SARS-CoV-2 Aerosol Transmission Indoors: A Closer Look at Viral Load, Infectivity, the Effectiveness of Preventive Measures and a Simple Approach for Practical Recommendations
Abstract
:1. Introduction
State of the Art
- The filter efficiency of the fabric;
- The leakage (i.e., air flow bypassing the mask) during exhalation;
- The leakage during inhalation.
- What viral loads are necessary to infect others via aerosol?
- Which are the most influencing factors regarding airborne transmission?
- Can a risk assessment model be simplified to allow practical recommendations?
- Is there a possibility to implement a simple measurement system for infection risk?
- What is the impact of different prevention measures on the risk of airborne transmission?
2. Materials and Methods
Dose–Response Model to Predict the Individual Infection Risk and the Predicted Attack Rate (PAR)
- Virus-related factor (VF)
- 2
- Situation-related factor (SF)
- 3
- Susceptible-person-related factor (SPF)
- 4
- Personal-protection-measures-related factor (PPF)
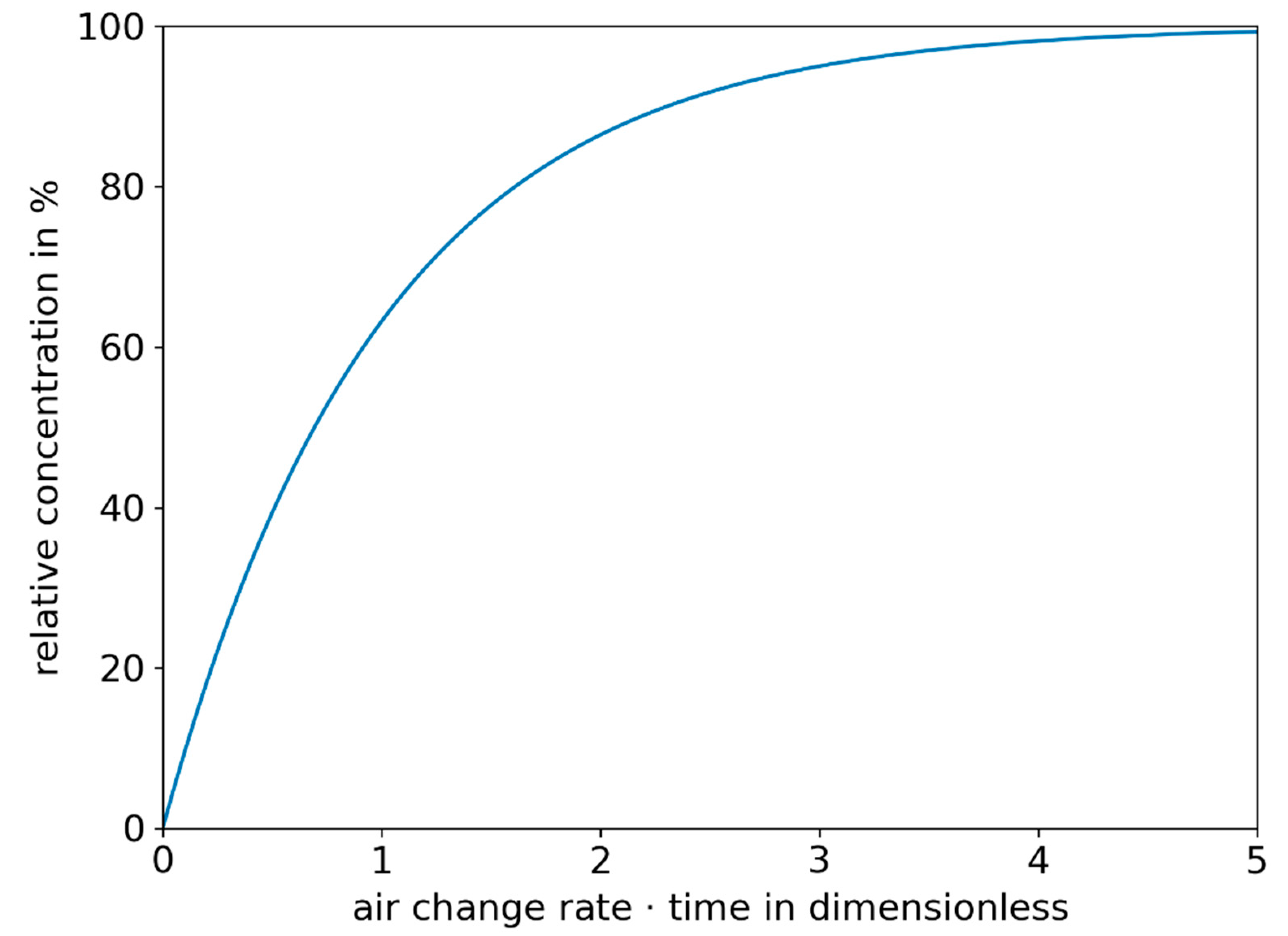
- The aerosol is ideally mixed in the room.
- The near field can contain a much higher virus-laden particle concentration, but it is neglected in the following.
- The air, which is introduced into the room, is free of virus-laden particles.
- A constant decay rate of deposition occurs (in this consideration )
- A constant decay rate because of inactivation occurs (in this investigation )
- The concentration of virus-laden particles at the beginning of unsteady cases is 0 virus-laden particles/m3.
3. Results
3.1. Derivation of Simplified Key Figures and Calculations for the Assessment of Infection Risks and Preventive Measures
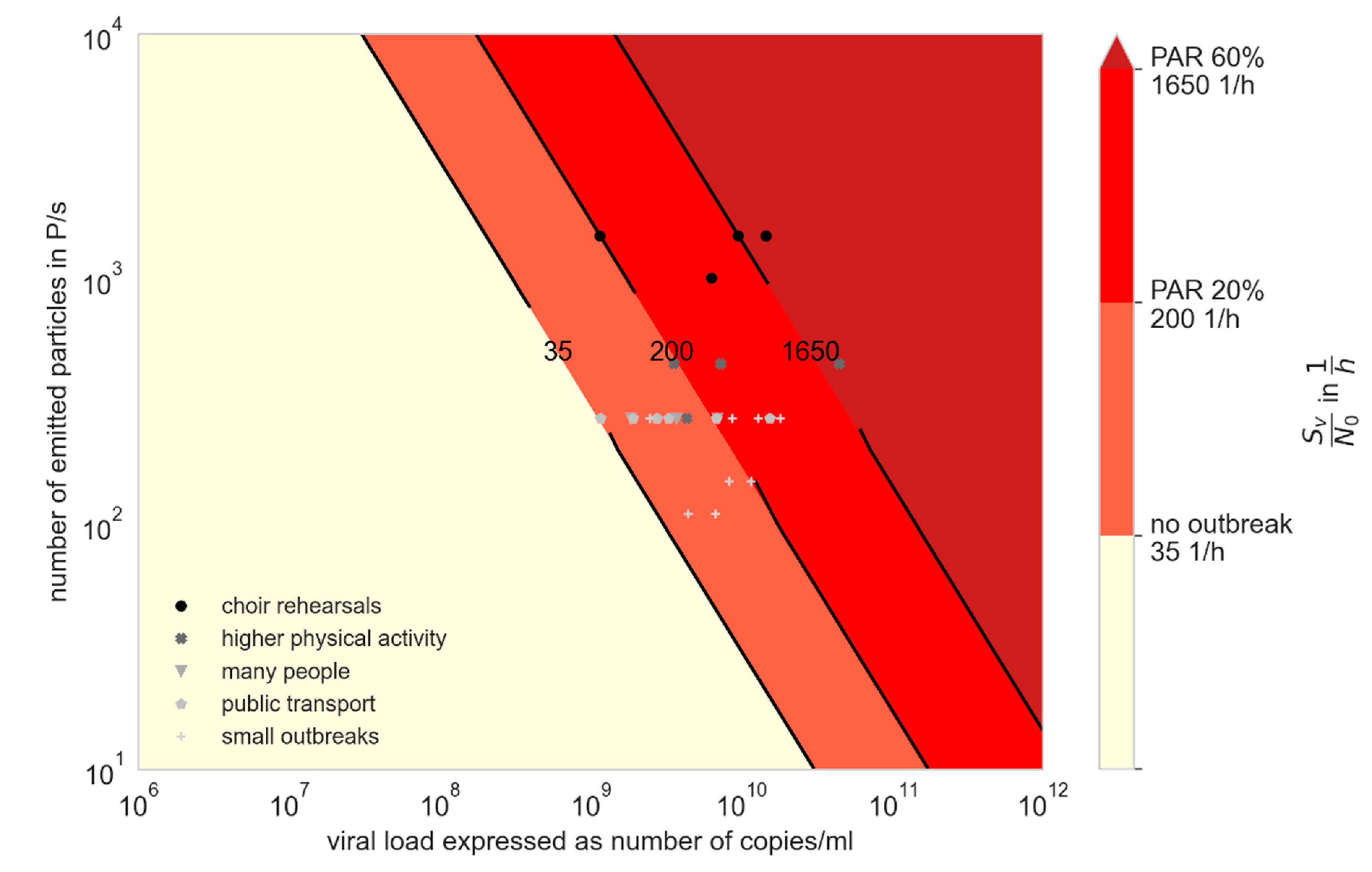
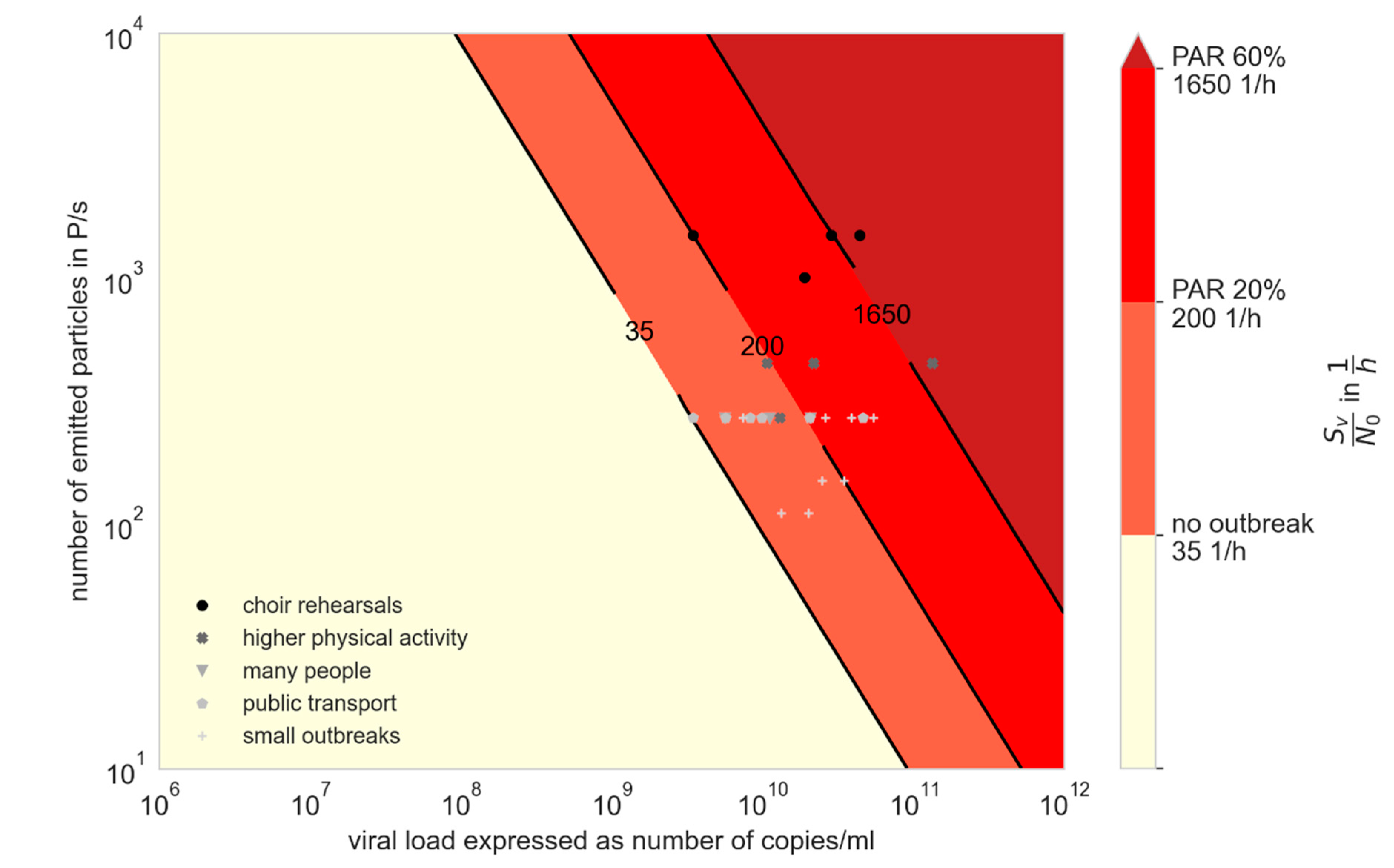
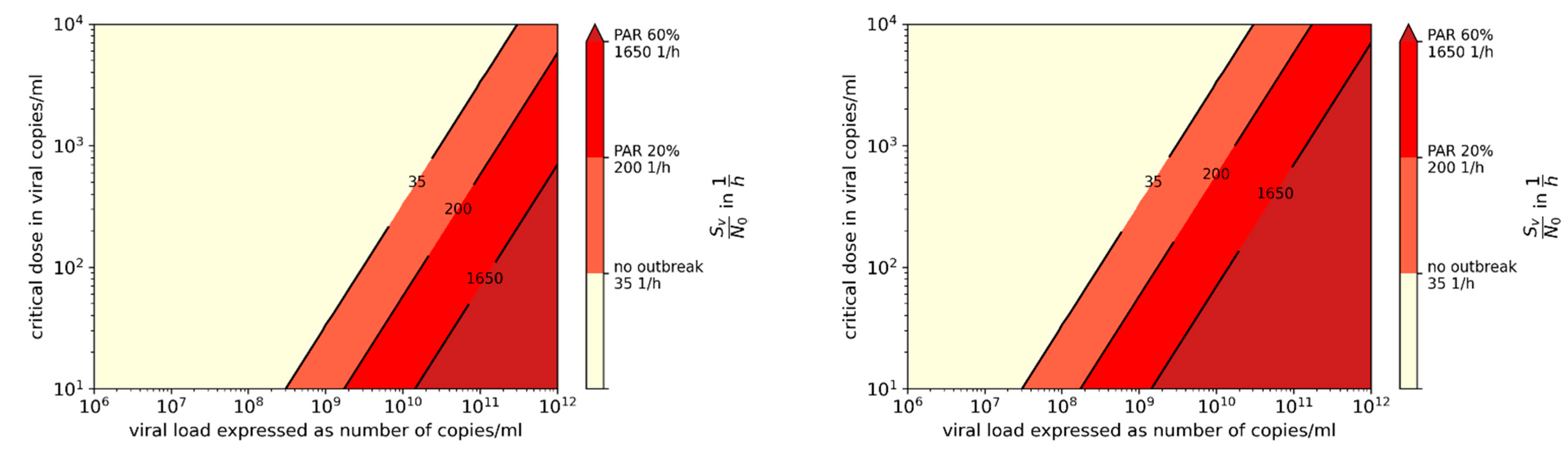
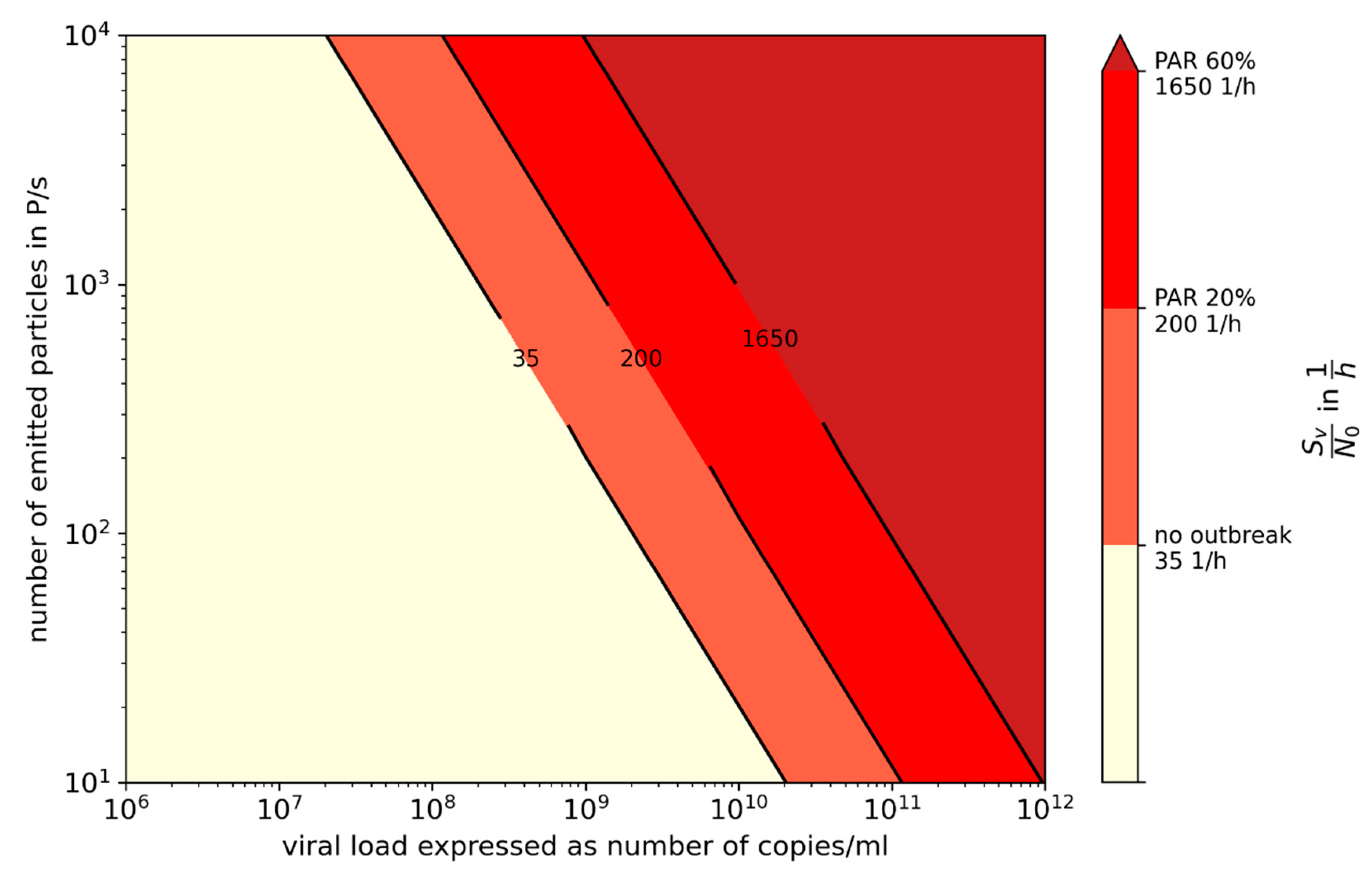
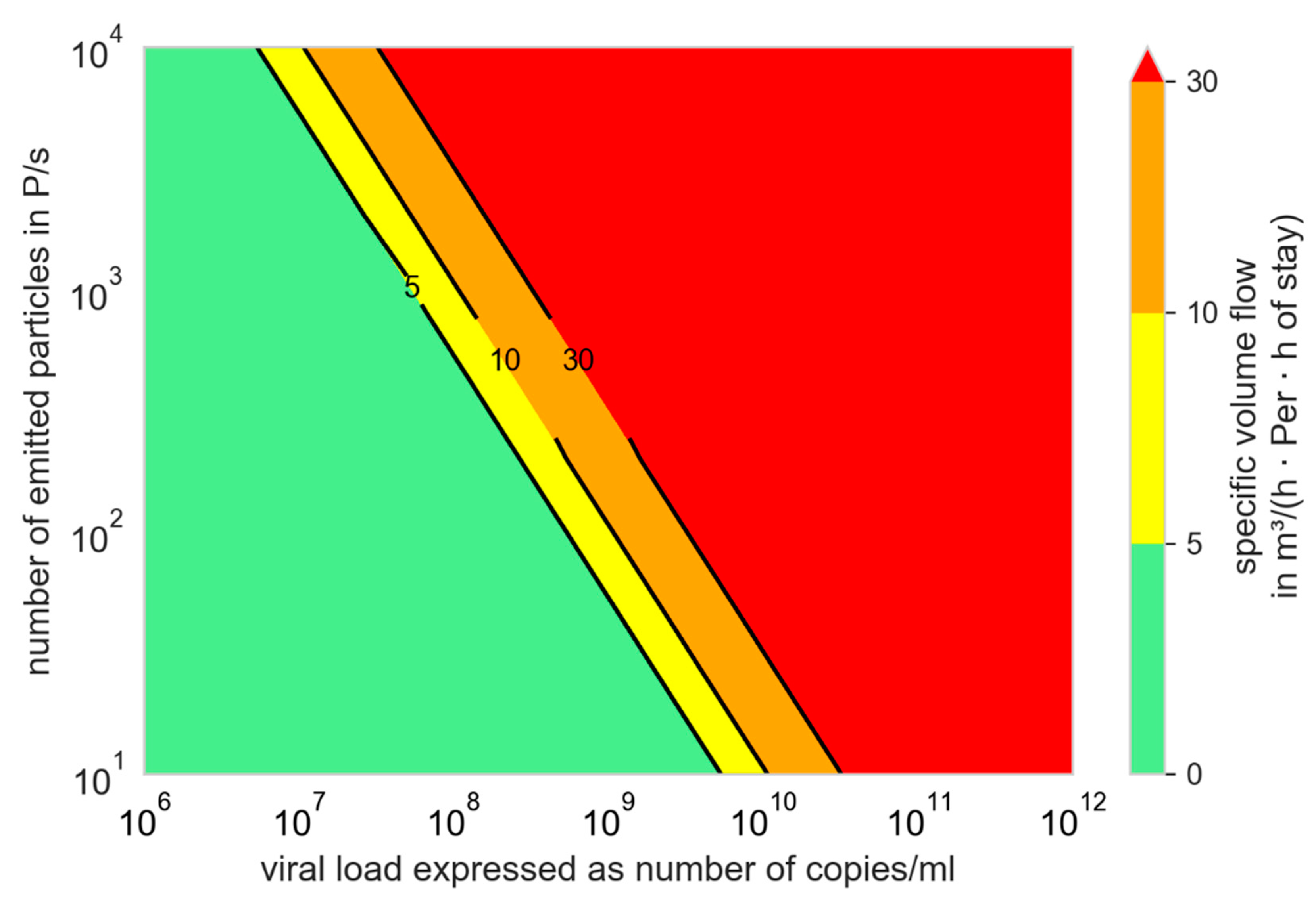
3.2. Influence of Variants of Concern (VOC)
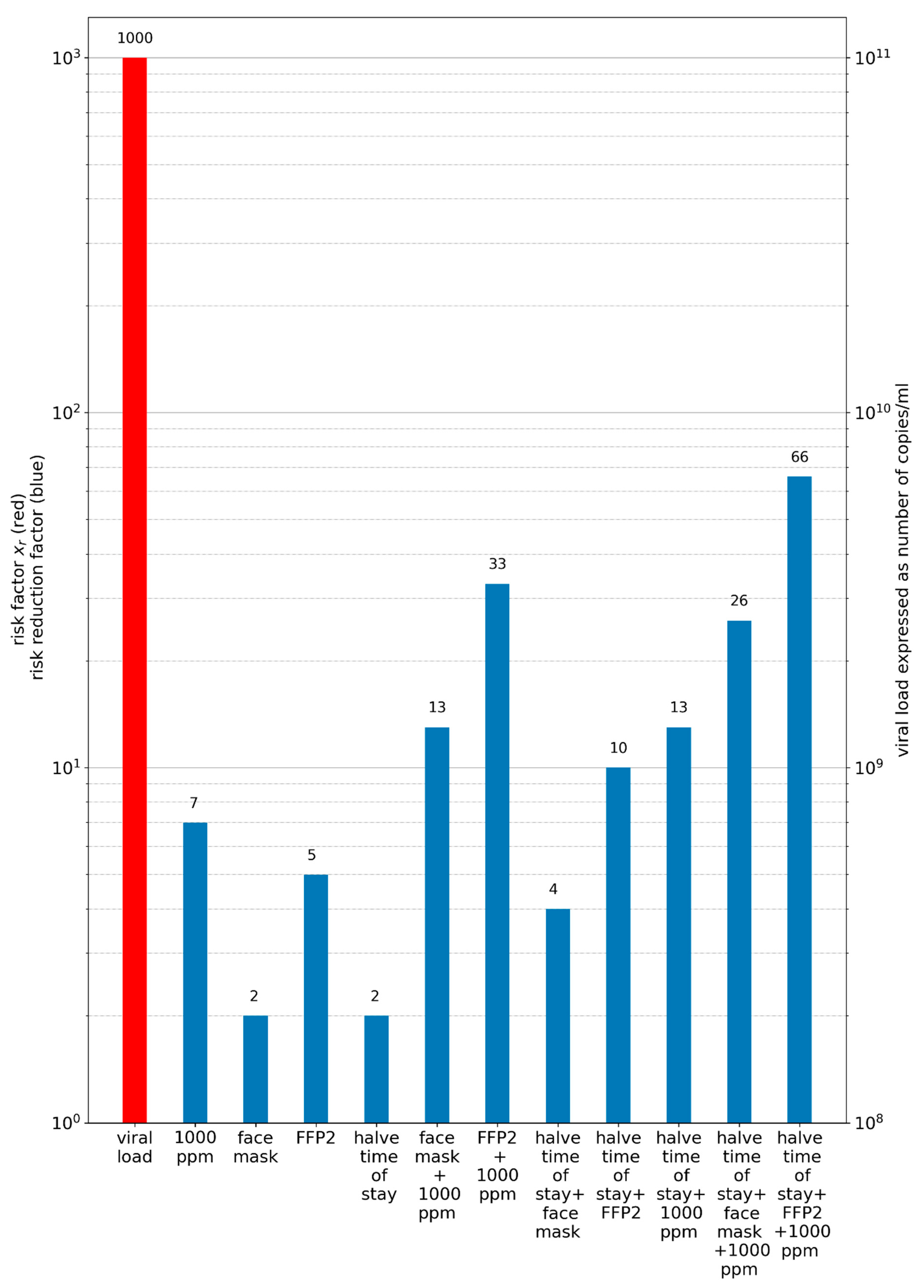
3.3. Comparison of Prevention Measures: Ag Testing, Wearing Masks, and Increasing Ventilation Rate
4. Discussion
5. Conclusions
- (1)
- For an outbreak due to aerosol transmission to happen, high viral loads are required, which regularly occurs with the Delta variant.
- (2)
- Preventive measures such as wearing masks and rising ventilation cannot prevent an outbreak when virus loads are very high, but are useful to mitigate it.
- (3)
- The person-related air flow rate per hour of stay is a favorable indicator for evaluating the preventive effect of ventilation measures. According to our observations, even volume flow rate and person-related volume flow rate have a more informative quality than the air change rate.
- (4)
- Instead of CO2 concentration, the CO2 dose (integration of the difference from the outdoor air concentration) is suitable for defining a limit value that should not be exceeded.
- (5)
- With a simplified approach it is easy to compare different indoor situations and preventive measures regarding aerosol transmission.
- (6)
- Ag tests possess an effective additional quality: they have a high sensitivity (detection rate) at virus loads of more than 106 viral copies/mL and are therefore able to detect infectious persons, providing the chance to isolate them before entering a room for a longer stay.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
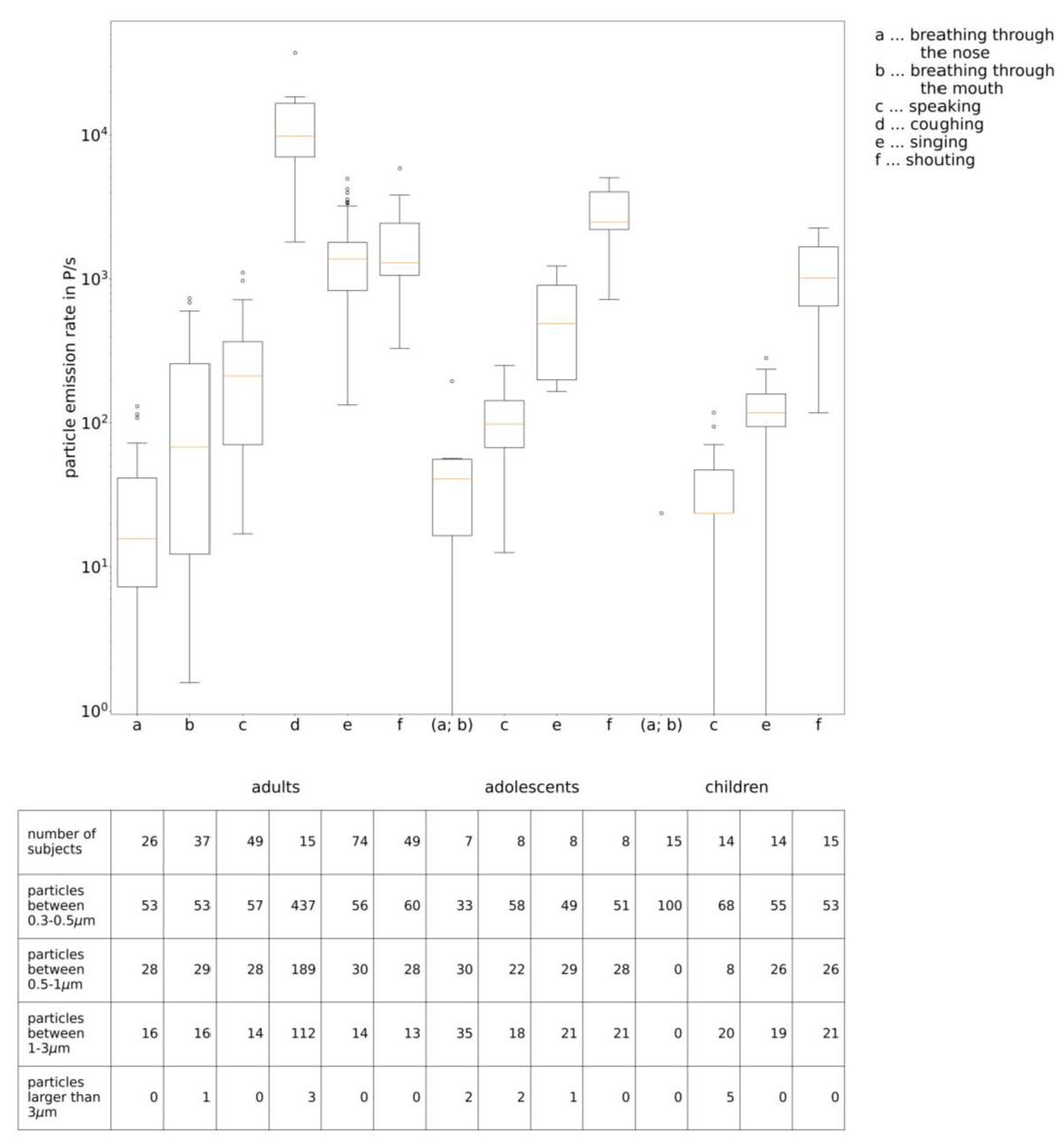
Calculation of the Conversion Factor from Number to Volume of the Particles
| Particle Emission Rate in Particles/s | |||||||
|---|---|---|---|---|---|---|---|
| Cumulative | 0.3–0.5 µm | 0.5–1.0 µm | 1.0–3.0 µm | >3.0 µm | |||
| Adults [35,35,39] | Breathing through the nose (26 subjects) | Average | 32 | 17 | 9 | 5 | 0 |
| Median | 16 | 12 | 4 | 2 | 0 | ||
| Min | 0 | 0 | 0 | 0 | 0 | ||
| Max | 740 | 314 | 262 | 159 | 12 | ||
| Breathing through the mouth (37 subjects) | Average | 164 | 87 | 48 | 27 | 1 | |
| Median | 68 | 36 | 16 | 10 | 0 | ||
| Min | 2 | 0 | 0 | 0 | 0 | ||
| Max | 1036 | 612 | 381 | 148 | 18 | ||
| Speaking (45 subjects) | Average | 268 | 152 | 77 | 38 | 1 | |
| Median | 212 | 118 | 49 | 24 | 0 | ||
| Min | 17 | 4 | 0 | 0 | 0 | ||
| Max | 1194 | 730 | 330 | 275 | 25 | ||
| Coughing * (7 subjects) | Average | 13,708 | 8047 | 3478 | 2057 | 126 | |
| Median | 9790 | 6494 | 2806 | 2315 | 98 | ||
| Min | 1805 | 1099 | 392 | 314 | 0 | ||
| Max | 287,697 | 196,781 | 71,826 | 18,933 | 353 | ||
| Singing (39 subjects) | Average | 1511 | 842 | 458 | 208 | 2 | |
| Median | 1376 | 742 | 396 | 166 | 0 | ||
| Min | 133 | 72 | 31 | 28 | 0 | ||
| Max | 6215 | 3677 | 1989 | 1457 | 23 | ||
| Shouting (15 subjects) | Average | 1843 | 1105 | 507 | 231 | 0 | |
| Median | 1295 | 777 | 353 | 165 | 0 | ||
| Min | 330 | 141 | 94 | 71 | 0 | ||
| Max | 5862 | 3743 | 1719 | 471 | 0 | ||
| Adolescents (13–15 years) [38] | Breathing (7 subjects) | Average | 54 | 18 | 16 | 19 | 1 |
| Median | 41 | 17 | 5 | 14 | 0 | ||
| Min | 0 | 0 | 0 | 0 | 0 | ||
| Max | 749 | 352 | 201 | 179 | 17 | ||
| Speaking (8 subjects) | Average | 112 | 65 | 25 | 20 | 3 | |
| Median | 98 | 51 | 20 | 11 | 1 | ||
| Min | 13 | 5 | 5 | 0 | 0 | ||
| Max | 251 | 137 | 55 | 53 | 13 | ||
| Singing (8 subjects) | Average | 577 | 284 | 166 | 122 | 5 | |
| Median | 490 | 286 | 127 | 72 | 3 | ||
| Min | 165 | 64 | 33 | 19 | 0 | ||
| Max | 1229 | 529 | 337 | 345 | 17 | ||
| Shouting (8 subjects) | Average | 2940 | 1491 | 820 | 603 | 26 | |
| Median | 2477 | 1417 | 697 | 461 | 9 | ||
| Min | 720 | 410 | 151 | 160 | 0 | ||
| Max | 5048 | 2486 | 1464 | 1088 | 104 | ||
| Children (8–10 years) [40] | Breathing (15 subjects) | Average | 2 | 2 | 0 | 0 | 0 |
| Median | 0 | 0 | 0 | 0 | 0 | ||
| Min | 0 | 0 | 0 | 0 | 0 | ||
| Max | 23 | 23 | 0 | 0 | 0 | ||
| Speaking (15 subjects) | Average | 40 | 27 | 3 | 8 | 2 | |
| Median | 24 | 24 | 0 | 0 | 0 | ||
| Min | 0 | 0 | 0 | 0 | 0 | ||
| Max | 118 | 71 | 47 | 47 | 23 | ||
| Singing (14 subjects) | Average | 131 | 72 | 34 | 25 | 0 | |
| Median | 118 | 59 | 35 | 23 | 0 | ||
| Min | 0 | 0 | 0 | 0 | 0 | ||
| Max | 800 | 400 | 235 | 165 | 0 | ||
| Shouting (15 subjects) | Average | 1166 | 614 | 298 | 250 | 5 | |
| Median | 1012 | 589 | 259 | 188 | 0 | ||
| Min | 23 | 23 | 0 | 0 | 0 | ||
| Max | 2260 | 1177 | 659 | 659 | 47 | ||
Appendix B
Necessary Boundary Conditions to Retrospectively Investigate Outbreaks
- (1)
- Virus-related aspects
- How many people were infected by the virus?
- How many people attended the event?
- How many people were vaccinated or had recovered from an infection?
- Has it been defined which type of virus caused the infection? If yes, which one?
- How high was the virus load during the infection event?
- (2)
- Room-related aspects
- How big is the room in which the event took place (area, volume)?
- Was the room ventilated mechanically?
- What volume flow or air change rate was available?
- Was the room ventilated by window opening?
- How often and for how long were the windows opened?
- Is there anything known about the outdoor conditions on that day? (Temperature, wind speed, wind direction.)
- (3)
- Event-related aspects
- How long did the event take?
- Did all attendees stay in the room together for the whole event? Otherwise, specify which part left and for how long.
- What was the main activity of the infectious person?
- What was the main activity of the susceptible persons?
- Were there any additional preventive measures (e.g., face masks)?
- Were the persons at fixed positions during the stay? What were the approximate positions of the persons?
Appendix C
| Choir Rehearsal Berlin 1 | Choir Rehearsal Berlin 2 | Skagit Valley Choir | French Choir | Korean Call Center | Korean Fitness Center | Hawaiian Fitness Class | German Slaughterhouse | |
|---|---|---|---|---|---|---|---|---|
| Source/s | [40] | [40] | [30,31] | [74] | [31,77] | [31,78] | [75] | [76] |
| Air change rate in 1/h | 0.17 | 0.45 | [31] 1.5, [30] 0.3–1.0 | unventilated, assumed 0.1 | [31] 1.5 | [31] 1.5 | unventilated, assumed 0.1 | 0.53 |
| 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | ++ ±5% | |
| Room volume in m3 | 1200 | 1720 | 810 | 135 | 1143 ** | 180 | 114 *** | 3000 |
| ++ ±5% | ++ ±5% | + ±20% | + ±20% | + ±20% | + ±20% | 0 ± 50% | + ±20% | |
| Number of previously infected persons | 1 | 1 | 1 | 1 * | 1 | 1 | 1 | 1 |
| ++ ±5% | ++ ±5% | ++ ±5% | 0 ± 50% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | |
| Number of susceptible persons | 77 | 42 | 61 | 25 | 89 | 192 | 10 | 78 |
| Main activity | Singing | Singing/Speaking | Singing | Singing | Speaking | Physical Activity | Physical Activity | Heavy working |
| Breathing volume flow (Qb,e and Qb,in) in m3/h | 0.65 | 0.61 | 0.65 | 0.65 | 0.54 | 0.9 | 0.9 | 0.9 |
| + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | |
| Mask efficiency in % | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| - | - | - | - | - | - | - | - | |
| Time of stay in h | 2.5 | 2 | 2.5 | 2 | 8 | 0.8 | 1 | 8 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | + ±20% | ++ ±5% | ++ ±5% | + ±20% | |
| Attack rate in % | 89 | 24 | 87 | 68 | 65 | 30 | 100 | 26 |
| ++ ±5% | ++ ±5% | + ±20% | + ±20% | ++ ±5% | + ±20% | + ±20% | ++ ±5% |
| School Israel | Courtroom | Wuhan Restaurant | Aircraft | Buddhist Bus | Wuhan (Bus 1) | Wuhan (Bus 2) | Minivan 1 | Minivan 2 | |
|---|---|---|---|---|---|---|---|---|---|
| Source/s | [79] | [80] | [1,85] | [81] | [31,82] | [31,83] | [31,83] | [84] | [84] |
| Air change rate in 1/h | 2.7 | unknown, assumed 0.3 | [1] 0.56–0.77 | 21 | 3 | 3 | 3 | 9 | 9 |
| 0 ± 50% | 0 ± 50% | 0 ± 50% | + ±20% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | |
| Room volume in m3 | 150 | 150 | [19] 431 * | 60 ** | 50 | 71 | 34 | 16 | 16 |
| ++ ±5% | ++ ±5% | + ±20% | ++ ±5% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | |
| Number of previously infected persons | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | |
| Number of susceptible persons | 66 | 9 | 21 | 19 | 68 | 48 | 12, 1 with mask | 4, all with cloth masks | 3, infected person w/o mask, other persons w/mask |
| Main activity | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking |
| Breathing volume flow (Qb,e and Qb,in) in m3/h | 0.5 | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 |
| + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | |
| Mask efficiency in % | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.7 | 0.85 |
| - | - | - | - | - | - | - | + ±20% | + ±20% | |
| Time of stay in h | 4.5 | 3 | 1.2 | 11 | 1.7 | 2.5 | 1.0 | 2 | 2 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | |
| Attack Rate in % | 43 | 33 | 45 | 6 | 34 | 15 | 17 | 100 | 33 |
| ++ ±5% | + ±20% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% |
| Club Meeting | School Berlin 1 | School Berlin 2 | Meeting Germany | School Hamburg 1 | School Hamburg 2 | School Hamburg 3 | School Hamburg 4 | |
|---|---|---|---|---|---|---|---|---|
| Source/s | [86] | [86] | [86] | [86] | [87] | [87] | [87] | [87] |
| Air change rate in 1/h | 0.20 | 8.3 | 10 | 1.2 | 1.9 | 3.3 | 3.2 | 3.2 |
| 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | 0 ± 50% | |
| Room volume in m3 | 254 | 180 | 150 | 170 | 154 | 157 | 157 | 157 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | |
| Number of previously infected persons | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | |
| Number of susceptible persons | 25 | 27 | 20 | 11 | 28 | 24 | 24 | 27 |
| Main activity | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking | Speaking |
| Breathing volume flow (Qb,e and Qb,in) in m3/h | 0.54 | 0.45 | 0.45 | 0.54 | 0.54/0.45 * | 0.54/0.45 * | 0.54/0.45 * | 0.54/0.45 * |
| + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | + ±20% | |
| Mask efficiency in % | 1 | 1 | 1 | 1 | 1 | 1 | 0.7 | 0.7 |
| - | - | - | - | - | - | + ±20% | + ±20% | |
| Time of stay in h | 1.5 | 4.5 | 1.5 | 2 | 3 | 1.5 | 1.5 | 0.75 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | |
| Attack rate in % | 58 | 10 | 6 | 17 | 57 | 33 | 13 | 4 |
| ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% | ++ ±5% |
Appendix D
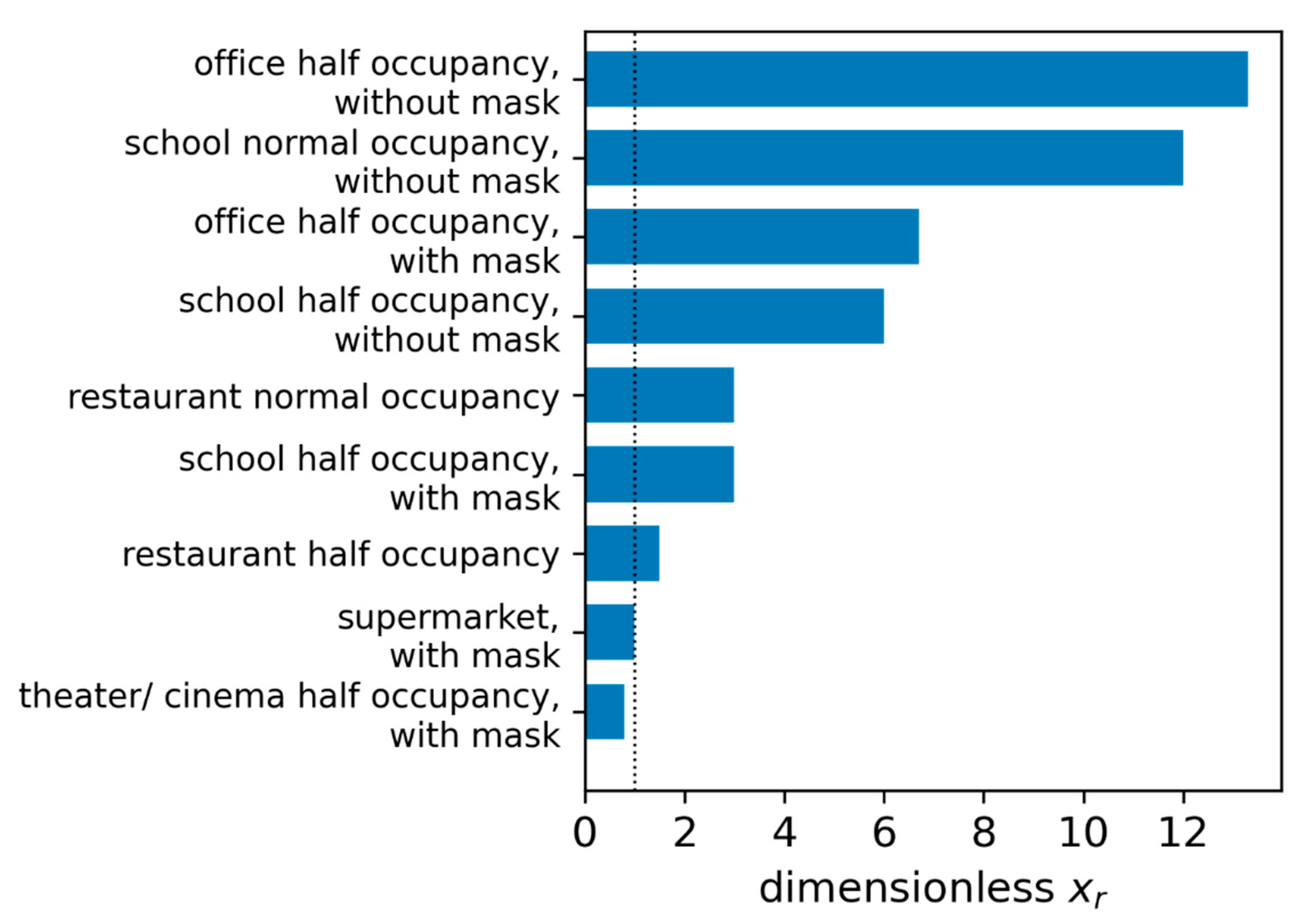
Comparison of Different Situations
| Np in P/s | Sv/N0 in Viral Copies/h | t in h | qpers in m3/h×Per | Qb,in in m3/h | fM in - | Rs in Per | xr in - | |
|---|---|---|---|---|---|---|---|---|
| Reference: supermarket, with mask | 160 | 184 | 0.5 | 25 | 0.54 | 0.5 | 1 | 1 |
| Office half occupancy, without mask | 160 | 184 | 8 | 60 | 0.54 | 1 | 13.2 | 13.2 |
| Office half occupancy, with mask | 160 | 184 | 8 | 60 | 0.54 | 0.5 | 6.6 | 6.6 |
| School normal occupancy, without mask | 80 | 92 | 6 | 25 | 0.54 | 1 | 11.9 | 11.9 |
| School half occupancy, without mask | 80 | 92 | 6 | 50 | 0.54 | 1 | 6 | 6 |
| School half occupancy, with mask | 80 | 92 | 6 | 50 | 0.54 | 0.5 | 3 | 3 |
| Restaurant normal occupancy | 160 | 184 | 1.5 | 50 | 0.54 | 1 | 6 | 6 |
| Restaurant half occupancy | 160 | 184 | 1.5 | 100 | 0.54 | 1 | 3 | 3 |
| Theater/cinema half occupancy, with mask | 80 | 92 | 2 | 60 | 0.54 | 0.5 | 0.8 | 0.8 |
References
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Hong, L.; Liang, P.; Wang, S.; Liang, P.; Li, J.; et al. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef]
- Correia, G.; Rodrigues, L.; da Silva, M.G.; Gonçalves, T. Airborne route and bad use of ventilation systems as non-neglible factors in SARS-CoV-2 transmission. Med. Hypotheses 2020, 141, 109781. [Google Scholar] [CrossRef]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.; Fraser, C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020, 368, eabb6936. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Leung, N.H. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Institut für Normung (DIN). DIN V 18599-10:2018-09: Energetische Bewertung von Gebäuden—Berechnung des Nutz-, End- und Primärenergiebedarfs für Heizung, Kühlung, Lüftung, Trinkwarmwasser und Beleuchtung; Deutsches Institut für Normung e.V. (DIN e.V.): Berlin, Germany, 2018. [Google Scholar]
- Müller, D.; Rewitz, K.; Derwein, D.; Burgholz, T.M.; Schweiker, M.; Bardey, J.; Tappler, P. Empfehlungen zum Erforderlichen Luftwechsel in Schulen, Großraumbüros, Härsälen und Turnhallen zur Reduktion Eines Aerosolgebundenen Infektionsrisikos. Available online: https://publications.rwth-aachen.de/record/804543 (accessed on 10 October 2021).
- Deutsches Institut für Normung e.V. (DIN e.V.). DIN 1946-4:2018-09 Ventilation and Air Conditioning—Part 4: Ventilation in Buildings and Rooms of Health Care; Beuth: Berlin, Germany, 2018. [Google Scholar]
- Scheer, F.A. Einfluss der Turbulenz einer Verdrängungsströmung in Operationsräumen auf Transport und Sedimentation von Mikroorganismen. Ph.D. Thesis, Technische Universität Berlin (TU Berlin), Berlin, Germany, 1998. [Google Scholar]
- Thatcher, T.L.; Lai, A.C.K.; Moreno-Jackson, R.; Sexto, R.G.; Nazaroff, W.W. Effects of room furnishings and air speed on particle depositon rates indoors. Atmos. Environ. 2002, 36, 1811–1819. [Google Scholar] [CrossRef] [Green Version]
- Offerman, P.-J.; Sexto, R.G.; Fisk, W.J.; Grimsrud, D.T.; Nazaroff, W.W.; Nero, A.V.; Revzan, K.L.; Yater, J. Control of Respiratory Particles in Indoor Air with Portable Air Cleaners. Atmos. Environ. 1984, 19, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.C.; Biele, G.; Mühlemann, B.; Veith, T.; Schneider, J.; Beheim-Schwarzbach, J.; Bleicker, T.; Tesch, J.; Schmidt, M.L.; Sander, L.E.; et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021, 373, abi5273. [Google Scholar] [CrossRef]
- Yang, Q.; Gonzales, T.K.S.P.K.; Lasda, E.; Decker, C.J.; Tat, K.L.; Fink, M.R.; Hager, C.R.; Davis, J.C.; Ozeroff, C.D.; Muhlrad, D.; et al. Just 2% of SARS-CoV-2−positive individuals carry 90% of the virus circulating in communities. Proc. Natl. Acad. Sci. USA 2021, 118, e2104547. [Google Scholar] [CrossRef]
- Ke, R.; Martinez, P.P.; Smith, R.L.; Gibson, L.L.; Mirza, A.; Conte, M.; Gallagher, N.; Luo, C.H.; Jarrett, J.; Conte, A.; et al. Daily Sampling of Early SARS-CoV-2 Infection Reveals Substantial Heterogeneity in Infectiousness. Available online: https://doi.org/10.1101/2021.07.12.21260208 (accessed on 10 October 2021).
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Xiong, Q.; Liu, Z.; Guo, Q.; Zou, L.; Zhang, H.; et al. Viral Infection and Transmission in a Large, Well-Documented Outbreak Caused by the SARS-CoV-2 Delta Variant. Available online: https://doi.org/10.1101/2021.07.07.21260122 (accessed on 10 October 2021).
- Chia, P.Y.; Ong, S.W.X.; Chiew, L.W.A.C.J.; Chavatte, J.-M.; Mak, T.-M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; Chai, L.Y.A.; et al. Virological and Serological Kinetics of SARS-CoV-2 Delta Variant Vaccine-Breakthrough Infections: A Multi-Center Cohort Study. Available online: https://doi.org/10.1101/2021.07.28.21261295 (accessed on 10 October 2021).
- Chau, N.V.V.; Ngoc, N.M.; Nguyet, L.A.; Quang, V.M.; Ny, N.T.H.; Khoa, D.B.; Phong, N.T.; Toan, L.M.; Hong, N.T.T.; Tuyen, N.T.K.; et al. Superspreading Event of SARS-CoV-2 Infection at a Bar. Emerg. Infect. Dis. 2021, 27, 310–314. [Google Scholar]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. The Impact of SARS-CoV-2 Vaccination on Alpha & Delta Variant Transmission. Available online: https://doi.org/10.1101/2021.09.28.2126426 (accessed on 10 October 2021).
- Van Doremalen, N.; Bushmaker, T.; Morris, D.; Holbrook, M.; Gamble, A.; Williamson, B.; Tamin, A.; Harcourt, J.T.N.; Gerber, S.; Lloyd-Smith, J.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Dabisch, P.; Schuit, M.; Herzog, A.; Beck, K.; Wood, S.; Krause, M.; Miller, D.; Weaver, W.; Freeburger, D.; Hooper, I.; et al. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci. Technol. 2021, 55, 142–153. [Google Scholar] [CrossRef]
- Riley, E.; Murphy, G.; Riley, R. Airborne Spread of Measles in a Suburban Elementary School. Am. J. Epidemiol. 1978, 107, 421–432. [Google Scholar] [CrossRef]
- Wells, W. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections; Harvard University Press: Cambridge, MA, USA, 1955. [Google Scholar]
- Karimzadeh, S.; Bhopal, R.; Huy, N.T. Review of infective dose, routes of transmission and outcome of COVID-19 caused by the SARS-CoV-2: Comparison with other respiratory viruses. Epidemiol. Infect. 2021, 149, E96. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Fitzner, K.; Rákóczy, T.; Sefker, T.; Reinhardt, A.; Baumgarth, S.; Schernus, G.-P.; Heckl, M.; Möser, M.; Höhenberger, L.; et al. Raumklimatechnik—Band 2: Raumluft- und Raumkühltechnik; Fitzner, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Panttelic, J.; Tham, K.W. Adequacy of air change rate as the sole indicator of an air distribution system’s effectiveness to mitigate airborne infectious disease transmission caused by a cough release in the room with overhead mixing ventilation: A case study. HVACR Res. 2013, 8, 947–961. [Google Scholar] [CrossRef]
- Mundt, E.; Mathisen, H.M.; Nielsen, P.V.; Moser, A. Ventilation Effectiveness: Rehva Guidebooks; Federation of European Heating and Ventilation Association (REHVA): Brussels, Belgium, 2004. [Google Scholar]
- Lichtner, E.; Kriegel, M. Pathogen Spread and Air Quality Indoors—Ventilation Effectiveness in a Classroom. Available online: https://doi.org/10.14279/depositonce-12437 (accessed on 10 October 2021).
- Miller, S.L.; Nazaroff, W.W.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Boerstra, A.; Dancer, S.J.; Kurnitski, J.; Marr, L.C.; Morawska, L.; et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021, 31, 314–323. [Google Scholar] [CrossRef]
- Prentiss, M.; Chu, A.; Berggren, K.K. Superspreading Events without Superspreaders: Using High Attack Rate Events to Estimate N0 for Airborne Transmission of COVID-19. MedRxiv 2020. Available online: https://doi.org/10.1101/2020.10.21.20216895 (accessed on 10 October 2021).
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of airborne viral transmission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Helleis, F.; Borrmann, S.; Cheng, Y.; Drewnick, F.; Haug, G.; Klimach, T.; Sciare, J.; Su, H.; Pöschl, U. Model Calculations of Aerosol Transmission and Infection Risk of COVID-19 in Indoor Environments. Int. J. Environ. Res. Public Health 2020, 17, 8114. [Google Scholar] [CrossRef]
- Mürbe, D.; Kriegel, M.; Lange, J.; Rotheudt, H.; Fleischer, M. Aerosol emission is inscreased in professional singing of classical music. Sci. Rep. 2021, 11, 14861. [Google Scholar] [CrossRef]
- Hartmann, A.; Lange, J.; Rotheudt, H.; Kriegel, M. Emission Rate and Particle Size of Bioaerosols during Breathing, Speaking and Coughing. Available online: http://dx.doi.org/10.14279/depositonce-10331 (accessed on 10 October 2021).
- Alsved, M.; Matamis, A.; Bohlin, R.; Richter, M.; Bengtsson, P.-E.; Fraenkel, C.-J.; Medstrand, P.; Löndahl, J. Exhaled respiratory particles during singing and talking. Aerosol Sci. Technol. 2020, 54, 1245–1248. [Google Scholar] [CrossRef]
- Gregson, F.K.A.; Watson, N.A.; Orton, C.M.; Haddrell, A.E.; McCarthy, L.P.; Finnie, T.J.R.; Gent, N.; Donaldson, G.C.; Shah, P.L.; Calder, J.D.; et al. Comparing Aerosol Concentrations and Particle Size Distributions Generated by Singing, Speaking and Breathing. Aerosol Sci. Technol. 2021, 55, 681–691. [Google Scholar] [CrossRef]
- Mürbe, D.; Kriegel, M.; Lange, J.; Schumann, L.; Hartmann, A.; Fleischer, M. Aerosol emission of adolescents voices during speaking, singing and shouting. PLoS ONE 2021, 16, e0246819. [Google Scholar] [CrossRef] [PubMed]
- Reichert, F.; Stier, O.; Hartmann, A.; Ruscher, C.; Brinkmann, A.; Grossegesse, M.; Neumann, M.; Werber, D.; Hausner, M.; Kunze, M.; et al. Analysis of Two Choir Outbreaks in Germany in 2020 Characterizes Longrange Transmission Risks through SARS-CoV-2. Available online: https://www.preprints.org/manuscript/202106.0518/v1 (accessed on 10 October 2021).
- Mürbe, D.; Schumann, L.; Hartmann, A.; Ifrim, L.; von Zadow, D.; Lüske, J.; Seybold, J.; Kriegel, M.; Fleischer, M. Vergleich der Aerosolpartikelemissionen von Grundschulkindern und Erwachsenen Beim Atmen, Sprechen, Singen und Rufen. Available online: http://doi.org/10.5281/zenodo.4770776 (accessed on 10 October 2021).
- Redrow, J.; Mao, S.; Celik, I.; Posada, J.A.; Feng, Z.-g. Modeling evaporation and dispersion of airborne sputum droplets expelled from a human cough. Build. Environ. 2011, 46, 2042–2051. [Google Scholar] [CrossRef]
- Kwong, L.H.; Wilson, R.; Kumar, S.; Crider, Y.S.; Sanchez, Y.R.; Rempel, D.; Pillarisetti, A. Review of the Breathability and Filtration Efficiency of Common Household Materials for Face Masks. ACS Nano 2021, 15, 5904–5924. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, K.; Obuchowski, N. Comparison of Fit for Sealed and Loose-Fitting Surgical Masks and N95 Filtering Facepiece Respirators. Ann. Work Expo. Health 2021, 65, 463–474. [Google Scholar] [CrossRef]
- Mueller, A.V.; Eden, M.J.; Oakes, J.M.; Bellini, C.; Fernandez, L.A. Quantitative Method for Comparative Assessment of Particle Removal Efficiency of Fabric Masks as Alternatives to Standard Surgical Masks for PPE. Matter 2020, 3, 950–962. [Google Scholar] [CrossRef]
- Asadi, S.; Cappa, C.D.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci. Rep. 2020, 10, 15665. [Google Scholar] [CrossRef] [PubMed]
- Cappa, C.D.; Asadi, S.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Expiratory aerosol particle escape from surgical masks due to imperfect sealing. Nat. Sci. Rep. 2021, 11, 12110. [Google Scholar] [CrossRef]
- Schumann, L.; Lange, J.; Rotheudt, H.; Hartmann, A.; Kriegel, M. Experimentelle Untersuchung der Leckage und Abscheideleistung von Typischen Mund-Nasen.Schutz und Mund-Nasen-Bedeckungen zum Schutz vor Luftgetragenen Krankheitserregern. Available online: https://doi.org/10.14279/depositonce-10857 (accessed on 10 October 2021).
- Dreller, S.; Jatzwauk, L.; Nassauer, A.; Paszkiewwicz, P.; Tobys, H.-U.; Rüden, H. Zur Frage des Geeigneten Atemschutzes vor Luftübertragenen Infektionserregern. Available online: https://www.dguv.de/medien/ifa/de/pub/grl/pdf/2006_003.pdf (accessed on 10 October 2021).
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, e00637-20. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Lin, C.-H.; Chen, Q. Characterizing exhaled airflow from breathing and talking. Indoor Air 2010, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Latasa, I. Respiratory flows as a method for safely preventing the coronvirus transmission (COVID-19). Apunt. Sports Med. 2020, 55, 81–85. [Google Scholar] [CrossRef]
- Jiang, J.; Hanna, R.; Willey, M.; Rieves, A. The measurement of airflow using Singing helmet that allows free movement of the jaw. J. Voice 2016, 30, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Dumond, R.; Gastinger, S.; Rahman, H.; le Faucheur, A.; Quinton, P.; Kang, H.; Prioux, J. Estimation of respiratory colume from thoracoabdominal breathing distances: Comparison of two models of machine learning. Eur. J. Appl. Physiol. 2017, 117, 1533–1555. [Google Scholar] [CrossRef]
- Binazzi, B.; Lanini, B.; Bianchi, R.; Romagnoli, I.; Nerini, M.; Gigliotti, F.; Duranti, R.M.-E.J.; Scano, G. Breathing Pattern and kinematics in normal subjects during speech, singing and loud whispering. Acta Physiol. 2006, 186, 233–246. [Google Scholar] [CrossRef]
- Spektrum Akademischer Verlag, Heidelberg. Lexikon der Biologie. Spektrum. De 1999. Available online: https://www.spektrum.de/lexika/showpopup.php?lexikon_id=9&art_id=5744&nummer=1988 (accessed on 5 October 2020).
- Taylor-Phillips, S.; Dinnes, J. Asymptomatic rapid testing for SARS-CoV-2. BMJ 2021, 374, n1733. [Google Scholar] [CrossRef]
- Osterman, A.; Baldauf, H.-M.; Eletreby, M.; Wettengel, J.M.; Afridi, S.Q.; Fuchs, T.; Holzmann, E.; Maier, A.; Döring, J.; Grzimek-Koschewa, N.; et al. Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting. Med. Microbiol. Immunol. 2021, 210, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, J.; Olaru, I.D.; Horner, S.; Schnitzler, P.; Heeg, K.; Denkinger, C.M. The Potential of SARS-CoV-2 Antigen-Detection Tests in the Screening of Asymptomatic Persons. Available online: https://doi.org/10.1101/2021.06.07.21258465 (accessed on 10 October 2021).
- Lindner, A.K.; Nikolai, O.; Kausch, F.; Wintel, M.; Hommes, F.; Gertler, M.; Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F.; et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021, 57, 2003961. [Google Scholar] [CrossRef]
- Lindner, A.K.; Nikolai, O.; Kausch, C.R.F.; Wintel, M.; Gertler, M.; Burock, S.; Hörig, M.; Bernhard, J.; Tobian, F.; Gaeddert, M.; et al. Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. J. Clin. Virol. 2021, 141, 104874. [Google Scholar] [CrossRef] [PubMed]
- Department of Health & Social Care. Asymptomatic Testing for SARS-CoV-2 Using Antigen-Detecting Lateral Flow Devices. Available online: https://www.gov.uk/government/publications/lateral-flow-device-performance-data/key-points-summary-asymptomatic-testing-for-sars-cov-2-using-antigen-detecting-lateral-flow-devices-evidence-from-performance-data-october-2020-to-m (accessed on 10 October 2021).
- Puyskens, A.; Krause, E.; Michel, J.; Nübling, M.; Scheiblauer, H.; Bourquain, D.; Grossegesse, M.; Valusenko, R.; Corman, V.; Drosten, C.; et al. Establishment of an Evaluation Panel for the Decentralized Technical Evaluation of the Sensitivity of 31 Rapid Detection Tests for SARS-CoV-2 Diagnostics. Available online: https://doi.org/10.1101/2021.05.11.21257021 (accessed on 10 October 2021).
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Lei, W.K.J.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of severn commercial SARS-CoV-2 rapid Point-of-Care Antigen tests. Lancet 2021, 2, e311–e319. [Google Scholar]
- Scheiblauer, H.; Filomena, A.; Nitsche, A.; Puyskens, A.; Corman, V.M.; Drosten, C.; Zwirglmaier, K.; Lange, C.; Emmerich, P.; Müller, M.; et al. Comparatie Sensitivity Evaluation for 122 CE-Marked SARS-CoV-2 Antigen Rapid Tests. Available online: https://doi.org/10.1101/2021.05.11.21257016 (accessed on 10 October 2021).
- Stockbridge, M.; Purver, M.; Solel, T.; Jian, A.; Arikan, D.; Ovens, R.; Swayamprakasam, A.; Chapman, D.; Vipond, R.; Brown, A.; et al. In Vitro and Clinical Post-Market Surveillance of Biotime SARS-CoV-2 Lateral Flow Antigen Device in Detecting the SARS-CoV-2 Delta Variant. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/999867/in-vitro-and-clinical-post-market-surveillance-of-Biotime-SARS-CoV-2-Lateral-Flow-Antigen-Device-in-detecting-the-SARS-CoV-2-Delta-variant-B.1.617.2.pdf (accessed on 10 October 2021).
- Jungnick, S.; Hobmaier, B.; Mautner, L.; Hoyos, M.; Haase, M.; Baiker, A.; Lahne, H.; Eberle, U.; Wimmer, C.; Hepner, S.; et al. Detection of the new SARS-CoV-2 variants of concern B.1.1.7 and B.1.351 in five SARS-CoV-2 rapid antigen tests (RATs), Germany, March 2021. Eur. Surveill. 2021, 6, 2100413. [Google Scholar] [CrossRef]
- Gabler, J.; Raabe, T.; Röhrl, K.; Gaudecker, H.-M.V. The Effectiveness of Strategies to Contain SARS-CoV-2: Testing, Vaccinations, and NPIs. Available online: https://arxiv.org/abs/2106.11129v2 (accessed on 10 October 2021).
- Smith, R.L.; Gibson, L.L.; Martinez, P.P.; Ke, R.; Mirza, A.; Conte, M.; Gallagher, N.; Conte, A.; Wang, L.; FredricksonM, R.; et al. Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection. J. Infect. Dis. 2021, 224, 976–982. [Google Scholar] [CrossRef]
- Young, B.C.; Eyre, D.W.; Kendrick, S.; White, C.; Smith, S.; Beveridge, G.; Nonnenmacher, T.; Ichofu, F.; Hillier, J.; Oakley, S.; et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: An open-label, cluster randomised trial. Lancet 2021, 398, 1217–1229. [Google Scholar] [CrossRef]
- Lemieux, C. Monte Carlo and Quasi-Monte Carlo Sampling; Springer Science+Buisness: New York, NY, USA, 2009. [Google Scholar]
- Kähler, C.J.; Fuchs, T.; Mutsch, B.; Hain, R. School Education during the SARS-CoV-2 Pandemic—Which Concept is Safe, Feasible and Environmentally Sound? Available online: https://doi.org/10.1101/2020.10.12.20211219 (accessed on 10 October 2021).
- Allen, J.G.; Ibrahim, A.M. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA Insights 2021, 325, 2112–2113. [Google Scholar] [CrossRef]
- Charlotte, N. High Rate of SARS-CoV-2 Transmission Due to Choir Practice in France at the Beginnung of the COVID-19-Pandemic. J. Voice 2020, 325, 2112–2113. [Google Scholar]
- Groves, L.M.; Usagawa, L.; Elm, J.; Low, E.; Manuzak, A.; Quint, J.; Center, K.E.; Buff, A.M.; Kemble, S.K. Community Transmission of SARS-CoV-2 at Three Fitness Facilities. Morb. Mortal. Wkly. Rep. 2021, 70, 316–320. [Google Scholar] [CrossRef]
- Günther, T.; Czech-Sioli, M.; Indenbirken, D.; Robitailles, A.; Tenhaken, P.; Exner, M.; Ottinger, M.; Fischer, N.; Grundhoff, A.; Brinkmann, M.M. Investigation of a Superspreading Event Preceding the Largest Meat Processing Plant-Related SARS-Coronavirus 2 Outbreak in Germany. Available online: http://dx.doi.org/10.2139/ssrn.3654517 (accessed on 10 October 2021).
- Park, S.Y.; Kim, Y.-M.; Lee, S.; Na, B.-J.; Kim, C.B.; Kim, J.-i.; Kim, H.S.; Kim, Y.B.; Park, Y.; Huh, I.S.; et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg. Infect. Dis. 2020, 26, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Han, S.H.; Rhee, J.-Y. Cluster of Coronavirus Disease Associated with Fitness Dance Classes, South Korea. Emerg. Infect. Dis. 2020, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Stein-Zamir, C.; Abramson, N.; Shoob, H.; Libal, E.; Bitan, M.; Cardash, T.; Cayam, R.; Miskin, I. A large COVID-19 outbreak in a high school 10 days after schools’ reopening. Eurosurveillance 2020, 25, 2001352. [Google Scholar] [CrossRef] [PubMed]
- Vernez, D.; Schwarz, S.; Sauvain, J.-J.; Petignat, C.; Suarez, G. Probable aerosol transmission of SARS-CoV-2 in a poorly ventilated courtroom. Indoor Air J. 2021, 31, 1776–1785. [Google Scholar] [CrossRef]
- Khanh, N.C.; Thai, P.Q.; Quach, H.-L.; Thi, N.-A.H.; Dinh, P.C.; Duong, T.N.; Mai, L.T.Q.; Nghia, N.D.; Quang, L.N. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 During Ling Flight. Emerg. Infect. Dis. 2020, 26, 11. [Google Scholar]
- Shen, Y.; Li, C.; Dong, H.; Wang, Z.; Martinez, L.; Sun, Z.; Handel, A.; Chen, Z.; Chen, E.; Ebel, M.; et al. Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA Int. Med. 2020, 180, 1665–1671. [Google Scholar] [CrossRef]
- Luo, K.; Lei, Z.; Hai, Z.; Xiao, S.; Jia, R.; Yang, H.; Jing, X.; Wang, H.; Xie, Z.; Luo, P.; et al. Transmission of SARS-CoV-2 in Public Transportation Vehicles: A Case Study in Hunan Province, China. Open Forum Infect. Dis. 2020, 7, ofaa430. [Google Scholar] [CrossRef]
- Jones, L.D.; Chan, E.R.; Zabarsky, T.F.; Cadnum, J.L.; Navas, M.E.; Redmond, S.N.; Kovach, J.D.; Linger, M.; Rutala, W.A.; Zimmerman, P.A.; et al. Transmission of SARS-CoV-2 on a Patient Transport Van. Infect. Dis. Soc. 2021, 4, ciab347. [Google Scholar]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Zhou, D.; Yu, C.; Xu, B.; Yang, Z. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China. Emerg. Infect. Dis. 2020, 26, 1628–1631. [Google Scholar] [CrossRef]
- Kriegel, M.; Buchholz, U.; Gastmeier, P.; Bischoff, P.; Abdelgawad, I.; Hartmann, A. Predicted Infection Risk for Aerosol Transmission of SARS-CoV-2. Available online: https://doi.org/10.1101/2020.10.08.20209106 (accessed on 10 October 2021).
- Baumgarte, S.; Hartkopf, F.; Hölzer, M.; von Kleist, M.; Sabine, N.; Kriegel, M.; Bollongino, K. Investigation of a Limited But Explosive COVID-19 Outbreak in a German Secondary School. Available online: https://doi.org/10.2139/ssrn.3946292 (accessed on 10 October 2021).

| AR in % | Situation-Related Factor (SF) | Personal-Protection-Measures-Related Factor (SPF) fM | Virus-Related Factor (VF) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | 25% Percentile | 75% Percentile | ||||||||
| Choir Rehearsal Berlin 1 (A) | 89 | 0.0013 | 0.0022 | 0.65 | 1 | 2529 | 1576 | 2594 | 2145 | 3220 |
| Choir Rehearsal Berlin 2 (B) | 24 | 0.006 | 0.0009 | 0.65 | 1 | 732 | 464 | 774 | 656 | 916 |
| Skagit Valley Choir (C) | 87 | 0.0019 | 0.0030 | 0.65 | 1 | 1649 | 1065 | 1932 | 1226 | 3335 |
| French Choir (D) | 68 | 0.0088 | 0.0165 | 0.65 | 1 | 199 | 107 | 200 | 144 | 281 |
| Korean Call Center (E) | 12 | 0.0018 | 0.0018 | 0.54 | 1 | 135 | 133 | 345 | 254 | 462 |
| Korean Fitness Center (F) | 30 | 0.0011 | 0.0019 | 0.9 | 1 | 378 | 205 | 375 | 283 | 495 |
| Hawaiian Fitness Class (G) | 100 | 0.0033 | 0.0098 | 0.9 | 1 | 2312 | 787 | 1014 | 523 | 1686 |
| German Slaughterhouse (H) | 26 | 0.0018 | 0.0020 | 0.9 | 1 | 185 | 167 | 184 | 150 | 226 |
| School Israel (I) | 43 | 0.0052 | 0.0058 | 0.54 | 1 | 216 | 195 | 140 | 103 | 184 |
| Courtroom (J) | 33 | 0.0115 | 0.0154 | 0.54 | 1 | 58 | 41 | 57 | 46 | 73 |
| Wuhan Restaurant (K) | 45 | 0.0096 | 0.0192 | 0.54 | 1 | 115 | 58 | 120 | 97 | 149 |
| Aircraft (L) | 62 | 0.0084 | 0.0084 | 0.54 | 1 | 214 | 213 | 212 | 173 | 261 |
| Buddhist Bus (M) | 34 | 0.0076 | 0.0090 | 0.54 | 1 | 102 | 86 | 99 | 72 | 133 |
| Wuhan (Bus 1) (N) | 15 | 0.0084 | 0.0094 | 0.54 | 1 | 36 | 32 | 35 | 25 | 48 |
| Wuhan (Bus 2) (O) | 17 | 0.0058 | 0.0079 | 0.54 | 1 | 59 | 44 | 59 | 45 | 78 |
| Minivan 1 (P) | 63 | 0.0124 | 0.0131 | 0.54 | 0.5 | 481 | 455 | 475 | 309 | 690 |
| Minivan 2 (Q) | 45 | 0.0124 | 0.0131 | 0.54 | 0.7 | 85 | 81 | 83 | 54 | 121 |
| Club Meeting (R) | 58 | 0.0029 | 0.0059 | 0.54 | 1 | 564 | 271 | 568 | 485 | 670 |
| School Berlin 1 (S) | 10 | 0.0038 | 0.0039 | 0.45 | 1 | 56 | 54 | 87 | 59 | 118 |
| School Berlin 2 (T) | 6 | 0.0015 | 0.0016 | 0.45 | 1 | 85 | 76 | 157 | 109 | 212 |
| Meeting Germany (U) | 17 | 0.0045 | 0.0060 | 0.54 | 1 | 77 | 58 | 79 | 64 | 97 |
| School Hamburg 1 (V) | 57 | 0.0062 | 0.0071 | 0.45 | 1 | 271 | 238 | 295 | 225 | 381 |
| School Hamburg 2 (W) | 33 | 0.0020 | 0.0024 | 0.45 | 1 | 401 | 334 | 456 | 344 | 592 |
| School Hamburg 3 (X) | 13 | 0.0020 | 0.0024 | 0.45 | 0.7 | 199 | 166 | 224 | 165 | 300 |
| School Hamburg 4 (Y) | 4 | 0.0008 | 0.0012 | 0.45 | 0.7 | 143 | 97 | 161 | 123 | 210 |
| Min | 4 | 0.0006 | 0.0009 | - | - | 36 | 32 | 35 | 25 | 48 |
| Max | 89 | 0.0160 | 0.0012 | - | - | 2529 | 1576 | 2594 | 2145 | 3320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriegel, M.; Hartmann, A.; Buchholz, U.; Seifried, J.; Baumgarte, S.; Gastmeier, P. SARS-CoV-2 Aerosol Transmission Indoors: A Closer Look at Viral Load, Infectivity, the Effectiveness of Preventive Measures and a Simple Approach for Practical Recommendations. Int. J. Environ. Res. Public Health 2022, 19, 220. https://doi.org/10.3390/ijerph19010220
Kriegel M, Hartmann A, Buchholz U, Seifried J, Baumgarte S, Gastmeier P. SARS-CoV-2 Aerosol Transmission Indoors: A Closer Look at Viral Load, Infectivity, the Effectiveness of Preventive Measures and a Simple Approach for Practical Recommendations. International Journal of Environmental Research and Public Health. 2022; 19(1):220. https://doi.org/10.3390/ijerph19010220
Chicago/Turabian StyleKriegel, Martin, Anne Hartmann, Udo Buchholz, Janna Seifried, Sigrid Baumgarte, and Petra Gastmeier. 2022. "SARS-CoV-2 Aerosol Transmission Indoors: A Closer Look at Viral Load, Infectivity, the Effectiveness of Preventive Measures and a Simple Approach for Practical Recommendations" International Journal of Environmental Research and Public Health 19, no. 1: 220. https://doi.org/10.3390/ijerph19010220
APA StyleKriegel, M., Hartmann, A., Buchholz, U., Seifried, J., Baumgarte, S., & Gastmeier, P. (2022). SARS-CoV-2 Aerosol Transmission Indoors: A Closer Look at Viral Load, Infectivity, the Effectiveness of Preventive Measures and a Simple Approach for Practical Recommendations. International Journal of Environmental Research and Public Health, 19(1), 220. https://doi.org/10.3390/ijerph19010220






