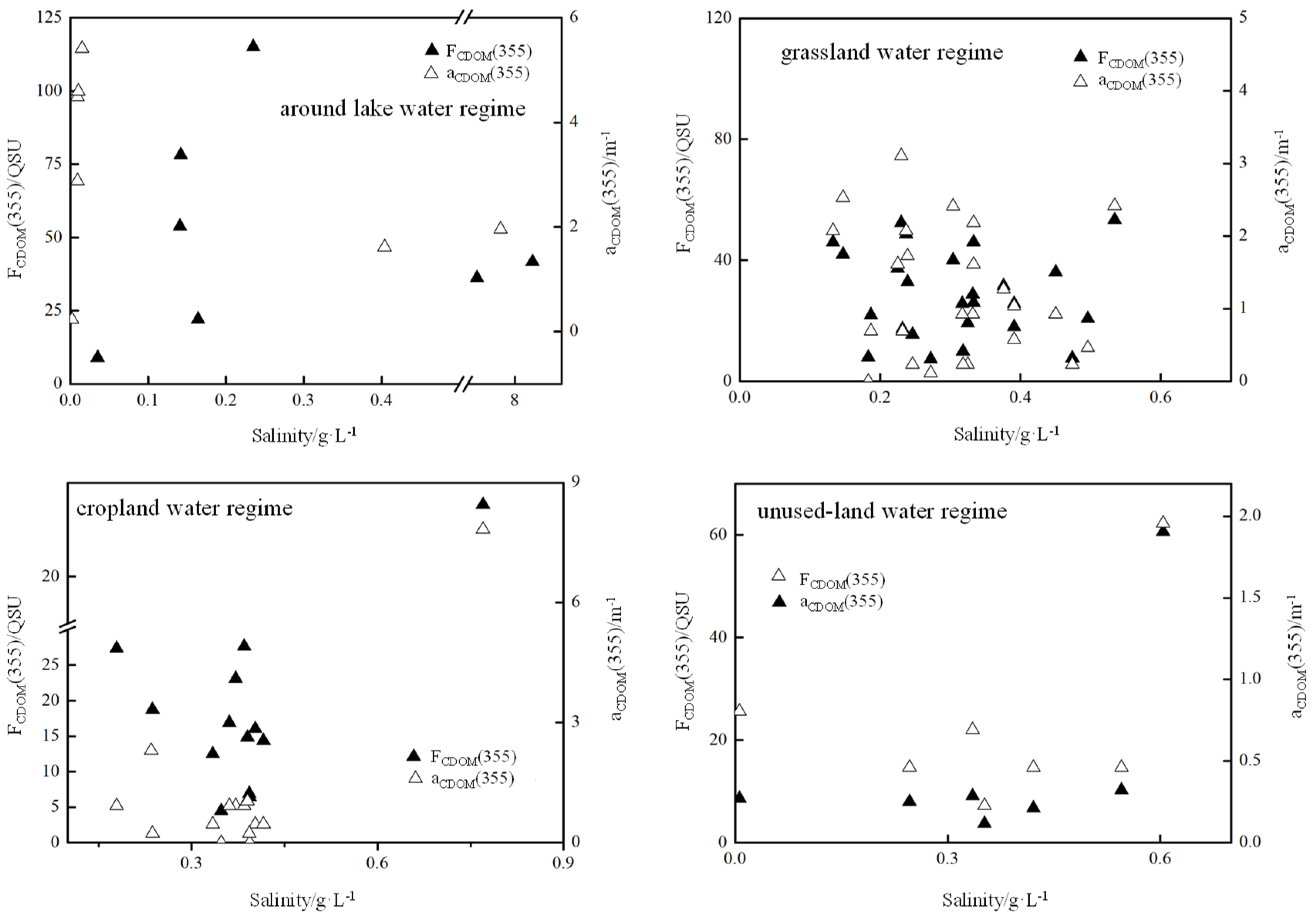
4.2. CDOM Absorption and Fluorescence Variability in Different Water Regimes
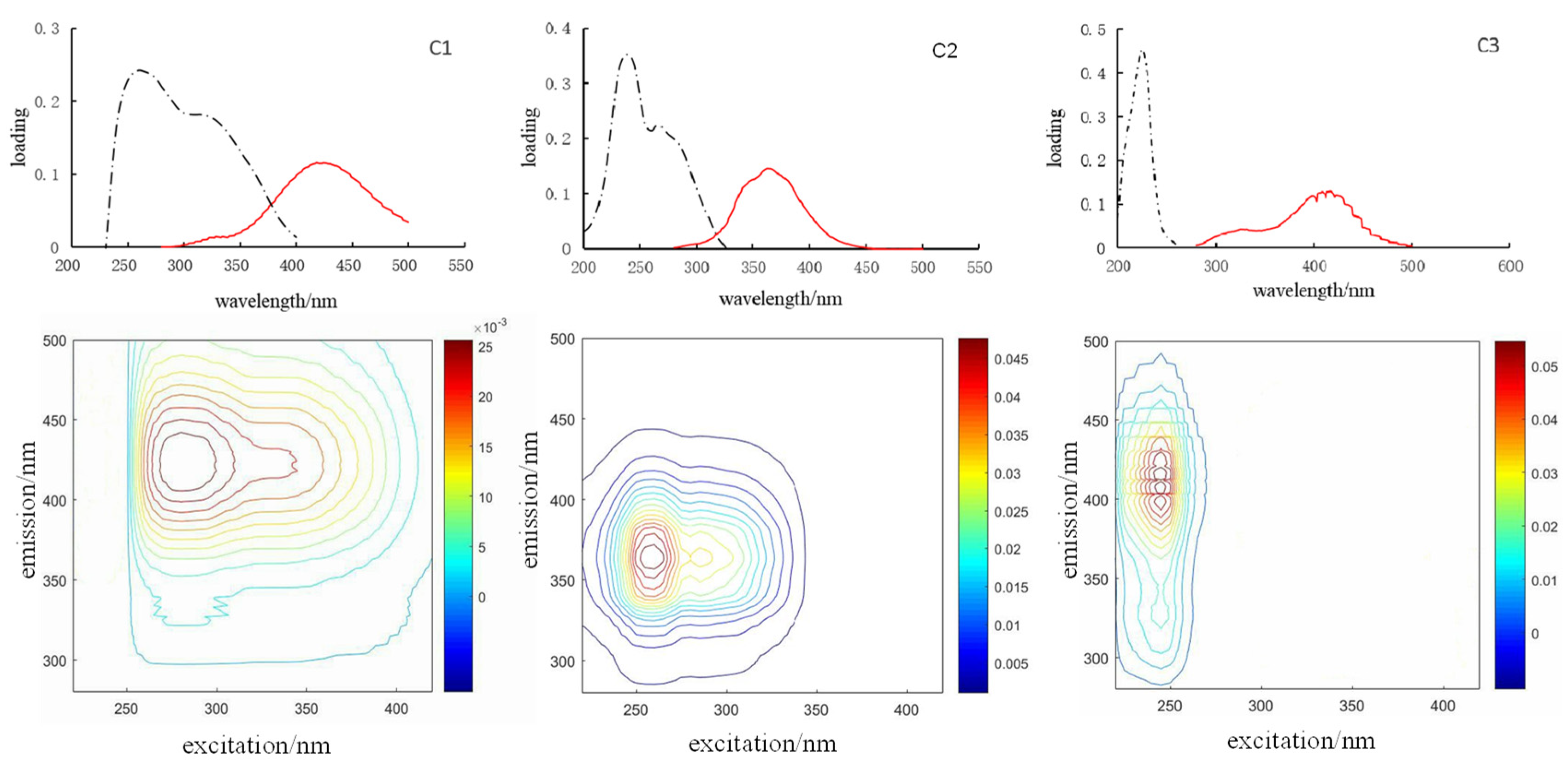
4.3. Component Variability along the Transect
4.4. Relationship between the Spectral Indices of DOM and Other Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOM | Dissolved Organic Matter |
| CDOM | Chromophoric Dissolved Organic Matter |
| PARAFAC | Parallel Factor |
| EEMS | Excitation–Emission Matrix Spectroscopy |
| HL | Humic Acid-Like |
| FL | Fulvic Acid-Like |
| EC | Electrical Conductivity |
| Eh | Oxidation and Reduction Potential (ORP) |
| DO | Dissolved Oxygen |
| SAL | Salinity |
References
- Zisopoulou, K.; Panagoulia, D. An In-Depth Analysis of Physical Blue and Green Water Scarcity in Agriculture in Terms of Causes and Events and Perceived Amenability to Economic Interpretation. Water 2021, 13, 1693. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Jones, E.R.; Florke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Lee, H.S.; Hur, J.; Shin, H.S. Dynamic exchange between particulate and dissolved matter following sequential resuspension of particles from an urban watershed under photo-irradiation. Environ. Pollut. 2021, 283, 117395. [Google Scholar] [CrossRef]
- Carstea, E.M.; Baker, A.; Bieroza, M.; Reynolds, D.M.; Bridgeman, J. Characterization of dissolved organic matter fluorescence properties by PARAFAC analysis and thermal quenching. Water Res. 2014, 61, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Wilske, C.; Herzsprung, P.; Lechtenfeld, O.; Kamjunke, N.; von Tumpling, W. Photochemically Induced Changes of Dissolved Organic Matter in a Humic-Rich and Forested Stream. Water 2020, 12, 331. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Su, R.G.; Shi, X.Y. Assessing the dynamics of chromophoric dissolved organic matter in the southern Yellow Sea by excitation-emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC). Cont. Shelf Res. 2014, 88, 103–116. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.M.; Xiu, W.; He, W.; Gao, Y.S.; Zhang, D.; Wang, A. Controls of organic matter bioreactivity on arsenic mobility in shallow aquifers of the Hetao Basin, P.R. China. J. Hydrol. 2019, 571, 448–459. [Google Scholar] [CrossRef]
- Kabir, M.M.; Akter, S.; Ahmed, F.T.; Mohinuzzaman, M.; Alam, M.D.U.; Mostofa, K.M.G.; Islam, A.R.M.T. Salinity-induced fluorescence dissolved organic matter influence co-contamination, quality and risk to human health of tube well water in southeast coastal Bangladesh. Chemosphere 2021, 275, 130053. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Sawade, E.; Aryal, R.; Chow, C.W.K.; van Leeuwen, J.; Drikas, M.; Cook, D.; Amal, R. Tracking changes in organic matter during nitrification using fluorescence excitation-emission matrix spectroscopy coupled with parallel factor analysis (FEEM/PARAFAC). J. Environ. Chem. Eng. 2018, 6, 1522–1528. [Google Scholar] [CrossRef]
- Sun, M.P.; Liu, S.Y.; Yao, X.J.; Guo, W.Q.; Xu, J.L. Glacier changes in the Qilian Mountains in the past half-century: Based on the revised first and second Chinese Glacier Inventory. J. Geogr. Sci. 2018, 28, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Yuan, Y.J.; Wei, W.S. Climatic response of Picea crassifolia tree-ring parameters and precipitation reconstruction in the western Qilian Mountains, China. J. Arid Environ. 2011, 75, 1121–1128. [Google Scholar] [CrossRef]
- Huai, B.J.; Wang, J.Y.; Sun, W.J.; Wang, Y.T.; Zhang, W.Y. Evaluation of the near-surface climate of the recent global atmospheric reanalysis for Qilian Mountains, Qinghai-Tibet Plateau. Atmos. Res. 2021, 250, 105401. [Google Scholar] [CrossRef]
- Son, K.; Lin, L.; Band, L.; Owens, E.M. Modelling the interaction of climate, forest ecosystem and hydrology to estimatecatchment dissolved organic carbon export. Hydrol. Process. 2019, 33, 1448–1464. [Google Scholar] [CrossRef]
- Tang, Y.T.; Zhang, Y.; Zhou, B.Z.; Wang, G.J.; Jian, S.L.; Li, K.M.; Zhao, K. Investigation of fish resources in Qilian Mountains of Qinghai Province. J. Gansu Agric. Univ. 2021, 56, 1–7. [Google Scholar]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Brym, A.; Paerl, H.W.; Montgometry, M.T.; Handsel, L.T.; Ziervogel, K.; Osburn, C.L. Optical and chemical characterization of base-extracted particulate organic matter in coastal marine environments. Mar. Chem. 2014, 162, 96–113. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.H.; Zhuang, W.E.; Chen, W.; Shi, W.X.; Zhu, Z.Y.; Yang, L.Y. Effects of fish culture on particulate organic matter in a reservoir-type river as revealed by absorption spectroscopy and fluorescence EEM-PARAFAC. Chemosphere 2020, 239, 124734. [Google Scholar] [CrossRef]
- Huang, S.B.; Wang, Y.X.; Ma, T.; Tong, L.; Wang, Y.Y.; Liu, C.R.; Zhao, L. Linking groundwater dissolved organic matter to sedimentary organic matter from a fluvio-lacustrine aquifer at Jianghan Plain, China by EEM-PARAFAC and hydrochemical analyses. Sci. Total Environ. 2015, 529, 131–139. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Mcknight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of DOM for indication of precursor material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Parlanti, E.; Giraudel, J.L.; Roumaillac, A.; Banik, A.; Huguet, A.; Vacher, L. Fluorescence and principal component analysis and parallel factor (PARAFAC) analysis: New criteria for the characterization of dissolved organic matter in aquatic environments. In Proceedings of the 13th Meeting of the International Humic Substances Society, Karlsruhe, Germany, 30 July–4 August 2006; pp. 369–372. [Google Scholar]
- Singh, S.; Sa, E.J.D.; Swenson, E.M. Chromophoric dissolved organic matter (CDOM) variability in Barataria Basin using excitation-emission matrix (EEM) fluorescence and parallel factor analysis (PARAFAC). Sci. Total Environ. 2010, 408, 3211–3222. [Google Scholar] [CrossRef]
- Parlanti, E.K.; Worz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Chi, Z.F.; Wang, W.J.; Li, H.; Wu, H.T.; Yan, B.X. Soil organic matter and salinityas critical factors affecting bacterial community and function of Phragmites australisdominated riparian and coastal wetlands. Sci. Total Environ. 2020, 762, 143156. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing DOM in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Clark, C.D.; De Bruyn, W.J.; Aiona, P.D. Temporal variation in optical properties of chromophoric dissolved organic matter (CDOM) in Southern California coastal waters with nearshore kelp and seagrass. Limnol. Oceanogr. 2015, 61, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.D.; De Bruyn, W.J.; Brahm, B.; Aiona, P. Optical properties of chromophoric dissolved organic matter (CDOM) and dissolved organic carbon (DOC) levels in constructed water treatment wetland systems in southern California, USA. Chemosphere 2020, 247, 125906. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P. OpenFluor- an online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Lambert, T.; Bouillon, S.; Darchambeau, F.; Massicotte, P.; Borges, A.V. Shift in the chemical composition of dissolved organic matter in the Congo River network. Biogeosciences 2016, 13, 5405–5420. [Google Scholar] [CrossRef] [Green Version]
- Stedmon, C.A.; Markager, S.; Kaas, H. Optical properties and signatures of chromophoric dissolved organic matter (CDOM) in Danish Coastal Waters. Estuar. Coast. Shelf Sci. 2000, 51, 267–278. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Zhang, Y.L.; Jeppesen, E.; Murphy, K.R.; Shi, K.; Liu, M.L.; Liu, X.H.; Zhu, G.W. Inflow rate-driven changes in the composition and dynamics of chromophoric dissolved organic matter in a large drinking water lake. Water Res. 2016, 100, 211–221. [Google Scholar] [CrossRef]
- Hu, B.; Wang, P.F.; Wang, C.; Qian, J.; Bao, T.L.; Shi, Y. Investigating spectroscopic and copper-binding characteristics of organic matter derived from sediments and suspended particles using EEM-PARAFAC combined with two-dimensional fluorescence/FTIR correlation analyses. Chemosphere 2019, 219, 45–53. [Google Scholar] [CrossRef]
- Wang, Z.G.; Liu, W.Q.; Zhao, N.J.; Li, H.B.; Zhang, Y.J.; Si-Ma, W.C.; Liu, J.G. Composition analysis of colored dissolved organic matter in Taihu Lake based on three dimension excitation-emission fluorescence matrix and PARAFAC model, and the potential application in water quality monitoring. J. Environ. Sci. 2007, 19, 787–791. [Google Scholar] [CrossRef]
- Cuss, C.W.; Gueguen, C. Relationships between molecular weight and fluorescence properties for size-fractionated dissolved organic matter from fresh and aged sources. Water Res. 2015, 68, 487–497. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Durako, M.J.; Young, H.; Kahn, A.E.; Cooper, W.J.; Gonsior, M. Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: Interannual variability. Mar. Chem. 2009, 113, 182–196. [Google Scholar] [CrossRef]
- Lee, M.H.; Osburn, C.L.; Shin, K.H.; Hur, J. New insight into the applicability of spectroscopic indices for dissolved organic matter (DOM) source discrimination in aquatic systems affected by biogeochemical processes. Water Res. 2018, 147, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, C.M.; Aeschbacher, M.; Page, S.E.; Wenk, J.; Sander, M.; McNeill, K. Photooxidation-induced changes in optical, electrochemical and photochemical properties of humic substances. Environ. Sci. Technol. 2014, 48, 2688–2696. [Google Scholar] [CrossRef] [Green Version]
- del Buey, P.; Sanz-Montero, M.E.; Braissant, O.; Cabestrero, O.; Visscher, P.T. The role of microbial extracellular polymeric substances on formation of sulfate minerals and fibrous Mg-clays. Chem. Geol. 2021, 581, 120403. [Google Scholar] [CrossRef]
- Osburn, C.L.; Retamal, L.; Vincent, W.F. Photoreactivity of chromophoric dissolved organic matter transported by the Mackenzie River to the Beaufort Sea. Mar. Chem. 2009, 115, 10–20. [Google Scholar] [CrossRef]
- Petrone, K.C.; Fellman, J.B.; Hood, E.; Donn, M.J.; Grierson, P.F. The origin and function of dissolved organic matter in agro-urban coastal streams. J. Geophys. Res. 2011, 116, 1–13. [Google Scholar] [CrossRef]


| Lake Surrounding Water | Cropland Water | Grassland Water | Unused Land Water | |
|---|---|---|---|---|
| C1 (QSU) | 231.99 ± 164.18 | 127.03 ± 169.59 | 146.26 ± 98.45 | 69.80 ± 91.73 |
| C2 (QSU) | 55.99 ± 32.96 | 63.58 ± 110.95 | 36.18 ± 18.66 | 265.96 ± 619.33 |
| C3(QSU) | 514.91 ± 345.80 | 170.48 ± 123.61 | 322.97 ± 233.78 | 142.69 ± 162.66 |
| a355 (m−1) | 3.02 ± 1.88 | 1.19 ± 2.00 | 1.38 ± 1.15 | 0.72 ± 0.57 |
| S275–295 (nm−1) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 | 0.02 |
| HIX | 4.28 ± 2.09 | 2.92 ± 1.47 | 4.18 ± 1.50 | 1.41 ± 0.52 |
| BIX | 0.93 ± 0.13 | 1.00 ± 0.1 | 0.91 ± 0.06 | 1.35 ± 0.80 |
| Components | Ex/Em | Description | References |
|---|---|---|---|
| Component 1 Humic acid-like | 265/334; 275/425; 315/425 | Terrestrial humic-like material Marine humic matter | C3: 310/380 [28]; C2: 265/475, C4: 275(355)/450 [34,35]; C3: <250/434 [18]; C1: 322/407 [36]; M: 310–320/380–420 [30] |
| Component 2 Protein | 240/363; 265/363 | Tryptophan-like substances free or bound in proteins Tyrosine-like materials | C5: 240/368 [27]; C8: 275/360 [28]; C3: ≤230(285)/340, C5: 275(≤230)/305 [37]; C4: 274/340 [18] |
| Component 3 Fulvic acid-like | 225/332; 225/408; 225/417; 225/423; 225/431 | Microbial humic-like substances | C1: ≤230(300)/418 [34,35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Chen, Z.; Zhang, Y.; Wen, Y.; Shang, L.; Zhong, J. The Optical Characterization and Distribution of Dissolved Organic Matter in Water Regimes of Qilian Mountains Watershed. Int. J. Environ. Res. Public Health 2022, 19, 59. https://doi.org/10.3390/ijerph19010059
Xiao M, Chen Z, Zhang Y, Wen Y, Shang L, Zhong J. The Optical Characterization and Distribution of Dissolved Organic Matter in Water Regimes of Qilian Mountains Watershed. International Journal of Environmental Research and Public Health. 2022; 19(1):59. https://doi.org/10.3390/ijerph19010059
Chicago/Turabian StyleXiao, Min, Zhaochuan Chen, Yuan Zhang, Yanan Wen, Lihai Shang, and Jun Zhong. 2022. "The Optical Characterization and Distribution of Dissolved Organic Matter in Water Regimes of Qilian Mountains Watershed" International Journal of Environmental Research and Public Health 19, no. 1: 59. https://doi.org/10.3390/ijerph19010059
APA StyleXiao, M., Chen, Z., Zhang, Y., Wen, Y., Shang, L., & Zhong, J. (2022). The Optical Characterization and Distribution of Dissolved Organic Matter in Water Regimes of Qilian Mountains Watershed. International Journal of Environmental Research and Public Health, 19(1), 59. https://doi.org/10.3390/ijerph19010059






