Start-Up of Chitosan-Assisted Anaerobic Sludge Bed Reactors Treating Light Oxygenated Solvents under Intermittent Operation
Abstract
:1. Introduction
2. Materials and Methods
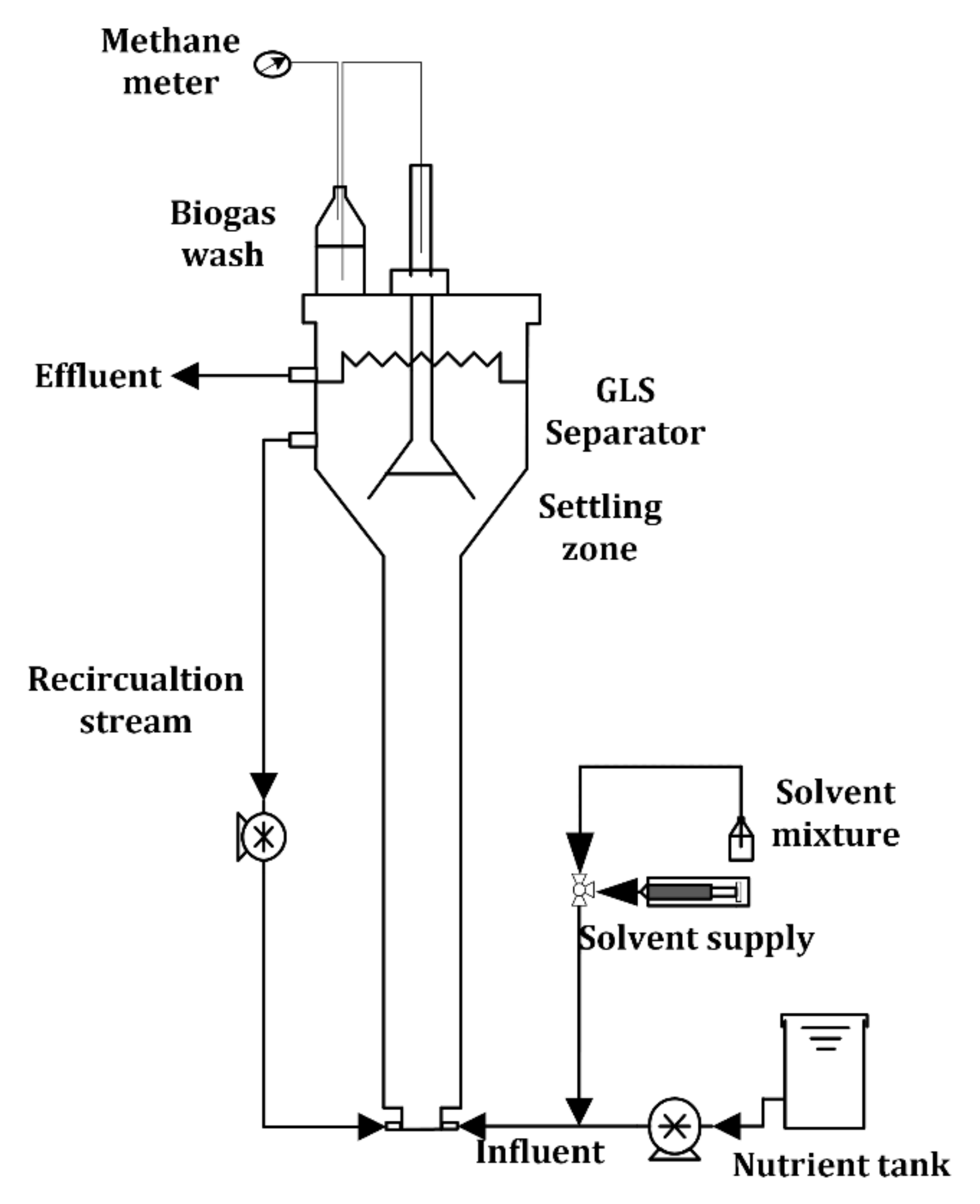
2.1. Experimental Setup
2.2. Inoculum and Feed Characteristics
2.3. Experimental Plan
2.4. Biochemical Methane Potential (BMP) and Specific Methanogenic Activity (SMA) Assays
2.5. Particle Size Distribution, Settling Velocity and Strength of Granules
2.6. Analytical Methods
2.7. Microbial Community Analysis
3. Results and Discussion
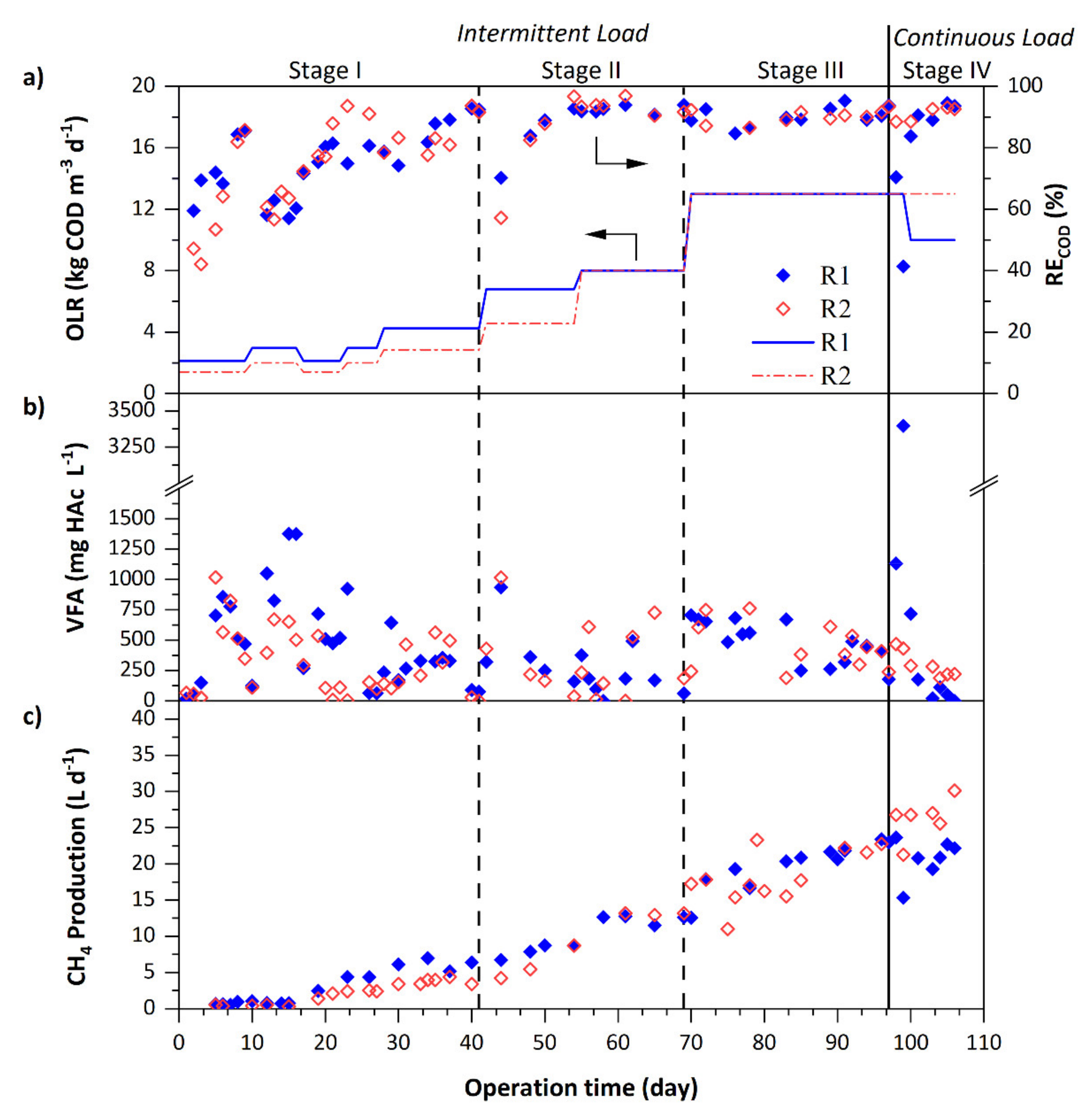
3.1. Performance of the Anaerobic Sludge Bed Reactors
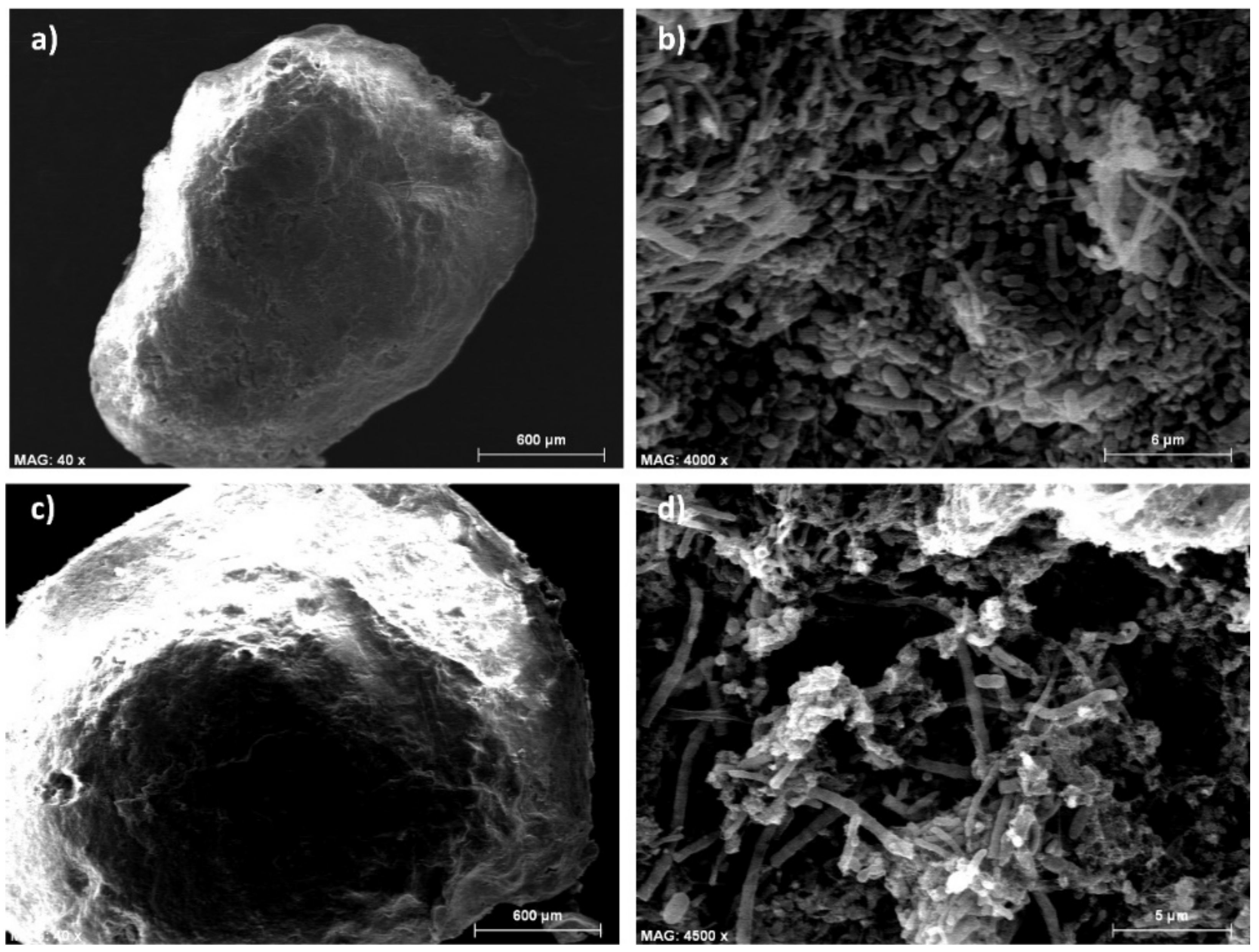
3.2. Formation of Anaerobic Granules
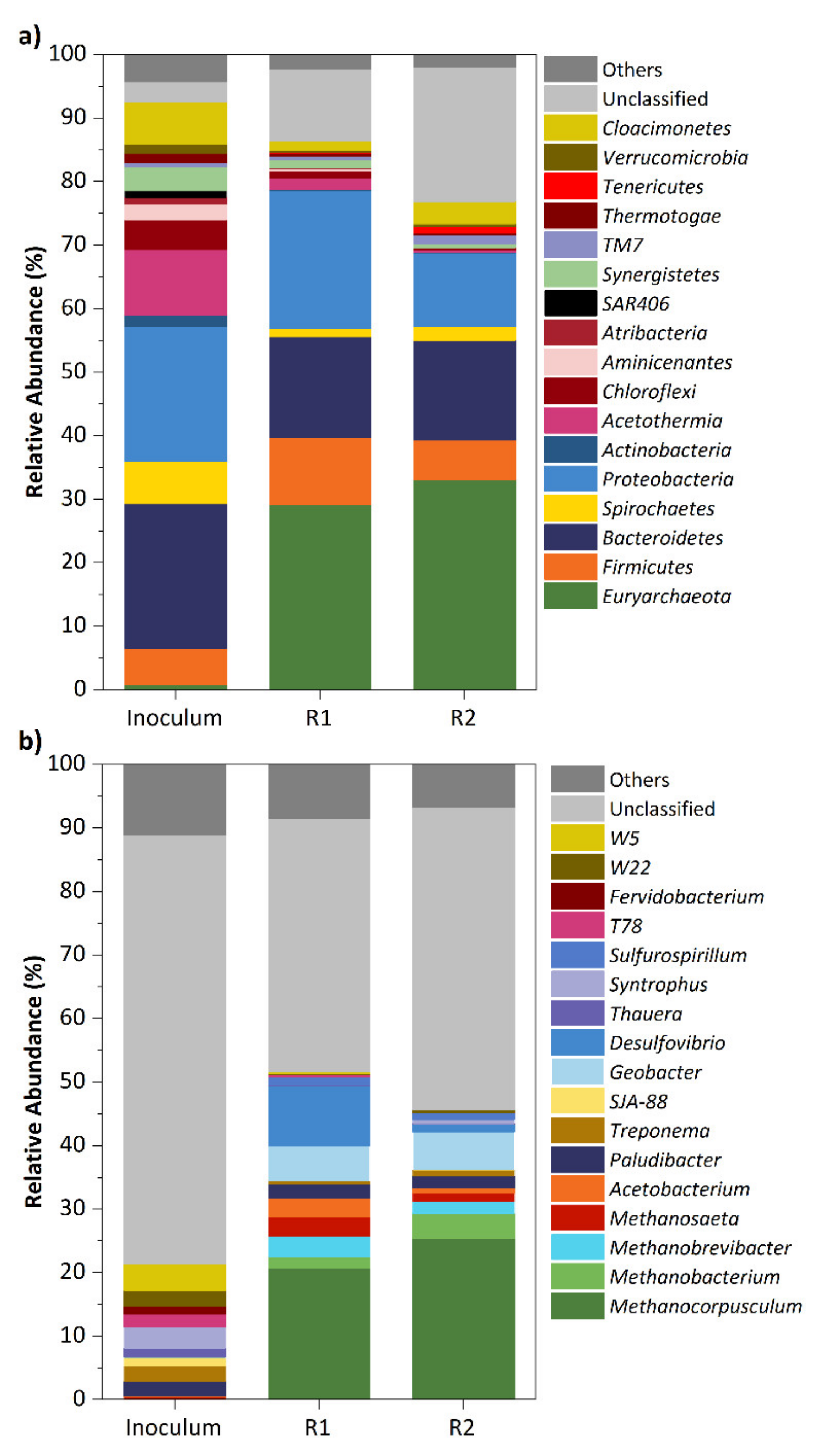
3.3. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. COM/2020/98 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2020:98:FIN (accessed on 12 March 2021).
- Flexographic Technical Association—Europe (FTA_Europe). Available online: https://fta-europe.eu (accessed on 12 March 2021).
- Bravo, D.; Álvarez-Hornos, F.J.; Penya-roja, J.M.; San-Valero, P.; Gabaldón, C. Aspen Plus process-simulation model: Producing biogas from VOC emissions in an anaerobic bioscrubber. J. Environ. Manag. 2018, 213, 530–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waalkens, A.; Gabaldón, C.; Penya-roja, J.M.; Álvarez-Hornos, F.J. Method for the Purification of Gases Containing Volatile Organic Compounds. Patent ES2542257, 22 June 2016. [Google Scholar]
- Bravo, D.; Ferrero, P.; Penya-Roja, J.; Hornos, F.J.A.; Gabaldón, C. Control of VOCs from printing press air emissions by anaerobic bioscrubber: Performance and microbial community of an on-site pilot unit. J. Environ. Manag. 2017, 197, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Lier, J.B.; van der Zee, F.P.; Frijters, C.T.M.J.; Ersahin, M.E. Celebrating 40 years anaerobic sludge bed reactors for indus-trial wastewater treatment. Rev. Environ. Sci. Biotechnol. 2015, 14, 681–702. [Google Scholar] [CrossRef] [Green Version]
- Enright, A.-M.; McGrath, V.; Gill, D.; Collins, G.; O’Flaherty, V. Effect of seed sludge and operation conditions on performance and archaeal community structure of low-temperature anaerobic solvent-degrading bioreactors. Syst. Appl. Microbiol. 2009, 32, 65–79. [Google Scholar] [CrossRef]
- Oktem, Y.A.; Ince, O.; Sallis, P.; Donnelly, T.; Ince, B.K. Anaerobic treatment of a chemical synthesis-based pharmaceutical wastewater in a hybrid upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2008, 99, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Siggins, A.; Enright, A.M.; O’Flaherty, V. Temperature dependent (37–15 °C) anaerobic digestion of a trichloroeth-ylene-contaminated wastewater. Bioresour. Technol. 2011, 102, 7645–7656. [Google Scholar] [CrossRef]
- Lafita, C.; Penya-Roja, J.M.; Gabaldón, C. Anaerobic removal of 1-methoxy-2-propanol under ambient temperature in an EGSB reactor. Bioprocess Biosyst. Eng. 2015, 38, 2137–2146. [Google Scholar] [CrossRef] [Green Version]
- Show, K.Y.; Yan, Y.; Yao, H.; Guo, H.; Li, T.; Show, D.Y.; Chang, J.S.; Lee, D.J. Anaerobic granulation: A review of granula-tion hypotheses, bioreactor designs and emerging green applications. Bioresour. Technol. 2020, 300, 122751. [Google Scholar] [CrossRef]
- Show, K.Y.; Wang, Y.; Foong, S.F.; Tay, J.H. Accelerated start-up and enhanced granulation in upflow anaerobic sludge blanket reactors. Water Res. 2004, 38, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Narvi, S.S.; Pandey, N.D. Influence of polymer addition on granulation in upflow anaerobic sludge blanket reac-tor: A review. Int. J. Appl. Environ. Sci. 2017, 12, 1561–1573. [Google Scholar]
- Liang, J.; Wang, Q.; Yoza, B.A.; Li, Q.X.; Chen, C.; Ming, J.; Yu, J.; Li, J.; Ke, M. Rapid granulation using calcium sulfate and polymers for refractory wastewater treatment in up-flow anaerobic sludge blanket reactor. Bioresour. Technol. 2020, 305, 123084. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treat-ment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Guha, S.; Harendranath, C.S.; Tripathi, S. Enhanced granulation by natural ionic polymer additives inUASB reactor treating low-strength wastewater. Water Res. 2005, 39, 3801–3810. [Google Scholar] [CrossRef]
- Hudayah, N.; Suraraksa, B.; Chaiprasert, P. Impact of EPS and chitosan combination on enhancement of anaerobic granule quality during simultaneous microbial adaptation and granulation. J. Chem. Technol. Biotechnol. 2019, 94, 3725–3735. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Gao, S. PVA/CS and PVA/CS/Fe gel beads’ synthesis mechanism and their performance in culti-vating anaerobic granular sludge. Chemosphere 2019, 219, 130–139. [Google Scholar] [CrossRef]
- Su, C.; Tao, A.; Zhao, L.; Wang, P.; Wang, A.; Huang, X.; Chen, M. Roles of modified biochar in the performance, sludge characteristics, and microbial community features of anaerobic reactor for treatment food waste. Sci. Total. Environ. 2021, 770, 144668. [Google Scholar] [CrossRef] [PubMed]
- Torres, K.; Álvarez-Hornos, F.; San-Valero, P.; Gabaldón, C.; Marzal, P. Granulation and microbial community dynamics in the chitosan-supplemented anaerobic treatment of wastewater polluted with organic solvents. Water Res. 2018, 130, 376–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, K.; Álvarez-Hornos, F.J.; Ferrero, P.; Gabaldón, C.; Marzal, P. Intermittent operation of UASB reactors treating wastewater polluted with organic solvents: Process performance and microbial community evaluation. Environ. Sci. Water Res. Technol. 2019, 5, 1270–1284. [Google Scholar] [CrossRef]
- Ghangrekar, M.; Asolekar, S.; Joshi, S. Characteristics of sludge developed under different loading conditions during UASB reactor start-up and granulation. Water Res. 2005, 39, 1123–1133. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Pena, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhunia, P.; Ghangrekar, M. Required minimum granule size in UASB reactor and characteristics variation with size. Bioresour. Technol. 2007, 98, 994–999. [Google Scholar] [CrossRef]
- Jeison, D.; Chamy, R. Comparison of the Behaviour of Expanded Granular Sludge Bed (EGSB) and Upflow Anaerobic Sludge Blanket (UASB) Reactors in Dilute and Concentrated Wastewater Treatment. Water Sci. Technol. 1999, 40, 91–97. [Google Scholar] [CrossRef]
- Ferrero, P.; San-Valero, P.; Gabaldón, C.; Martínez-Soria, V.; Penya-Roja, J. Anaerobic degradation of glycol ether-ethanol mixtures using EGSB and hybrid reactors: Performance comparison and ether cleavage pathway. J. Environ. Manag. 2018, 213, 159–167. [Google Scholar] [CrossRef]
- Enright, A.-M.; McHugh, S.; Collins, G.; O’Flaherty, V. Low-temperature anaerobic biological treatment of solvent-containing pharmaceutical wastewater. Water Res. 2005, 39, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Doyle, R.J.; Rosenberg, M. Mechanism of enhancement of microbial cell hydrophobicity by cationic polymers. J. Bacteriol. 1990, 172, 5650–5654. [Google Scholar] [CrossRef] [Green Version]
- Thaveesri, J.; Daffonchio, D.; Liessens, B.; Vandermeren, P.; Verstraete, W. Granulation and sludge bed stability in upflow anaerobic sludge bed reactors in relation to surface thermodynamics. Appl. Environ. Microbiol. 1995, 61, 3681–3686. [Google Scholar] [CrossRef] [Green Version]
- Lafita, C.; Marzal, P.; Gabaldón, C.; San-Valero, P.; Penya-Roja, J.M. Enhancement of biomass retention in an EGSB reactor used to treat 1-methoxy-2-propanol. J. Chem. Technol. Biotechnol. 2017, 93, 1044–1049. [Google Scholar] [CrossRef]
- Pol, L.H.; Lopes, S.D.C.; Lettinga, G.; Lens, P. Anaerobic sludge granulation. Water Res. 2004, 38, 1376–1389. [Google Scholar] [CrossRef]
- Alphenaar, P.A.; Visser, A.; Lettinga, G. The effect of liquid upward velocity and hydraulic retention time on granulation in UASB reactors treating wastewater with a high sulphate content. Bioresour. Technol. 1993, 43, 249–258. [Google Scholar] [CrossRef]
- O’Flaherty, V.; Lens, P.N.L.; de Beer, D.; Colleran, E. Effect of feed composition and upflow velocity on aggregate charac-teristics in anaerobic upflow reactors. Appl. Microbiol. Biotechnol. 1997, 47, 102–107. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Guha, S.; Harendranath, C.S.; Tripathi, S. Influence of extrinsic factors on granulation in UASB reactor. Appl. Microbiol. Biotechnol. 2006, 71, 145–154. [Google Scholar] [CrossRef]
- van Lier, J.B.; Mahmoud, N.; Zeeman, G. Anaerobic Wastewater Treatment. In Biological Wastewater Treatment: Principles, Modelling and Design, 1st ed.; Henze, M., van Loosdrecht, M.C.M., Ekama, G.A., Brdjamovic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 401–442. [Google Scholar]
- Schmidt, J.E.; Ahring, B.K. Granular sludge formation in upflow anaerobic sludge blanket (UASB) reactors. Biotechnol. Bioeng. 1996, 49, 229–246. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Asolekar, S.R.; Ranganathan, K.R.; Joshi, S.G. Experience with UASB reactor start-up under different operating conditions. Water Sci. Technol. 1996, 34, 421–428. [Google Scholar] [CrossRef]
- Sudmalis, D.; Gagliano, M.; Pei, R.; Grolle, K.; Plugge, C.; Rijnaarts, H.; Zeeman, G.; Temmink, H. Fast anaerobic sludge granulation at elevated salinity. Water Res. 2018, 128, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Antwi, P.; Li, J.; Boadi, P.O.; Meng, J.; Shi, E.; Xue, C.; Zhang, Y.; Ayivi, F. Functional bacterial and archaeal diversity revealed by 16S rRNA gene pyrosequencing during potato starch processing wastewater treatment in an UASB. Bioresour. Technol. 2017, 235, 348–357. [Google Scholar] [CrossRef]
- Connelly, S.; Shin, S.G.; Dillon, R.J.; Ijaz, U.Z.; Quince, C.; Sloan, W.T.; Collins, G. Bioreactor Scalability: Laboratory-Scale Bioreactor Design Influences Performance, Ecology, and Community Physiology in Expanded Granular Sludge Bed Bioreactors. Front. Microbiol. 2017, 8, 664. [Google Scholar] [CrossRef]
- Ferrero, P.; Carrascosa, C.; Marzal, P.; Gabaldón, C.; Penya-Roja, J.M.; Martínez-Soria, V. Effect of substrate composition on the stability and microbial community of an anaerobic expanded granular sludge bed reactor treating printing solvent mix-tures of ethanol and glycol ethers. Int. Biodeter. Biodegr. 2019, 145, 104815. [Google Scholar] [CrossRef]
- Luo, G.; Li, J.; Li, Y.; Wang, Z.; Li, W.T.; Li, A.M. Performance, kinetics behaviors and microbial community of internal cir-culation anaerobic reactor treating wastewater with high organic loading rate: Role of external hydraulic circulation. Bioresour. Technol. 2016, 222, 470–477. [Google Scholar] [CrossRef]
- Song, G.; Xi, H.; Zhou, Y.; Fu, L.; Xing, X.; Wu, C. Influence of organic load rate (OLR) on the hydrolytic acidification of 2-butenal manufacture wastewater and analysis of bacterial community structure. Bioresour. Technol. 2017, 243, 502–511. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y.; Gao, D.; Liu, G.; Chen, C.; Ren, N.; van Lier, J.B.; de Kreuk, M. Microbial population dynamics in re-sponse to increasing loadings of pre-hydrolyzed pig manure in an expanded granular sludge bed. Water Res. 2015, 87, 29–37. [Google Scholar] [CrossRef]
- Regueiro, L.; Veiga, P.; Figueroa, M.; Alonso-Gutierrez, J.; Stams, A.J.M.; Lema, J.M.; Carballa, M. Relationship between mi-crobial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 2012, 167, 581–589. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, Q.; Wu, X.; Li, X.; Li, Q.; Wang, Y. Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar] [CrossRef]
- Eichler, B.; Schink, B. Fermentation of primary alcohols and diols and pure culture of syntrophically alcohol-oxidizing anaer-obes. Arch. Microbiol. 1985, 143, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Kampmann, K.; Ratering, S.; Kramer, I.; Schmidt, M.; Zerr, W.; Schnell, S. Unexpected Stability of Bacteroidetes and Firmicutes Communities in Laboratory Biogas Reactors Fed with Different Defined Substrates. Appl. Environ. Microbiol. 2012, 78, 2106–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fykse, E.M.; Aarskaug, T.; Madslien, E.H.; Dybwad, M. Microbial community structure in a full-scale anaerobic treatment plant during start-up and first year of operation revealed by high-throughput 16S rRNA gene amplicon sequencing. Bioresour. Technol. 2016, 222, 380–387. [Google Scholar] [CrossRef]
- Song, M.; Shin, S.G.; Hwang, S. Methanogenic population dynamics assessed by real-time quantitative PCR in sludge granule in upflow anaerobic sludge blanket treating swine wastewater. Bioresour. Technol. 2010, 101, S23–S28. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Kuang, X.Z.; Shi, X.S.; Yuan, X.Z.; Guo, R.B. Paludibacter jiangxiensis sp. nov., a strictly anaerobic, propio-nate-producing bacterium isolated from rice paddy field. Arch. Microbiol. 2014, 196, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, Gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Owusu-Agyeman, I.; Eyice, Ö.; Cetecioglu, Z.; Plaza, E. The study of structure of anaerobic granules and methane producing pathways of pilot-scale UASB reactors treating municipal wastewater under sub-mesophilic conditions. Bioresour. Technol. 2019, 290, 121733. [Google Scholar] [CrossRef]
- Liu, S.; Suflita, J.M. Anaerobic biodegradation of methyl esters byAcetobacterium woodii andEubacterium limosum. J. Ind. Microbiol. Biotechnol. 1994, 13, 321–327. [Google Scholar] [CrossRef]
- Schramm, E.; Schink, B. Ether-cleaving enzyme and diol dehydratase involved in anaerobic polyethylene glycol degradation by a new Acetobacterium sp. Biodegradation 1991, 2, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevin, K.P.; Holmes, D.E.; Woodard, T.L.; Hinlein, E.S.; Ostendorf, D.W.; Lovley, D.R. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates. Int. J. Syst. Evol. Microbiol. 2005, 55, 1667–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.B.; He, Z.; Yang, Z.K.; Ringbauer, J.A., Jr.; He, Q.; Zhou, J.; Voordouw, G.; Wall, J.D.; Arkin, A.P.; Hazen, T.C.; et al. The electron transfer system of syntrophically grown Desulfovibrio vulgaris. J. Bacteriol. 2009, 191, 5793–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, P.M.; Malvankar, N.S.; Werner, J.J.; Franks, A.E.; Elena-Rotaru, A.; Shrestha, M.; Liu, F.; Nevin, K.P.; Angenent, L.T.; Lovley, D.R. Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour. Technol. 2014, 174, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Yu, Q.; Dang, Y.; Li, Y.; Quan, X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016, 102, 475–484. [Google Scholar] [CrossRef]


| Stage I | Stage II | Stage III | Stage IV | |||||
|---|---|---|---|---|---|---|---|---|
| Operational day | 0–41 | 42–69 | 70–97 | 98–106 | ||||
| Reactor | R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 |
| Operational mode | Intermittent load 16 h d−1 a | Continuous load | ||||||
| OLR (kg COD m−3 d−1) a | 2.1–4.3 | 1.4–2.9 | 6.8–8.0 | 4.6–8.0 | 13.0 | 13.0 | 10.0 | 13.0 |
| Influent COD (g L−1) | 4.3 | 2.9–2.6 | 4.3–5.1 | 3.6–5.1 | 8.3 | 8.3 | 6.4 | 8.3 |
| Up-flow velocity, UL (m h−1) | 0.05–0.10 | 3.00 | 0.12–0.15 | 3.00 | 0.15 | 3.00 | 0.15 | 3.00 |
| BMP (NmL CH4 g COD−1) | ||||
| Reactor | Ethanol | Ethyl Acetate | E2P a | Mixture |
| R1 | 299 ± 5 | 292 ± 5 | 341 ± 9 | 300 ± 5 |
| R2 | 303 ± 4 | 308 ± 10 | 321 ± 5 | 314 ± 13 |
| SMA (NmL CH4 g VSS−1 d−1) | ||||
| Reactor | Ethanol | Ethyl Acetate | E2P a | Mixture |
| R1 | 574 ± 20 | 520 ± 13 | 110 ± 1 | 510 ± 6 |
| R2 | 536 ± 6 | 518 ± 19 | 142 ± 11 | 500 ± 6 |
| Granules (%) | Mean Diameter (µm) | ||||
|---|---|---|---|---|---|
| Day | R1 | R2 | R1 | R2 | |
| Stage I | 0 | 5.7 | 5.7 | 85 | 85 |
| 20 | 62.6 | 51.9 | 475 | 354 | |
| 41 | 21.8 | 31.6 | 187 | 238 | |
| Stage II | 61 | 72.4 | 46.3 | 465 | 307 |
| Stage III | 82 | 41.4 | 29.5 | 312 | 224 |
| Stage IV | 106 | 71.1 | 52.2 | 562 | 386 |
| Granular Strength | |||||
|---|---|---|---|---|---|
| Reactor | Mean Diameter (µm) | Settling Velocity (m h−1) | IC2 (%) a | IC3 (%) a | IC5 (%) a |
| R1 | 562 | 19.9 | 13 | 18 | 38 |
| R2 | 386 | 22.4 | 24 | 33 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, K.; Álvarez-Hornos, F.J.; Gabaldón, C.; Marzal, P. Start-Up of Chitosan-Assisted Anaerobic Sludge Bed Reactors Treating Light Oxygenated Solvents under Intermittent Operation. Int. J. Environ. Res. Public Health 2021, 18, 4986. https://doi.org/10.3390/ijerph18094986
Torres K, Álvarez-Hornos FJ, Gabaldón C, Marzal P. Start-Up of Chitosan-Assisted Anaerobic Sludge Bed Reactors Treating Light Oxygenated Solvents under Intermittent Operation. International Journal of Environmental Research and Public Health. 2021; 18(9):4986. https://doi.org/10.3390/ijerph18094986
Chicago/Turabian StyleTorres, Keisy, Francisco Javier Álvarez-Hornos, Carmen Gabaldón, and Paula Marzal. 2021. "Start-Up of Chitosan-Assisted Anaerobic Sludge Bed Reactors Treating Light Oxygenated Solvents under Intermittent Operation" International Journal of Environmental Research and Public Health 18, no. 9: 4986. https://doi.org/10.3390/ijerph18094986
APA StyleTorres, K., Álvarez-Hornos, F. J., Gabaldón, C., & Marzal, P. (2021). Start-Up of Chitosan-Assisted Anaerobic Sludge Bed Reactors Treating Light Oxygenated Solvents under Intermittent Operation. International Journal of Environmental Research and Public Health, 18(9), 4986. https://doi.org/10.3390/ijerph18094986






