Vaginal Aging—What We Know and What We Do Not Know
Abstract
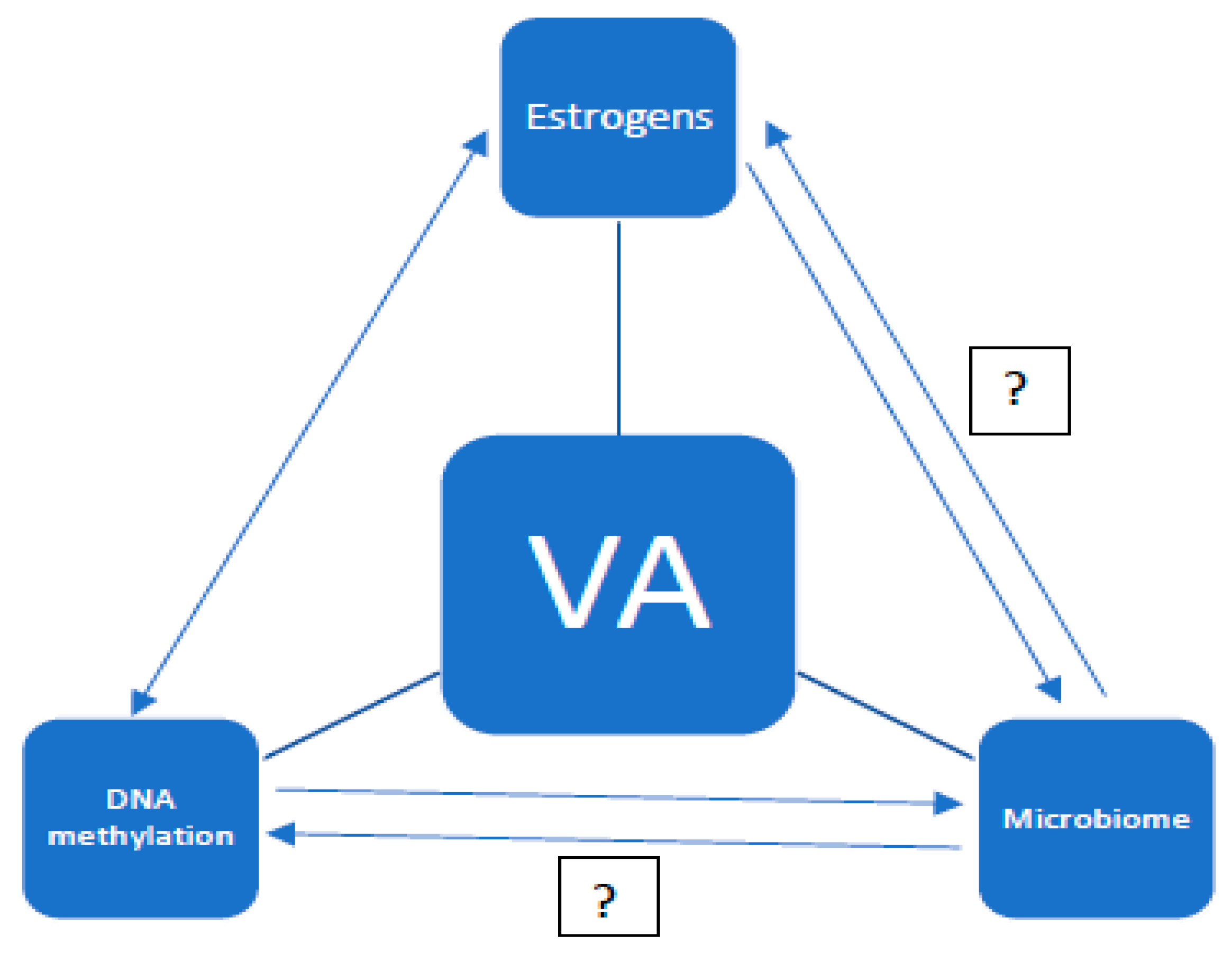
1. Introduction
2. Changes in the Field of Microbiota
3. The Influence of Hormones on the Vaginal Epithelium
4. Genetic Aspects of Aging
- Chromatin remodeling regulated by histone GlcNAcylation;
- Transcriptional activation induced by the GlcNAcylation of HCF1 (host cell factor 1) promoted by the interaction of TET2/3 with OGT;
- Facilitation of the biding of transcriptional factor-like molecules to the promoter of associated genes by the OGT–TET3 complex [48].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niehrs, C.; Calkhoven, C.F. Emerging Role of C/EBPβ and Epigenetic DNA Methylation in Ageing. Trends Genet. 2020, 36, 71–80. [Google Scholar] [CrossRef]
- Borkowska, J.; Domaszewska-Szostek, A.; Kołodziej, P.; Wicik, Z.; Połosak, J.; Buyanovskaya, O.; Charzewski, L.; Stańczyk, M.; Noszczyk, B.; Puzianowska-Kuznicka, M. Alterations in 5 hmC level and genomic distribution in aging-related epigenetic drift in human adipose stem cells. Epigenomics 2020, 12, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Szymański, J.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. [Google Scholar] [CrossRef]
- Kranich, J.; Maslowski, K.M.; Mackay, C.R. Commensal flora and the regulation of inflammatory and autoimmune responses. Semin. Immunol. 2011, 23, 139–145. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Franceschi, C.; Brigidi, P. The aging gut microbiota: New perspectives. Ageing Res. Rev. 2011, 10, 428–429. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef]
- Borgogna, J.C.; Shardell, M.D.; Santori, E.K.; Nelson, T.M.; Rath, J.M.; Glover, E.D.; Ravel, J.; Gravitt, P.E.; Yeoman, C.J. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: A cross-sectional analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Masha, S.C.; Owuor, C.; Ngoi, J.M.; Cools, P.; Sanders, E.J.; Vaneechoutte, M.; Crucitti, T.; De Villiers, E.P. Comparative analysis of the vaginal microbiome of pregnant women with either Trichomonas vaginalis or Chlamydia trachomatis. PLoS ONE 2019, 14, e0225545. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.C.; Crucitti, T.; Smekens, T.; Hansen, C.H.; Andreasen, A.; Jespers, V.; Hardy, L.; Irani, J.; Changalucha, J.; Baisley, K.; et al. The Vaginal Microbiota Among Adolescent Girls in Tanzania Around the Time of Sexual Debut. Front. Cell. Infect. Microbiol. 2020, 10, 305. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; De Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between Vaginal Microbiome and Female Health: A review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal Microbiota of Adolescent Girls Prior to the Onset of Menarche Resemble Those of Reproductive-Age Women. mBio 2015, 6, e00097-15. [Google Scholar] [CrossRef]
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.L.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Hummelen, R.; Macklaim, J.M.; Bisanz, J.E.; Hammond, J.-A.; McMillan, A.; Vongsa, R.; Koenig, D.; Gloor, G.B.; Reid, G. Vaginal Microbiome and Epithelial Gene Array in Post-Menopausal Women with Moderate to Severe Dryness. PLoS ONE 2011, 6, e26602. [Google Scholar]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Gliniewicz, K.; Schneider, G.M.; Ridenhour, B.J.; Williams, C.J.; Song, Y.; Farage, M.A.; Miller, K.; Forney, L.J. Comparison of the Vaginal Microbiomes of Premenopausal and Postmenopausal Women. Front. Microbiol. 2019, 10, 193. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Srinivasan, S.; Plantinga, A.; Wu, M.C.; Reed, S.D.; Guthrie, K.A.; LaCroix, A.Z.; Fiedler, T.; Munch, M.; Liu, C.; et al. Associations between improvement in genitourinary symptoms of menopause and changes in the vaginal ecosystem. Menopause 2018, 25, 500–507. [Google Scholar] [CrossRef]
- Montoya, T.I.; Maldonado, P.A.; Acevedo, J.F.; Word, R.A. Effect of Vaginal or Systemic Estrogen on Dynamics of Collagen Assembly in the Rat Vaginal Wall. Biol. Reprod. 2015, 92, 43. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Brandi, H.; Gomez, V.; Luque, D. Efficacy of Erbium: YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg. Med. 2017, 49, 160–168. [Google Scholar] [CrossRef]
- Chen, G.-D.; Oliver, R.H.; Leung, B.S.; Lin, L.-Y.; Yeh, J. Estrogen receptor α and β expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil. Steril. 1999, 71, 1099–1102. [Google Scholar] [CrossRef]
- Fu, X.; Rezapour, M.; Wu, X.; Li, L.; Sjörgen, C. Expression of estrogen receptor-α and -β in anterior vaginal walls of genuine stress incontinent women. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2003, 14, 276–281. [Google Scholar] [CrossRef]
- Cavallini, A.; Di Naro, E.; Giocolano, A.; Caringella, A.M.; Ferreri, R.; Tutino, V.; Loverro, G. Estrogen receptor (ER) and ER-related receptor expression in normal and atrophic human vagina. Maturitas 2008, 59, 219–225. [Google Scholar] [CrossRef]
- Gebhart, J.B.; Rickard, D.J.; Barrett, T.J.; Lesnick, T.G.; Webb, M.J.; Podratz, K.C.; Spelsberg, T.C. Expression of estrogen receptor isoforms α and β messenger RNA in vaginal tissue of premenopausal and postmenopausal women. Am. J. Obstet. Gynecol. 2001, 185, 1325–1331. [Google Scholar] [CrossRef]
- Fagan, D.H.; Yee, D. Crosstalk Between IGF1R and Estrogen Receptor Signaling in Breast Cancer. J. Mammary Gland. Biol. Neoplasia 2008, 13, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fagan, D.H.; Uselman, R.R.; Sachdev, D.; Yee, D. Acquired Resistance to Tamoxifen Is Associated with Loss of the Type I Insulin-like Growth Factor Receptor: Implications for Breast Cancer Treatment. Cancer Res. 2012, 72, 3372–3380. [Google Scholar] [CrossRef]
- Fan, P.; Wang, J.; Santen, R.J.; Yue, W. Long-term Treatment with Tamoxifen Facilitates Translocation of Estrogen Receptor α out of the Nucleus and Enhances its Interaction with EGFR in MCF-7 Breast Cancer Cells. Cancer Res. 2007, 67, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Maximov, P.Y.; Curpan, R.F.; Abderrahman, B.; Jordan, V.C. The molecular, cellular and clinical consequences of targeting the estrogen receptor following estrogen deprivation therapy. Mol. Cell. Endocrinol. 2015, 418, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Massarweh, S.; Osborne, C.K.; Wakeling, A.E.; Ali, S.; Weiss, H.; Schiff, R. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J. Natl. Cancer Inst. 2004, 96, 926–935. [Google Scholar] [CrossRef]
- Sehl, M.E.; Ganz, P.A. Potential Mechanisms of Age Acceleration Caused by Estrogen Deprivation: Do Endocrine Therapies Carry the Same Risks? JNCI Cancer Spectr. 2018, 2, pky035. [Google Scholar] [CrossRef]
- Gorodeski, G.I. Effects of estrogen on proton secretion via the apical membrane in vaginal-ectocervical epithelial cells of postmenopausal women. Menopause 2005, 12, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Gorodeski, G.I. Aging and Estrogen Effects on Transcervical-Transvaginal Epithelial Permeability. J. Clin. Endocrinol. Metab. 2005, 90, 345–351. [Google Scholar] [CrossRef]
- Gorodeski, G.I. Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women. Menopause 2007, 14, 1012–1019. [Google Scholar] [CrossRef][Green Version]
- Musicki, B.; Liu, T.; Lagoda, G.A.; Bivalacqua, T.J.; Strong, T.D.; Burnett, A.L. Endothelial Nitric Oxide Synthase Regulation in Female Genital Tract Structures. J. Sex. Med. 2009, 6, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zong, W.; Luan, H.; Liu, J.-H.; Zhang, A.-Z.; Li, X.-L.; Liu, S.-Y.; Zhang, S.-Q.; Gao, J.-G. Decreased expression of elastin and lysyl oxidase family genes in urogenital tissues of aging mice: Elastin and LOX family genes decreased in aging. J. Obstet. Gynaecol. Res. 2014, 40, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Sun, D.; Kandhi, S.; Groogh, G.; Zhuge, J.; Huang, W.; Hammock, B.D.; Huang, A. Estrogen-dependent epigenetic regulation of soluble epoxide hydrolase via DNA methylation. Proc. Natl. Acad. Sci. USA 2018, 115, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Noblin, D.J.; Serebrenik, Y.V.; Adams, A.; Zhao, C.; Crews, C.M. Targeted protein destabilization reveals an estrogen-mediated ER stress response. Nat. Chem. Biol. 2014, 10, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, W.; Malejczyk, J.; Wróblewska, M. Starzenie: Mechanizmy epigenetyczne i genetyczne. Aging: Epigenetic and genetic mechamisms. Ginekol. Pol. 2015, 2, 47–52. [Google Scholar]
- Truong, T.P.; Sakata-Yanagimoto, M.; Yamada, M.; Nagae, G.; Enami, T.; Nakamoto-Matsubara, R.; Aburatani, H.; Chiba, S. Age-Dependent Decrease of DNA Hydroxymethylation in Human T Cells. J. Clin. Exp. Hematop. 2015, 55, 1–6. [Google Scholar] [CrossRef]
- Li, D.; Guo, B.; Wu, H.; Tan, L.; Lu, Q. TET Family of Dioxygenases: Crucial Roles and Underlying Mechanisms. Cytogenet. Genome Res. 2015, 146, 171–180. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Pei, D. The Battle between TET Proteins and DNA Methylation for the Right Cell. Trends Cell Biol. 2018, 28, 973–975. [Google Scholar] [CrossRef]
- Kagiwada, S.; Kurimoto, K.; Hirota, T.; Yamaji, M.; Saitou, M. Replication-coupled passive DNA demethylation for the erasure of genome imprints in mice. EMBO J. 2012, 32, 340–353. [Google Scholar] [CrossRef]
- Parker, M.J.; Weigele, P.R.; Saleh, L. Insights into the Biochemistry, Evolution, and Biotechnological Applications of the Ten-Eleven Translocation (TET) Enzymes. Biochemistry 2019, 58, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; An, J.; Bandukwala, H.S.; Chavez, L.; Äijö, T.; Pastor, W.A.; Segal, M.F.; Li, H.; Koh, K.P.; Lähdesmäki, H.; et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 2013, 497, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Zhao, Z.J. Structure, regulation, and function of TET family proteins. In Epigenetic Gene Expression and Regulation; Huang, S., Litt, M.D., Blakey, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 379–395. [Google Scholar]
- Ulrich, C.M.; Toriola, A.T.; Koepl, L.M.; Sandifer, T.; Poole, E.M.; Duggan, C.; McTiernan, A.; Issa, J.-P.J. Metabolic, hormonal and immunological associations with global DNA methylation among postmenopausal women. Epigenetics 2012, 7, 1020–1028. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymański, J.K.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Vaginal Aging—What We Know and What We Do Not Know. Int. J. Environ. Res. Public Health 2021, 18, 4935. https://doi.org/10.3390/ijerph18094935
Szymański JK, Słabuszewska-Jóźwiak A, Jakiel G. Vaginal Aging—What We Know and What We Do Not Know. International Journal of Environmental Research and Public Health. 2021; 18(9):4935. https://doi.org/10.3390/ijerph18094935
Chicago/Turabian StyleSzymański, Jacek K., Aneta Słabuszewska-Jóźwiak, and Grzegorz Jakiel. 2021. "Vaginal Aging—What We Know and What We Do Not Know" International Journal of Environmental Research and Public Health 18, no. 9: 4935. https://doi.org/10.3390/ijerph18094935
APA StyleSzymański, J. K., Słabuszewska-Jóźwiak, A., & Jakiel, G. (2021). Vaginal Aging—What We Know and What We Do Not Know. International Journal of Environmental Research and Public Health, 18(9), 4935. https://doi.org/10.3390/ijerph18094935





