Comparing Advanced with Basic Telerehabilitation Technologies for Patients with Rett Syndrome—A Pilot Study on Behavioral Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
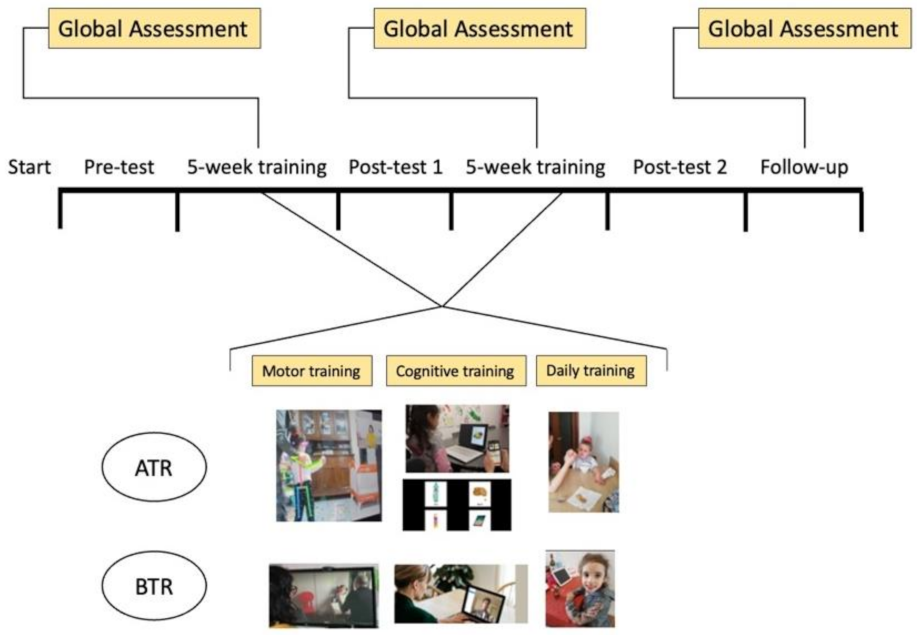
2.2. Study Design
2.3. Assessment and Measures
2.3.1. Attention
2.3.2. Type and Duration of Stereotypies
2.3.3. Global Evaluation
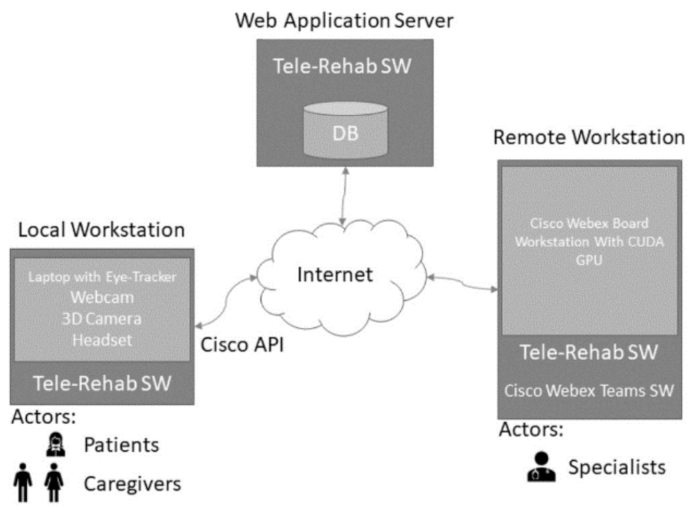
2.3.4. The Local Workstation
2.3.5. Technological Architecture
2.3.6. The Telerehabilitation Software
2.3.7. Procedure
2.3.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwamm, L.H.; Holloway, R.G.; Amarenco, P.; Audebert, H.J.; Bakas, T.; Chumbler, N.R.; Handschu, R.; Jauch, E.C.; Levine, S.R.; Mayberg, M.; et al. A review of the evidence for the use of telemedicine within stroke systems of care: A scientific statement from the American Heart Association/American Stroke Association. Stroke 2009, 40, 2616–2634. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.M.; Mawson, S.; Brownsell, S. Telerehabilitation: Enabling the remote delivery of healthcare, rehabilitation, and self management. Stud. Health Technol. Inform. 2009, 145, 231–248. [Google Scholar]
- Theodoros, D.; Russell, T. Telerehabilitation: Current perspectives. Stud. Health Technol. Inform. 2008, 131, 191–209. [Google Scholar] [PubMed]
- Marzano, G.; Ochoa-Siguencia, L.; Pellegrino, A. Towards a new wave of telerehabilitation applications. Perspective 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Pramuka, M.; van Roosmalen, L. Telerehabilitation technologies: Accessibility and usability. Int. J. Telerehabilitation 2009, 1, 85–98. [Google Scholar] [CrossRef] [Green Version]
- How, T.V.; Hwang, A.S.; Green, R.E.A.; Mihailidis, A. Envisioning future cognitive telerehabilitation technologies: A co-design process with clinicians. Disabil. Rehabil. Assist. Technol. 2017, 12, 244–261. [Google Scholar] [CrossRef]
- Hosseiniravandi, M.; Kahlaee, A.; Karim, H.; Ghamkhar, L.; Safdari, R. Home-based telerehabilitation software systems for remote supervising: A systematic review. Int. J. Technol. Assess. Health Care 2016, 36, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Kairy, D.; Lehoux, P.; Vincent, C.; Visintin, M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil. Rehabil. 2000, 31, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.; Caute, A.; Haigh, Z.; Galliers, J.; Wilson, S.; Kessie, A.; Hirani, S.; Hegarty, B.; Marshall, J. A comparison of remote therapy, face to face therapy and an attention control intervention for people with aphasia: A quasi-randomised controlled feasibility study. Clin. Rehabil. 2016, 30, 359–373. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the state-of-the-art and areas of application. JMIR Rehabil. Assist. Technol. 2017, 4, 4–7. [Google Scholar] [CrossRef]
- Caprì, T.; Nucita, A.; Iannizzotto, G.; Stasolla, F.; Romano, A.; Semino, M.; Giannatiempo, S.; Canegallo, V.; Fabio, R.A. Telerehabilitation for improving adaptive skills of children and young adults with multiple disabilities: A systematic review. Rev. J. Autism Dev. Disord. 2021, 8, 244–252. [Google Scholar] [CrossRef]
- Corti, C.; Oldrati, V.; Oprandi, M.C.; Ferrari, E.; Poggi, G.; Borgatti, R.; Urgesi, C.; Bardoni, A. Remote technology-based training programs for children with acquired brain injury: A systematic review and a meta-analytic exploration. Behav. Neurol. 2019, 1, 346–987. [Google Scholar] [CrossRef] [Green Version]
- Agostini, M.; Moja, L.; Banzi, R.; Pistotti, V.; Tonin, P.; Venneri, A.; Turolla, A. Telerehabilitation and recovery of motor function: A systematic review and meta-analysis. J. Telemed. Telecare 2015, 21, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Giordano, A.; Bonometti, G.P.; Vanoglio, F.; Paneroni, M.; Bernocchi, P.; Comini, L.; Giordano, A. Feasibility and cost-effectiveness of a multidisciplinary home-telehealth intervention programme to reduce falls among elderly discharged from hospital: Study protocol for a randomized controlled trial. BMC Geriatry 2016, 16, 209. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.J.; Frymark, T.; Franceschini, N.M.; Theodoros, D.G. Assessment and Treatment of Cognition and Communication Skills in Adults with Acquired Brain Injury via Telepractice: A Systematic Review. Am. J. Speech-Lang. Pathol. 2015, 24, 295–315. [Google Scholar] [CrossRef]
- Van De Sandt-Koenderman, W.M.E. Aphasia rehabilitation and the role of computer technology: Can we keep up with modern times? Int. J. Speech-Lang. Pathol. 2011, 13, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Bluma, S.M. Portage Guide to Early Education; Cooperative Education Service Agency: Portage, WI, USA, 1976; p. 12.
- Solana, J.; Caceres, C.; Garcia-Molina, A.; Opisso, E.; Roig, T.; Tormos, J.M.; Gomez, E.J. Improving brain injury cognitive rehabilitation by personalized telerehabilitation services: Guttmann neuropersonal trainer. IEEE J. Biomed. Health Inform. 2015, 19, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, E.L.; Carney, A. Integrating the iPad into an intensive, comprehensive aphasia program. Semin. Speech Lang. 2014, 35, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caprì, T.; Fabio, R.A.; Iannizzotto, G.; Nucita, A. The TCTRS Project: A Holistic Approach for Telerehabilitation in Rett Syndrome. Electronics 2020, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Cardullo, S.; Gamberini, L.; Milan, S.; Mapelli, D. Rehabilitation tool: A pilot study on a new neuropsychological interactive training system. Stud. Health Technol. Inform. 2015, 219, 168–173. [Google Scholar] [PubMed]
- Valentine, A.Z.; Hall, S.S.; Young, E.; Brown, B.J.; Groom, M.J.; Hollis, C.; Hall, C.L. Implementation of Telehealth Services to Assess, Monitor, and Treat Neurodevelopmental Disorders: Systematic Review. J. Med. Internet Res. 2021 2021, 23, e22619. [Google Scholar] [CrossRef] [PubMed]
- Maresca, G.; Maggio, M.G.; De Luca, R.; Manuli, A.; Tonin, P.; Pignolo, L.; Calabrò, R.S. Tele-Neuro-Rehabilitation in Italy: State of the Art and Future Perspectives. Front. Neurol. 2020, 11, 563375. [Google Scholar] [CrossRef]
- Stasolla, F. Virtual Reality and Wearable Technologies to Support Adaptive Responding of Children and Adolescents With Neurodevelopmental Disorders: A Critical Comment and New Perspectives. Front. Psychol. 2021, 12, 720626. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.; Caprì, T.; Iannizzotto, G.; Nucita, A.; Semino, M.; Giannatiempo, S.; Zocca, L.; Fabio, R.A. Social and cognitive interactions through an interactive school service for RTT patients at the COVID-19 time. Front. Psychol. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iannizzotto, G.; Nucita, A.; Fabio, R.A.; Caprì, T.; Bello, L.L. Remote eye-tracking for cognitive telerehabilitation and interactive school tasks in times of COVID-19. Information 2020, 11, 296. [Google Scholar] [CrossRef]
- Castelli, I.; Antonietti, A.; Fabio, R.A.; Lucchini, B.; Marchetti, A. Do Rett syndrome girls possess Theory of Mind? Some evidence from not-treated girls. Life Span Disabil. 2013, XVI2, 157–168. [Google Scholar]
- Fabio, R.A.; Caprì, T.; Buzzai, C.; Pittalà, V.; Gangemi, A. Auditory and visual oddball paradigm evaluated through P300 in five girls with Rett syndrome. Neuroquantology 2018, 17, 40–49. [Google Scholar] [CrossRef]
- Fabio, R.A.; Giannatiempo, S.; Caprì, T. Attention and identification of the same and the similar visual stimuli in Rett Syndrome. Life Span Disabil. 2019, 1, 113–127. [Google Scholar]
- Fabio, R.A.; Giannatiempo, S.; Semino, M.; Caprì, T. Longitudinal cognitive rehabilitation applied with eye-tracker for patients with Rett Syndrome. Res. Dev. Disabil. 2021, 111, 103891. [Google Scholar] [CrossRef]
- Hagberg, B.; Goutières, F.; Hanefeld, F.; Rett, A.; Wilson, J. Rett syndrome: Criteria for inclusion and exclusion. Brain Dev. 1985, 7, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S.; Balla, D.A.; Cicchetti. Vineland ABS; Giunti Editore: Firenze, Italy, 2013. [Google Scholar]
- Fabio, R.A.; Martinazzoli, C.; Antonietti, A. Development and standardization of the “r.a.r.s” (Rett assessment rating scale). Life Span Disabil. 2005, 8, 257–281. [Google Scholar]
- Cervi, F.; Saletti, V.; Turner, K.; Peron, A.; Bulgheroni, S.; Taddei, M.; Vignoli, A. The TAND checklist: A useful screening tool in children with tuberous sclerosis and neurofibromatosis type 1. Orphanet J. Rare Dis. 2020, 15, 1–237. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Stahlhut, M.; Wong, K.; Syhler, B.; Bisgaard, A.; Jacoby, P.; Leonard, H. Validating the rett syndrome gross motor scale. PLoS ONE 2016, 11, e0147555. [Google Scholar] [CrossRef] [Green Version]
- Vignoli, A.; Fabio, R.A.; La Briola, F.; Giannatiempo, S.; Antonietti, A.; Maggiolini, S.; Canevini, M.P. Correlations between neurophysiological, behavioral, and cognitive function in rett syndrome. Epilepsy Behav. 2010, 17, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kozloff, M.; Bates, S. A Program for Families of Children with Learning and Behavior Problems. Cogn. Behav. Ther. 1981, 10, 56–57. [Google Scholar] [CrossRef]
- Rodocanachi Roidi, M.L.; Isaias, I.U.; Cozzi, F.; Grange, F.; Scotti, F.M.; Gestra, V.F.; Ripamonti, E. A new scale to evaluate motor function in rett syndrome: Validation and psychometric properties. Pediatric Neurol. 2019, 100, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S. Vineland Adaptive Behavior Scales. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Nucita, A.; Bernava, G.M.; Giglio, P.; Peroni, M.; Bartolo, M.; Orlando, S.; Marazzi, M.C.; Palombi, L. A Markov chain based model to predict HIV/AIDS epidemiological trends. In International Conference on Model and Data Engineering; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8216, pp. 225–236. [Google Scholar]
- Fisher, R.A. Statistical Methods for Research Workers, 14th ed.; Hafner: New York, NY, USA, 1973. [Google Scholar]
- Fabio, R.A.; Caprì, T.; Nucita, A.; Iannizzotto, G.; Mohammadhasani, N. Eye gaze digital games to improve motivational and attentional ability in Rett syndrome. J. Spec. Educ. Rehabil. 2018, 19, 105–126. [Google Scholar] [CrossRef]
- Fabio, R.A.; Gangemi, A.; Caprì, T.; Budden, S.; Falzone, A. Neurophysiological and cognitive effects of Transcranial Direct Current Stimulation in three girls with Rett Syndrome with chronic language impairments. Res. Dev. Disabil. 2018, 76, 76–87. [Google Scholar] [CrossRef]
- Fabio, R.A.; Gangemi, A.; Semino, M.; Vignoli, A.; Priori, A.; Canevini, M.P.; Di Rosa, G.; Caprì, T.; Veneselli, E. Effects of combined transcranial direct current stimulation with cognitive training in girls with Rett syndrome. Brain Sci. 2020, 10, 276. [Google Scholar] [CrossRef]
- Fabio, R.A.; Pergolizzi, G.; Nucita, A.; Iannizzotto, G.; Caprì, T. The role of a virtual avatar in attention and memory tasks in Rett syndrome. BMC Neurol. 2021, 21, 223. [Google Scholar] [CrossRef]
- Gangemi, A.; Caprí, T.; Fabio, R.A.; Puggioni, P.; Falzone, A.M.; Martino, G. Transcranial direct current stimulation (TDCS) and cognitive empowerment for the functional recovery of diseases with chronic impairment and genetic etiopathogenesis. Adv. Genet. Res. 2018, 18, 179–196. Available online: www.scopus.com (accessed on 2 December 2021).
| Participants | Name | Clinical Stage | Age | MeCP2 Mutation | Level of Severity (RARS) | Functional Ability Level |
|---|---|---|---|---|---|---|
| ATR Group | ||||||
| 1 | L.G | IV | 25 | T158M | 75.5 | 75 |
| 2 | L.A | IV | 25 | T158M | 75.5 | 75 |
| 3 | D.D | IV | 31 | R306C | 75 | 90 |
| 4 | C.A | III | 5 | T158M | 58 | 84 |
| 5 | A.C | III | 5 | ---- | 71 | 71 |
| 6 | C.L | III | 4 | P152R | 69.5 | 109 |
| 7 | F.D | IV | 18 | T158M | 64 | 136 |
| 8 | S.M | III | 14 | T158M | 62 | 91 |
| 9 | D.F | IV | 25 | R255X | 64 | 111 |
| 10 | C.M | III | 7 | P322L | 65.5 | 104 |
| 11 | S.D | IV | 15 | P133C | 72 | 151 |
| BTR Group | ||||||
| 1 | B.C | III | 5 | R255X | 71 | 75 |
| 2 | S.A | III | 10 | P322L | 75 | 108 |
| 3 | B.G | IV | 24 | P152R | 75 | 74 |
| 4 | G.L | IV | 10 | R255X | 75.5 | 84 |
| 5 | S.L | IV | 9 | T158M | 70 | 78 |
| 6 | B.A | III | 10 | P152R | 75 | 71 |
| 7 | P.V | III | 8 | ---- | 65.5 | 69 |
| 8 | L.M | III | 9 | P322L | 58 | 136 |
| 9 | M.S | IV | 24 | T158M | 64 | 110 |
| 10 | S.P | IV | 22 | T158M | 62 | 105 |
| 1. Basic Behaviors Area: Evaluates the prerequisite behaviors for learning and communication, they are: spontaneous eye contact, eye contact on request, looking at objects, tracking objects and faces, functional gestures, cooperation with simple spoken requests (reply to their name, look for mother), sitting long enough to complete a task, object permanence, be able to wait for their turn before starting an activity, and be able to communicate basic needs (need to eat, drink, sleep, play, walk, go to the bathroom, and feel good or bad). |
| 2. Neuropsychological Area: Evaluates brain-based skills which are needed in acquisition of knowledge, manipulation of information, and reasoning. They have more to do with the mechanisms of how people learn, remember, problem-solve, and pay attention, rather than with actual knowledge. This area includes selective attention, types and intensity of stereotypes, lateralization, temporal orientation, spatial orientation, memory span, logical sequences, and categorization (animals, dress, foods, drinks, objects, places, actions). |
| 3. Basic Cognitive Area: Evaluates the basic cognitive concepts that allow the understanding of reality (spatial concepts, topological concepts, etc.). This area includes object recognition, color discrimination, geometric form discrimination measure concepts, spatial concepts, human body discriminations, time concepts, and cause-effect relationship. |
| 4. Advanced Cognitive Area: Evaluates the concepts of school learning that include the sub-areas of writing and mathematics. This area includes global words recognition, syllables recognition, recompleting words through syllables, alphabetic symbols recognition, recompleting words with alphabetic symbols, recognition of words representing actions, using words to communicate, math pre-requisite concepts, recognition of numbers, and biunivocal relation between number and quantity. |
| 5. Communication Area: Evaluates the development of language by measuring responses to environmental sounds and speech, as well as the production of sounds and words. The skills of communication, comprehension and expression that allow the person to interact with others. This area includes expressing a basic need at a corporal level, recognizing, and expressing a basic need through pictures, understanding the biunivocal relation between the corpora, recognizing and expressing a basic need through word, understanding the biunivocal relation of a basic need between a picture and the word that expresses it, verbal comprehension, and verbal production. |
| 6. Emotional Area: Evaluates the person’s abilities and ways of experiencing, expressing, and understanding their own emotions and those of others are analyzed. This area includes identify emotions and express emotions. |
| 7. Hand motor Area: Evaluates the ability to make movements using the small muscles in our hands and wrists. Children rely on these skills to do key tasks in school and in everyday life. Fine motor skills are complex, however. They involve the coordinated efforts of the brain and muscles and they are built on the gross motor skills that allow us to make bigger movements. This area includes musculoskeletal alterations, hand-eye coordination during motor tasks lateralization, reaching movement, touching ability, grasping ability, releasing movement, repositioning movement, bimanual coordination, and the ability to push and pull an object. |
| 8. Graphomotor Area: Evaluates the fine motor skills incorporating, among others, graphomotor skills (GS) which, in turn, involve strength and control of the finger muscles, and incorporates important daily skills such as writing and drawing that are necessary for the academic achievement of all students. This area includes grasping of pencil, drawing patterns, and the use of school tools. |
| 9. Global Motor Area: Evaluates the gross-motor skills which are important for an upright posture, walking, running, and climbing. It allows for the observation of physical weakness or disability or defects of movement. This area includes: standing, sitting, parachute reactions, rolling supine—on one side, rolling supine—prone, supine—to seated on the floor, seated on the floor—to standing, seated on a chair—to standing, standing—to seated on the floor, standing—to seated on a chair, walking, body spatial orientation in standing, stepping, running, climbing upstairs, descending stairs, jumping, picking up an object from the ground (small ball), playing with a ball, and walking on a slope. |
| 10. Autonomy in Daily Life Area: Measures early adaptive and self-help behavior typically seen at home, as well as social behavior that develops through early adult-child interactions; therefore, this area analyses the level of autonomy in the praxis of daily life This area includes daily autonomy such as, eating, drinking, coughing or difficulty breathing during meal, type of food’s consistence, washing, autonomy in the bathroom and dressing, and other skills such as, playing and socialization skills, and advanced autonomy activities. |
| Pre-Test | Post-Test1 | Post-Test2 | p | ||||
|---|---|---|---|---|---|---|---|
| Measures | Experimental | Control | Experimental | Control | Experimental | Control | |
| Attention time | 11.36 (7.88) | 12.17 (7.33) | 18.82 (8.74) | 16.67 (7.37) | 29.64 (8.64) | 19.17 (4.92) | 0.05 |
| Intensity of trainer aids | 21.91 (4.46) | 22.50 (7.58) | 10.09 (3.86) | 18.83 (9.40) | 8.64 (4.93) | 16.83 (7.78) | 0.00 |
| Intensity of stereotypes | 2.64 (1.12) | 2.87 (1.11) | 2.91 (1.04) | 2.43 (0.98) | 3.00 (1.00) | 2.86 (1.07) | 0.001 |
| Pre-Test | Post-Test1 | Post-Test2 | p | ||||
|---|---|---|---|---|---|---|---|
| Measures | Experimental | Control | Experimental | Control | Experimental | Control | |
| Basic Behaviors Prerequisites Area | 3.58 (0.47) | 3.47 (0.59) | 4.06 (0.42) | 3.72 (0.53) | 4.24 (0.27) | 3.92 (0.41) | 0.01 |
| Neuropsychological Area | 1.90 (0.28) | 1.72 (0.48) | 2.45(0.40) | 2.05(0.55) | 2.86 (0.50) | 2.29 (0.66) | 0.01 |
| Basic Cognitive Area | 2.29 (0.84) | 2.06 (0.92) | 2.79 (0.74) | 2.45 (0.88) | 3.22 (0.74) | 2.79 (0.88) | 0.001 |
| Advanced Cognitive Area | 1.00 (0.00) | 1.08 (0.18) | 1.01 (0.03) | 1.10 (0.17) | 1.03 (0.07) | 1.15 (0.17) | 0.08 |
| Communication Area | 2.16 (0.44) | 1.87 (0.59) | 2.60 (0.37) | 2.23 (0.69) | 2.81 (0.35) | 2.37 (0.79) | 0.001 |
| Emotional Area | 2.88 (0.58) | 2.92 (0.74) | 3.38 (0.74) | 3.25 (0.82) | 3.66 (0.76) | 3.42 (0.75) | 0.01 |
| Hand motor Area | 2.82 (0.67) | 2.87 (0.56) | 3.27 (0.83) | 3.20 (0.52) | 3.52 (0.78) | 3.41 (0.61) | 0.01 |
| Graphomotor Area | 1.36 (0.41) | 1.24 (0.37) | 1.55 (0.56) | 1.38 (0.36) | 1.58 (0.56) | 1.48 (0.57) | 0.09 |
| Global Motor Area | 2.61 (0.32) | 3.01 (0.40) | 2.85 (0.32) | 3.19 (0.30) | 2.94 (0.32) | 3.26 (0.29) | 0.01 |
| Autonomy in Daily Life Area | 2.37 (0.41) | 2.10 (0.70) | 2.42 (0.38) | 2.15 (0.74) | 2.47 (0.39) | 2.17 (0.77) | 0.08 |
| Total Score GAIRS | 2.29 (0.23) | 2.30 (0.44) | 2.66 (0.27) | 2.51 (0.44) | 2.84 (0.26) | 2.67 (0.49) | 0.01 |
| Pre-Test | Post-Test1 | Post-Test2 | p | ||||
|---|---|---|---|---|---|---|---|
| Measures | Experimental | Control | Experimental | Control | Experimental | Control | |
| Vineland Score | 98.70 (26.95) | 95.25 (4.57) | 102.50 (25.23) | 97.25 (7.50) | 105.60 (26.30) | 95.24 (6.44) | 0.001 |
| RARS Score | 67.70 (5.90) | 67.00 (8.80) | 65.75 (6.58) | 66.30 (9.10) | 64.60(5.80) | 65.90 (9.70) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabio, R.A.; Semino, M.; Giannatiempo, S.; Caprì, T.; Iannizzotto, G.; Nucita, A. Comparing Advanced with Basic Telerehabilitation Technologies for Patients with Rett Syndrome—A Pilot Study on Behavioral Parameters. Int. J. Environ. Res. Public Health 2022, 19, 507. https://doi.org/10.3390/ijerph19010507
Fabio RA, Semino M, Giannatiempo S, Caprì T, Iannizzotto G, Nucita A. Comparing Advanced with Basic Telerehabilitation Technologies for Patients with Rett Syndrome—A Pilot Study on Behavioral Parameters. International Journal of Environmental Research and Public Health. 2022; 19(1):507. https://doi.org/10.3390/ijerph19010507
Chicago/Turabian StyleFabio, Rosa Angela, Martina Semino, Samantha Giannatiempo, Tindara Caprì, Giancarlo Iannizzotto, and Andrea Nucita. 2022. "Comparing Advanced with Basic Telerehabilitation Technologies for Patients with Rett Syndrome—A Pilot Study on Behavioral Parameters" International Journal of Environmental Research and Public Health 19, no. 1: 507. https://doi.org/10.3390/ijerph19010507
APA StyleFabio, R. A., Semino, M., Giannatiempo, S., Caprì, T., Iannizzotto, G., & Nucita, A. (2022). Comparing Advanced with Basic Telerehabilitation Technologies for Patients with Rett Syndrome—A Pilot Study on Behavioral Parameters. International Journal of Environmental Research and Public Health, 19(1), 507. https://doi.org/10.3390/ijerph19010507










