Risk Indicators Improve the Prescription Quality of Drugs with Anticholinergic Properties in Nursing Homes
Abstract
:1. Introduction
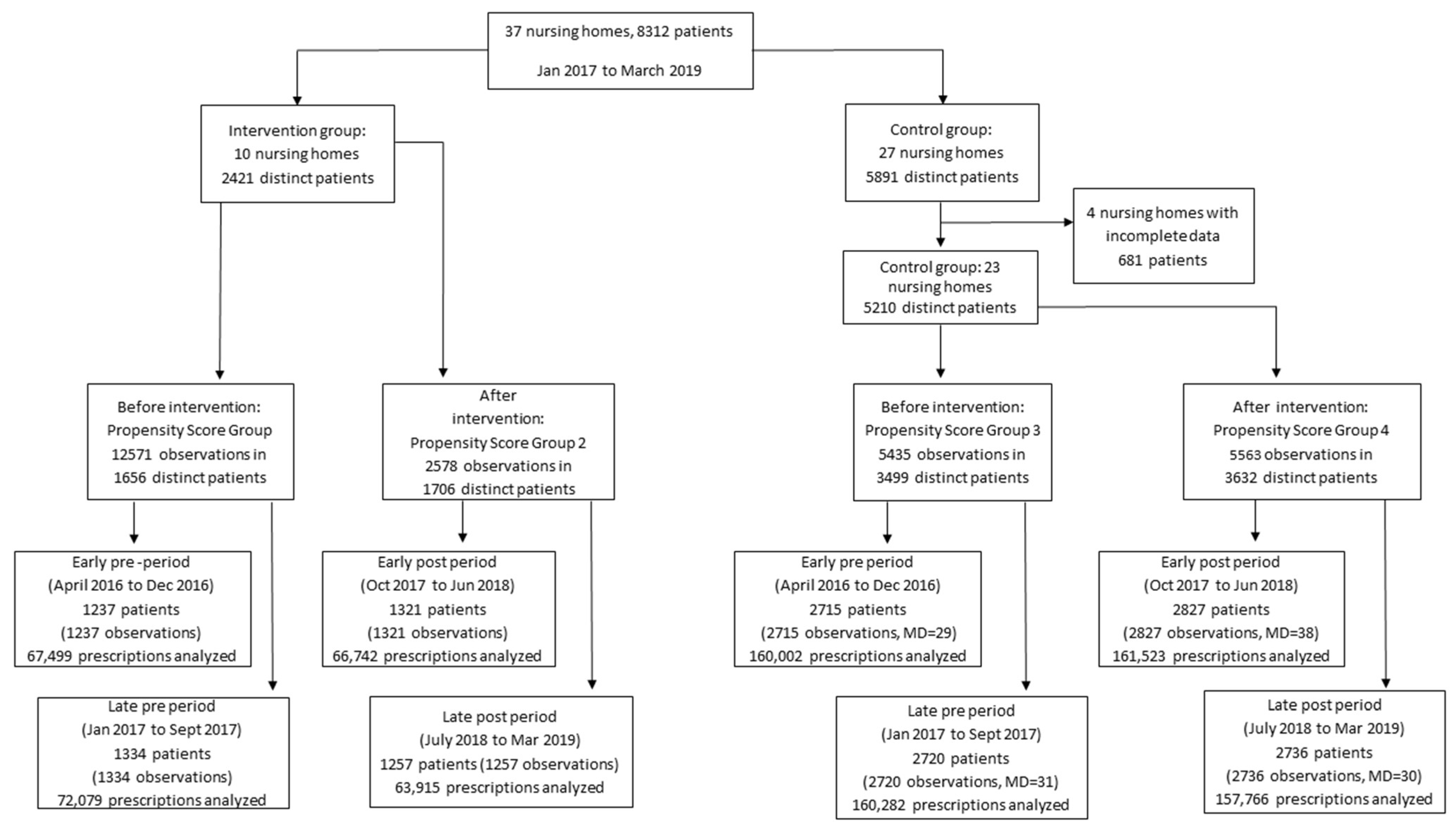
2. Methods
2.1. Study Design
2.2. Intervention-Exposure
- -
- The overall proportion of patients receiving anticholinergics (numerator: number of patients receiving anticholinergics; denominator: total number of patients);
- -
- The number of patients who received at least 1 drug with anticholinergic properties (numerator: number of patients receiving at one least 1 drug with anticholinergic properties; denominator: total number of patients);
- -
- Among these patients, the total number of drugs with anticholinergic properties per patient (calculated as the total number of drugs with anticholinergic properties divided by the total number of patients);
- -
- The average number of drug prescriptions per patient (calculated as the total number of all drugs prescribed divided by the total number of patients considered, i.e., all nursing home residents during the study period). The Quality Indicators were developed in collaboration with the medical directors of the intervention nursing homes. They were chosen because they can easily be retrieved from the information stored in the electronic pill dispensers, and also because they represent variables that are commonly reported as outcomes in other trials of interventions to reduce anticholinergic burden or prescription of anticholinergics in the literature.
2.3. Study Population
2.4. Data Collection
2.5. Statistical Analysis
2.6. Ethical Considerations
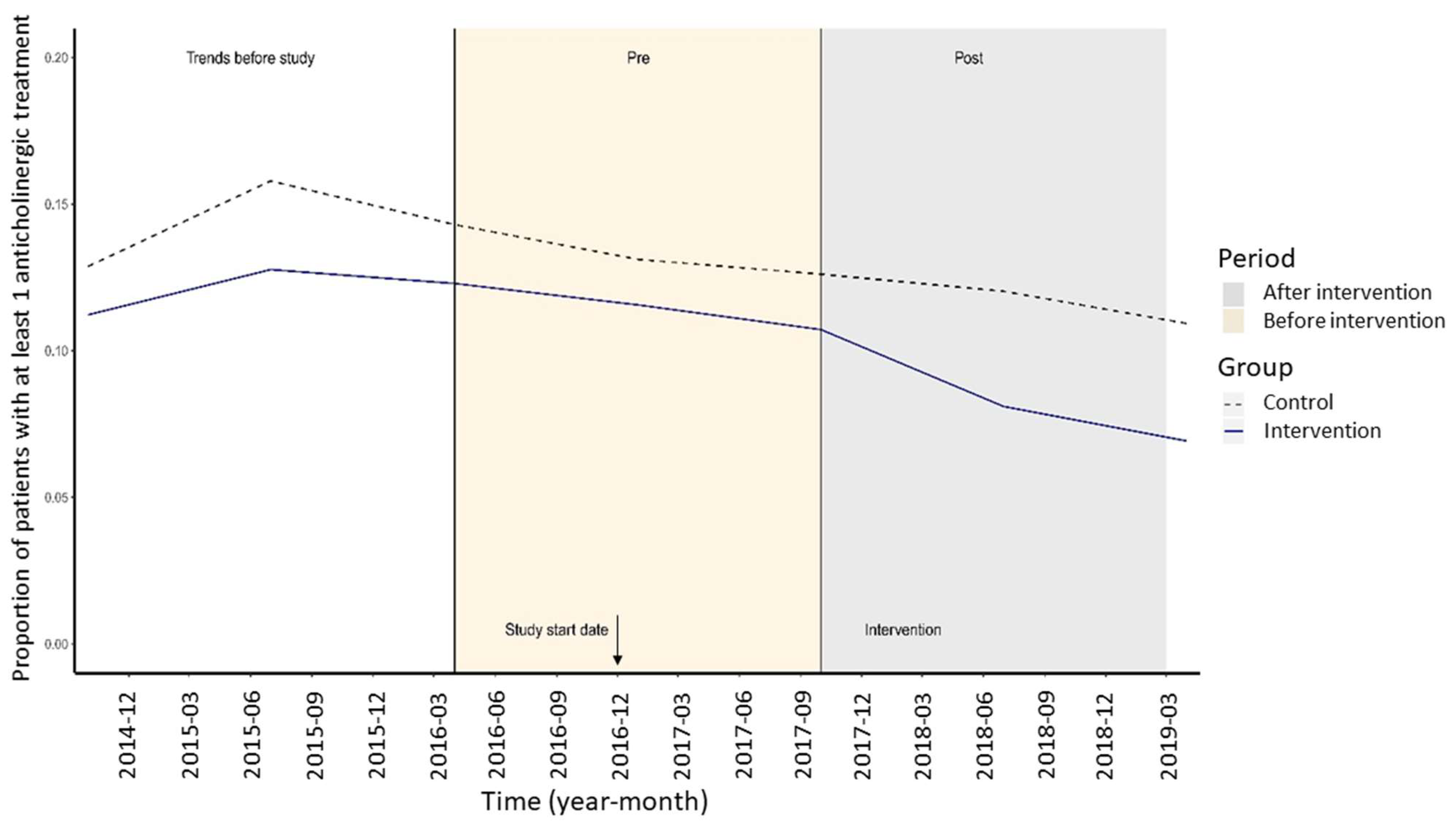
3. Results
4. Discussion
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laroche, M.L.; Charmes, J.P.; Merle, L. Potentially inappropriate medications in the elderly: A French consensus panel list. Eur. J. Clin. Pharmacol. 2007, 63, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.; Ryan, C.; Byrne, S.; Kennedy, J.; O’Mahony, D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int. J. Clin. Pharmacol. Ther. 2008, 46, 72–83. [Google Scholar] [CrossRef]
- Renom-Guiteras, A.; Meyer, G.; Thurmann, P.A. The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. Clin. Pharmacol. 2015, 71, 861–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancelli, I.; Beltrame, M.; Gigli, G.L.; Valente, M. Drugs with anticholinergic properties: Cognitive and neuropsychiatric side-effects in elderly patients. Neurol. Sci. 2009, 30, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lechevallier-Michel, N.; Molimard, M.; Dartigues, J.F.; Fabrigoule, C.; Fourrier-Reglat, A. Drugs with anticholinergic properties and cognitive performance in the elderly: Results from the PAQUID Study. Br. J. Clin. Pharmacol. 2005, 59, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Ness, J.; Hoth, A.; Barnett, M.J.; Shorr, R.I.; Kaboli, P.J. Anticholinergic medications in community-dwelling older veterans: Prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am. J. Geriatr. Pharmacother. 2006, 4, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Paque, K.; Elseviers, M.; Vander Stichele, R.; Dilles, T.; Pardon, K.; Deliens, L.; Christiaens, T. Associations of potentially inappropriate medication use with four year survival of an inception cohort of nursing home residents. Arch. Gerontol. Geriatr. 2019, 80, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, D.L.; La Carpia, D.; Grande, G.; Casucci, P.; Bacelli, T.; Bernabei, R.; Onder, G.; Italian Group for Appropriate Drug Prescription in the Elderly (I-GrADE). Anticholinergic Medication Burden and 5-Year Risk of Hospitalization and Death in Nursing Home Elderly Residents With Coronary Artery Disease. J. Am. Med. Dir. Assoc. 2016, 17, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bali, V.; Carnahan, R.M.; Chen, H.; Johnson, M.L.; Aparasu, R.R. Risk of Mortality Associated with Anticholinergic Use in Elderly Nursing Home Residents with Depression. Drugs Aging 2017, 34, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Fox, C.; Kwok, C.S.; Luben, R.N.; Wareham, N.J.; Khaw, K.T. Total anticholinergic burden and risk of mortality and cardiovascular disease over 10 years in 21,636 middle-aged and older men and women of EPIC-Norfolk prospective population study. Age Ageing 2015, 44, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- Alldred, D.P.; Kennedy, M.C.; Hughes, C.; Chen, T.F.; Miller, P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst. Rev. 2016, 2, CD009095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotty, M.; Halbert, J.; Rowett, D.; Giles, L.; Birks, R.; Williams, H.; Whitehead, C. An outreach geriatric medication advisory service in residential aged care: A randomised controlled trial of case conferencing. Age Ageing 2004, 33, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Nakham, A.; Myint, P.K.; Bond, C.M.; Newlands, R.; Loke, Y.K.; Cruickshank, M. Interventions to Reduce Anticholinergic Burden in Adults Aged 65 and Older: A Systematic Review. J. Am. Med. Dir. Assoc. 2020, 21, 172–180.e175. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, R.M.; Brown, G.D.; Letuchy, E.M.; Rubenstein, L.M.; Gryzlak, B.M.; Smith, M.; Reist, J.C.; Kelly, M.W.; Schultz, S.K.; Weckmann, M.T.; et al. Impact of programs to reduce antipsychotic and anticholinergic use in nursing homes. Alzheimer’s Dement. 2017, 3, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Tay, H.S.; Soiza, R.L.; Mangoni, A.A. Minimizing anticholinergic drug prescribing in older hospitalized patients: A full audit cycle. Ther. Adv. Drug Saf. 2014, 5, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boustani, M.; Campbell, N.; Munger, S.; Maidment, I.; Fox, C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health 2008, 4, 311–320. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch. Intern. Med. 2008, 168, 508–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Pollock, B.G.; Culp, K.R. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J. Clin. Pharmacol. 2006, 46, 1481–1486. [Google Scholar] [CrossRef]
- Dimick, J.B.; Ryan, A.M. Methods for evaluating changes in health care policy: The difference-in-differences approach. JAMA 2014, 312, 2401–2402. [Google Scholar] [CrossRef]
- Stuart, E.A.; Huskamp, H.A.; Duckworth, K.; Simmons, J.; Song, Z.; Chernew, M.; Barry, C.L. Using propensity scores in difference-in-differences models to estimate the effects of a policy change. Health Serv. Outcomes Res. Methodol. 2014, 14, 166–182. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Pope, G.; Wall, N.; Peters, C.M.; O’Connor, M.; Saunders, J.; O’Sullivan, C.; Donnelly, T.M.; Walsh, T.; Jackson, S.; Lyons, D.; et al. Specialist medication review does not benefit short-term outcomes and net costs in continuing-care patients. Age Ageing 2011, 40, 307–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cool, C.; Cestac, P.; McCambridge, C.; Rouch, L.; de Souto Barreto, P.; Rolland, Y.; Lapeyre-Mestre, M. Reducing potentially inappropriate drug prescribing in nursing home residents: Effectiveness of a geriatric intervention. Br. J. Clin. Pharmacol. 2018, 84, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Lapane, K.L.; Hughes, C.M.; Daiello, L.A.; Cameron, K.A.; Feinberg, J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J. Am. Geriatr. Soc. 2011, 59, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Trygstad, T.K.; Christensen, D.B.; Wegner, S.E.; Sullivan, R.; Garmise, J.M. Analysis of the North Carolina long-term care polypharmacy initiative: A multiple-cohort approach using propensity-score matching for both evaluation and targeting. Clin. Ther. 2009, 31, 2018–2037. [Google Scholar] [CrossRef] [PubMed]
- Jamtvedt, G.; Young, J.M.; Kristoffersen, D.T.; O’Brien, M.A.; Oxman, A.D. Does telling people what they have been doing change what they do? A systematic review of the effects of audit and feedback. Qual. Saf. Health Care 2006, 15, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Tuti, T.; Nzinga, J.; Njoroge, M.; Brown, B.; Peek, N.; English, M.; Paton, C.; van der Veer, S.N. A systematic review of electronic audit and feedback: Intervention effectiveness and use of behaviour change theory. Implement. Sci. 2017, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Christina, V.; Baldwin, K.; Biron, A.; Emed, J.; Lepage, K. Factors influencing the effectiveness of audit and feedback: Nurses’ perceptions. J. Nurs. Manag. 2016, 24, 1080–1087. [Google Scholar] [CrossRef]
- Gude, W.T.; van Engen-Verheul, M.M.; van der Veer, S.N.; de Keizer, N.F.; Peek, N. How does audit and feedback influence intentions of health professionals to improve practice? A laboratory experiment and field study in cardiac rehabilitation. BMJ Qual. Saf. 2017, 26, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Buntinx, F.; Winkens, R.; Grol, R.; Knottnerus, J.A. Influencing diagnostic and preventive performance in ambulatory care by feedback and reminders. A review. Fam. Pract. 1993, 10, 219–228. [Google Scholar] [CrossRef]
- Ivers, N.; Jamtvedt, G.; Flottorp, S.; Young, J.M.; Odgaard-Jensen, J.; French, S.D.; O’Brien, M.A.; Johansen, M.; Grimshaw, J.; Oxman, A.D. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Selbaek, G.; Janus, S.I.M.; Bergh, S.; Engedal, K.; Ruths, S.; Helvik, A.S.; Saltyte Benth, J.; Zuidema, S.U. Change in psychotropic drug use in Norwegian nursing homes between 2004 and 2011. Int. Psychogeriatr. 2018, 30, 385–394. [Google Scholar] [CrossRef]
- Kleipool, E.E.F.; Nielen, M.M.J.; Korevaar, J.C.; Harskamp, R.E.; Smulders, Y.M.; Serne, E.; Thijs, A.; Peters, M.J.L.; Muller, M. Prescription patterns of lipid lowering agents among older patients in general practice: An analysis from a national database in the Netherlands. Age Ageing 2019, 48, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, K.H.; Selbaek, G.; Ruths, S. Trends in potentially inappropriate medication prescribing to nursing home patients: Comparison of three cross-sectional studies. Pharmacoepidemiol. Drug Saf. 2017, 26, 192–200. [Google Scholar] [CrossRef] [PubMed]
| Intervention Group * | Control Group * | p-Value | |
|---|---|---|---|
| Number of nursing homes | 10 | 23 | - |
| Number of nursing home residents | 1656 | 3499 | - |
| Number of patients with ≥1 anticholinergic drug during pre-intervention period: mean (SD) | 210 (12.7) | 500 (14.3) | 0.13 |
| Male sex of residents; n (%) | 593 (35.8) | 1144 (32.7) | 0.03 |
| Age ≥ 80 years of residents; n (%) | 1433 (86.5) | 3107 (88.8) | 0.02 |
| Number of beds; median [Q1, Q3] | 86.50 [84.00, 101.00] | 92.00 [80.00, 101.00] | 0.95 |
| Absenteeism; mean (SD) | 0.10 (0.03) | 0.10 (0.04) | 0.06 |
| Full-time or equivalent medical staff; mean, (SD) | 27.70 (5.75) | 25.55 (6.07) | <0.001 |
| Full-time or equivalent non-medical staff; median [Q1, median Q3] | 16.00 [12.00, 18.75] | 16.40 [13.49, 20.50] | 0.003 |
| Bed occupancy; median [Q1, Q3] | 0.97 [0.96, 0.99] | 0.96 [0.93, 0.99] | 0.01 |
| Full-time or equivalent temporary workers; mean (SD) | 12.49 (3.55) | 12.48 (4.86) | 0.62 |
| Full-time or equivalent permanent staff; mean (SD) | 50.71 (8.95) | 50.07 (9.74) | 0.48 |
| Mortality rate; median [Q1, Q3] | 2.00 [1.00, 3.00] | 2.00 [1.00, 3.00] | 0.03 |
| Turnover; median [Q1, Q3] | 0.15 [0.10, 0.20] | 0.15 [0.10, 0.21] | 0.84 |
| Variable | Unadjusted Analysis † | Fully Adjusted PS-Weighted Analysis ‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value (Wald Test) | Odds Ratio | 95% CI | p-Value (Wald Test) | |||
| Period * | 0.04 § | 0.41 § | ||||||
| 2016-04 to 2016-12 (baseline) | 1 | (Reference) | 1 | (Reference) | ||||
| 2017-01 to 2017-09 (second pre-period) | 0.947 | 0.828 | 1.084 | 0.927 | 0.810 | 1.062 | ||
| 2017-10 to 2018-06 (early intervention period) | 0.910 | 0.783 | 1.057 | 0.894 | 0.762 | 1.049 | ||
| 2018-07 to 2019-03 (late intervention period) | 0.805 | 0.690 | 0.939 | 0.848 | 0.698 | 1.030 | ||
| Intervention group (Reference: control group) | 0.850 | 0.734 | 0.985 | 0.03 | 0.826 | 0.635 | 1.075 | 0.16 |
| Effect of intervention in the intervention group: interaction product term | 0.737 | 0.589 | 0.921 | <0.01 | 0.685 | 0.533 | 0.880 | <0.01 |
| Male sex (Reference: female) | 1.069 | 0.964 | 1.186 | 0.21 | 1.024 | 0.886 | 1.183 | 0.75 |
| Age ≥ 80 years (Reference: <80 years) | 0.442 | 0.390 | 0.501 | <0.001 | 0.437 | 0.348 | 0.548 | <0.001 |
| Mortality rate in the nursing home during period || | 1.000 | 0.953 | 1.051 | 0.97 | 0.943 | 0.882 | 1.009 | 0.09 |
| Number of beds || | 1.020 | 0.968 | 1.068 | 0.50 | 1.037 | 0.893 | 1.204 | 0.64 |
| Bed occupancy || | 1.010 | 0.960 | 1.059 | 0.74 | 0.878 | 0.785 | 0.983 | 0.02 |
| Medical full-time equivalents per bed || | 0.954 | 0.908 | 1.002 | 0.06 | 0.997 | 0.863 | 1.151 | 0.96 |
| Non-medical full-time equivalents per bed || | 1.040 | 0.992 | 1.092 | 0.10 | 0.987 | 0.850 | 1.147 | 0.87 |
| Absenteeism || | 0.912 | 0.868 | 0.958 | <0.01 | 0.954 | 0.883 | 1.032 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, S.; Chrusciel, J.; Ndiongue, B.M.; Blochet, C.; Forget, J.F.; Letty, A.; Hay, P.E.; Novella, J.L. Risk Indicators Improve the Prescription Quality of Drugs with Anticholinergic Properties in Nursing Homes. Int. J. Environ. Res. Public Health 2022, 19, 423. https://doi.org/10.3390/ijerph19010423
Sanchez S, Chrusciel J, Ndiongue BM, Blochet C, Forget JF, Letty A, Hay PE, Novella JL. Risk Indicators Improve the Prescription Quality of Drugs with Anticholinergic Properties in Nursing Homes. International Journal of Environmental Research and Public Health. 2022; 19(1):423. https://doi.org/10.3390/ijerph19010423
Chicago/Turabian StyleSanchez, Stéphane, Jan Chrusciel, Biné Mariam Ndiongue, Caroline Blochet, Jean François Forget, Aude Letty, Paul Emile Hay, and Jean Luc Novella. 2022. "Risk Indicators Improve the Prescription Quality of Drugs with Anticholinergic Properties in Nursing Homes" International Journal of Environmental Research and Public Health 19, no. 1: 423. https://doi.org/10.3390/ijerph19010423
APA StyleSanchez, S., Chrusciel, J., Ndiongue, B. M., Blochet, C., Forget, J. F., Letty, A., Hay, P. E., & Novella, J. L. (2022). Risk Indicators Improve the Prescription Quality of Drugs with Anticholinergic Properties in Nursing Homes. International Journal of Environmental Research and Public Health, 19(1), 423. https://doi.org/10.3390/ijerph19010423






