Endometriosis: New Perspective for the Diagnosis of Certain Cytokines in Women and Adolescent Girls, as Well as the Progression of Disease Outgrowth: A Systematic Review
Abstract
1. Introduction
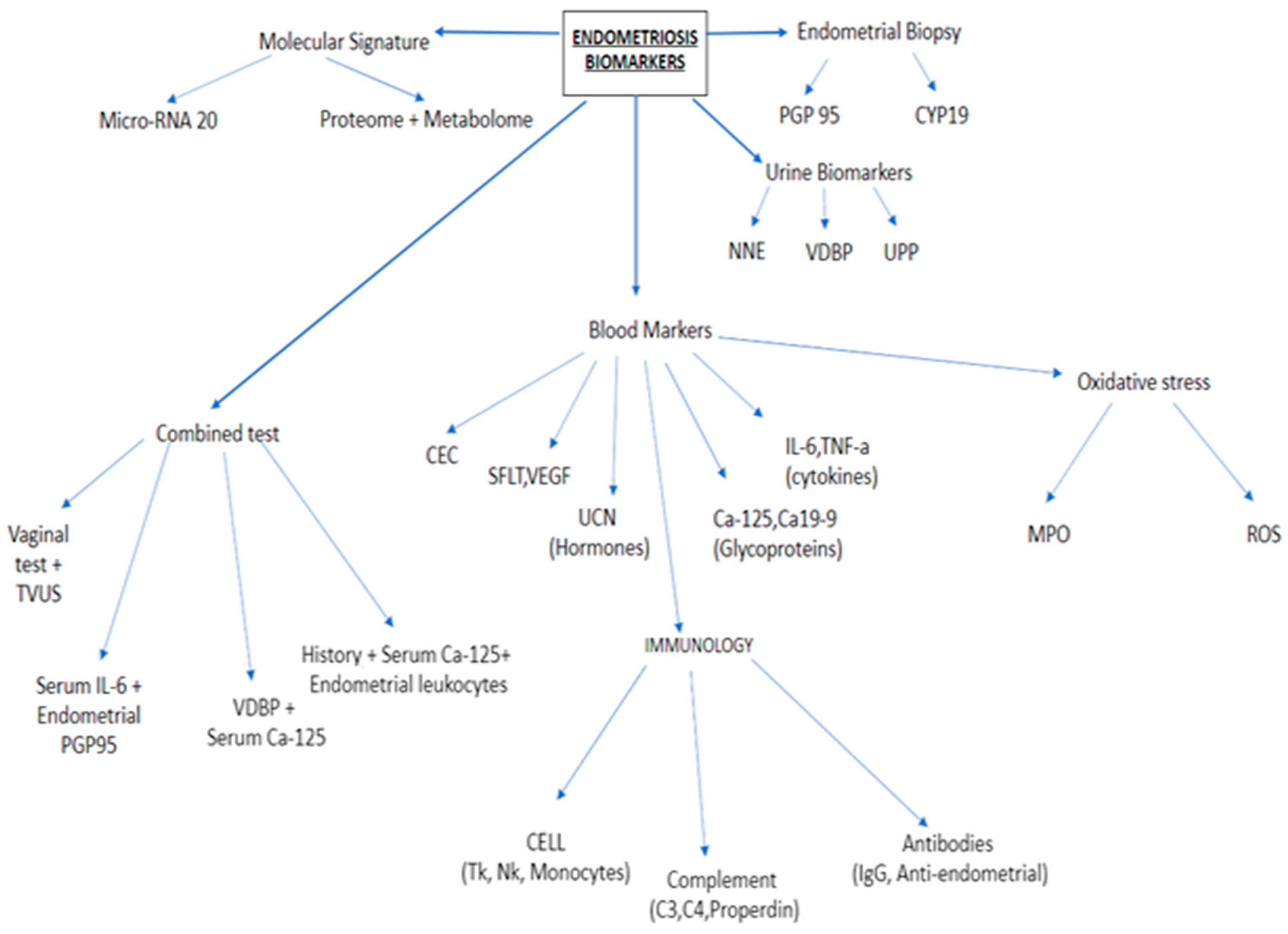
2. Classical Blood Biomarkers
3. Role of Inflammation Cytokines and Immunological Molecules as Serum/Plasma Biomarkers
3.1. Glycoproteins
3.2. Inflammatory Cytokines
- (1)
- IL-6 is one of the pro-inflammatory cytokines found at increased levels in affected women. Most studies estimate that, in stages I–II of the disease, there is an increased level of this particular cytokine, which has a fairly high level of sensitivity, at 76% [13].
- (2)
- IL-8 belongs to the family of chemokines produced and released by cells associated with chronic inflammation—called monocytes/macrophages—and is considered to be a non-invasive biomarker. Many studies have determined that this parameter is drastically augmented during stages I–II of the disease, especially in endometriomas [14].
- (3)
- TNF-alpha is another pro-inflammatory cytokine with extraordinary potential as a pro-angiogenic molecule. Some studies revealed an increased serum level, with the urine level remaining unchanged. The severity of endometriosis greatly depends on the serum level of TNF-alpha. Current research suggests that the soluble TNF-alpha receptor in affected patients shows a potent increase during the follicular phase of the menstrual cycle [15].
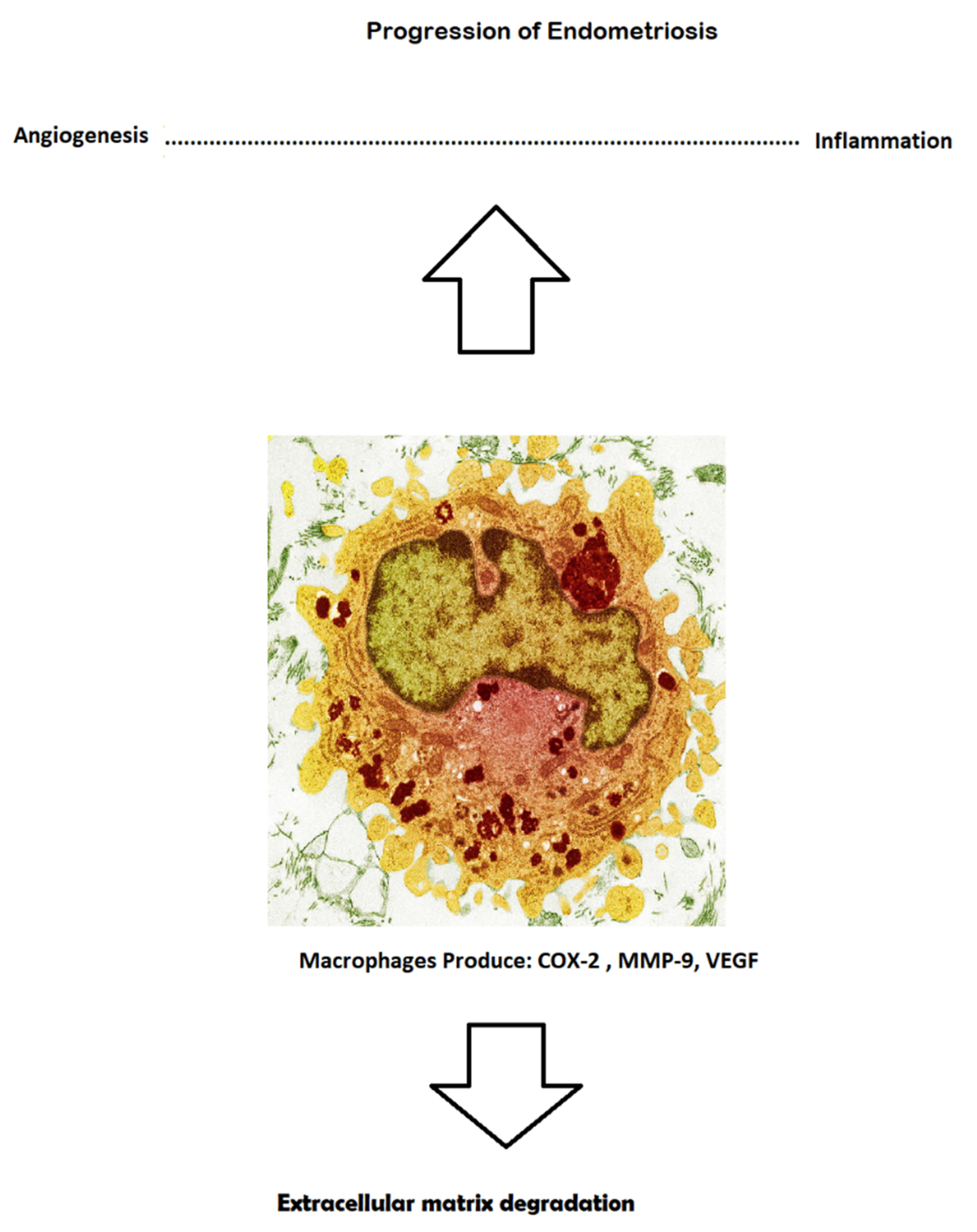
3.3. Angiogenic Molecules
4. New Endometriosis Markers
4.1. Urocortin
4.2. Circulating Endometrial Cells (CECs)
5. New Molecular Signatures
5.1. Proteome
5.2. Metabolome
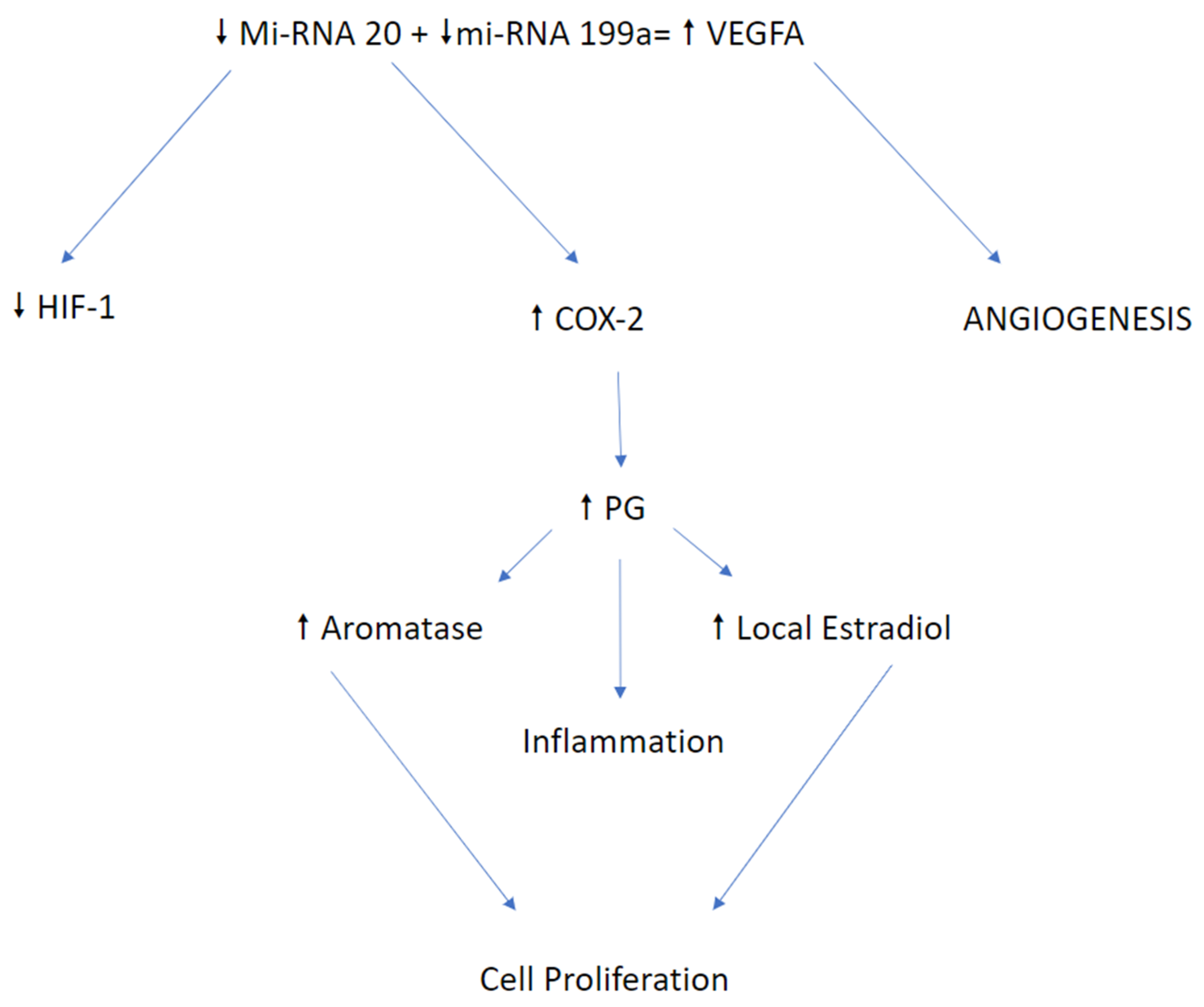
5.3. MicroRNA
6. New Hope as a Biomarker
7. Role of MI-RNA and Different Expression in Endometriosis
8. Factors Impacting miRNA Expression
9. MiRNA-20a: Crucial Role of Circulating Candidate
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FGF | Fibroblast Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
| PEDF | Pigmented Epithelial Dermal Factor |
| IL-1 | Interleukin 1 |
| IL-1R | Interleukin 1 Receptor |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-17 | Interleukin 17 |
| TNF-alpha/beta | Tumor Necrosis Factor-alpha/beta |
| IFN-gamma | Interferon-gamma |
| UCN-1 | Urocortin 1 |
| CEC | Circulating Endometrial Cell |
| COX-2 | Cyclooxygenase 2 |
| TH2 | T-Lymphocytes CD40 + helper 2 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| HIF-1 | Hypoxia Induced Factor-1 |
| CA 125 | Carbohydrate Antigen 125 |
| CA 19-9 | Carbohydrate Antigen 19-9 |
| CCND-1 | Cyclin D1 |
References
- Laganà, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Frangež, H.B.; Bokal, E.V.; Vitale, S.G. Anxiety and depression in patients with endometriosis: Impact and management challenges. Int. J. Women’s Health 2017, 9, 323–330. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.C.; Laganà, A.S. Impact of endometriosis on quality of life and psychological well-being. J. Psychosom. Obstet. Gynecol. 2016, 38, 317–319. [Google Scholar] [CrossRef]
- La Rosa, V.L.; Barra, F.; Chiofalo, B.; Platania, A.; Di Guardo, F.; Conway, F.; Antonio, S.D.A.; Lin, L.-T. An overview on the relationship between endometriosis and infertility: The impact on sexuality and psychological well-being. J. Psychosom. Obstet. Gynecol. 2020, 41, 93–97. [Google Scholar] [CrossRef]
- Bsc, P.A.W.R.; D’Hooghe, T.M.; Fazleabas, A.; Giudice, L.C.; Montgomery, G.W.; Petraglia, F.; Taylor, R.N. Defining Future Directions for Endometriosis Research. Reprod. Sci. 2013, 20, 483–499. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitale, S.G.; Trovato, M.A.; Palmara, V.I.; Rapisarda, A.M.C.; Granese, R.; Sturlese, E.; De Dominici, R.; Alecci, S.; Padula, F.; et al. Full-Thickness Excision versus Shaving by Laparoscopy for Intestinal Deep Infiltrating Endometriosis: Rationale and Potential Treatment Options. BioMed Res. Int. 2016, 2016, 3617179. [Google Scholar] [CrossRef]
- Coutinho, L.M.; Ferreira, M.C.; Rocha, A.L.L.; Carneiro, M.M.; Reis, F.M. New biomarkers in endometriosis. Adv. Clin. Chem. 2019, 89, 59–77. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Frangež, H.B.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef]
- Vitale, S.G.; Capriglione, S.; Peterlunger, I.; La Rosa, V.L.; Vitagliano, A.; Noventa, M.; Valenti, G.; Sapia, F.; Angioli, R.; Lopez, S.; et al. The Role of Oxidative Stress and Membrane Transport Systems during Endometriosis: A Fresh Look at a Busy Corner. Oxidative Med. Cell. Longev. 2018, 2018, 7924021. [Google Scholar] [CrossRef]
- Mihalyi, A.; Gevaert, O.; Kyama, C.M.; Simsa, P.; Pochet, N.; De Smet, F.; De Moor, B.; Meuleman, C.; Billen, J.; Blanckaert, N.; et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum. Reprod. 2009, 25, 654–664. [Google Scholar] [CrossRef]
- Agic, A.; Djalali, S.; Wölfler, M.M.; Halis, G.; Diedrich, K.; Hornung, D. Combination of CCR1 mRNA, MCP1, and CA125 Measurements in Peripheral Blood as a Diagnostic Test for Endometriosis. Reprod. Sci. 2008, 15, 906–911. [Google Scholar] [CrossRef]
- Vodolazkaia, A.; El-Aalamat, Y.; Popovic, D.; Mihalyi, A.; Bossuyt, X.; Kyama, C.M.; Fassbender, A.; Bokor, A.; Schols, D.; Huskens, D.; et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum. Reprod. 2012, 27, 2698–2711. [Google Scholar] [CrossRef]
- Othman, E.E.-D.R.; Hornung, D.; Salem, H.T.; Khalifa, E.A.; El-Metwally, T.H.; Al-Hendy, A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 240–246. [Google Scholar] [CrossRef]
- Kalu, E.; Sumar, N.; Giannopoulos, T.; Patel, P.; Croucher, C.; Sherriff, E.; Bansal, A. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J. Obstet. Gynaecol. Res. 2007, 33, 490–495. [Google Scholar] [CrossRef]
- Daraï, E.; Detchev, R.; Hugol, D.; Quang, N.T. Serum and cyst fluid levels of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum. Reprod. 2003, 18, 1681–1685. [Google Scholar] [CrossRef]
- Asante, A.; Taylor, R.N. Endometriosis: The Role of Neuroangiogenesis. Annu. Rev. Physiol. 2011, 73, 163–182. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Ahn, S.H.; Edwards, A.K.; Singh, S.S.; Young, S.L.; Lessey, B.A.; Tayade, C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J. Immunol. 2015, 195, 2591–2600. [Google Scholar] [CrossRef]
- Gupta, R.-Y.Q.M.K. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144–1155. [Google Scholar] [CrossRef]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D.; et al. The Functional Role of the ELR Motif in CXC Chemokine-mediated Angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef]
- Carrarelli, P.; Luddi, A.; Funghi, L.; Arcuri, F.; Batteux, F.; Dela Cruz, C.; Tosti, C.; Reis, F.M.; Chapron, C.; Petraglia, F. Urocortin and corticotrophin-releasing hormone receptor type 2 mRNA are highly expressed in deep infiltrating endometriotic lesions. Reprod. Biomed. Online 2016, 33, 476–483. [Google Scholar] [CrossRef]
- Novembri, R.; Carrarelli, P.; Toti, P.; Rocha, A.L.L.; Borges, L.E.; Reis, F.M.; Piomboni, P.; Florio, P.; Petraglia, F. Urocortin 2 and urocortin 3 in endometriosis: Evidence for a possible role in inflammatory response. Mol. Hum. Reprod. 2011, 17, 587–593. [Google Scholar] [CrossRef]
- DeSitter, I.; Guerrouahen, B.S.; Benali-Furet, N.; Wechsler, J.; Jänne, P.A.; Kuang, Y.; Yanagita, M.; Wang, L.; Berkowitz, J.A.; Distel, R.J.; et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011, 31, 427–441. [Google Scholar]
- Mascalchi, M.; Falchini, M.; Maddau, C.; Salvianti, F.; Nistri, M.; Bertelli, E.; Sali, L.; Zuccherelli, S.; Vella, A.; Matucci, M.; et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J. Cancer Res. Clin. Oncol. 2016, 142, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Bobek, V.; Kolostova, K.; Kucera, E. Circulating endometrial cells in peripheral blood. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 267–274. [Google Scholar] [CrossRef]
- Seeber, B.; Sammel, M.D.; Fan, X.; Gerton, G.L.; Shaunik, A.; Chittams, J.; Barnhart, K.T. Proteomic analysis of serum yields six candidate proteins that are differentially regulated in a subset of women with endometriosis. Fertil. Steril. 2010, 93, 2137–2144. [Google Scholar] [CrossRef][Green Version]
- Vicente-Muñoz, S.; Morcillo, I.; Puchades-Carrasco, L.; Payá, V.; Pellicer, A.; Pineda-Lucena, A. Pathophysiologic processes have an impact on the plasma metabolomic signature of endometriosis patients. Fertil. Steril. 2016, 106, 1733–1741.e1. [Google Scholar] [CrossRef]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Updat. 2018, 24, 497–515. [Google Scholar] [CrossRef]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. Mol. Mech. Mutagen. 2011, 717, 85–90. [Google Scholar] [CrossRef]
- Mishra, P.J. MicroRNAs as promising biomarkers in cancer diagnostics. Biomark. Res. 2014, 2. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Rekker, K.; Saare, M.; Roost, A.M.; Kaart, T.; Sõritsa, D.; Karro, H.; Sõritsa, A.; Simón, C.; Salumets, A.; Peters, M. Circulating miR-200–family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil. Steril. 2015, 104, 938–946.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-T.; Zhao, Y.-N.; Han, B.-W.; Hong, S.-J.; Chen, Y.-Q. Circulating MicroRNAs Identified in a Genome-Wide Serum MicroRNA Expression Analysis as Noninvasive Biomarkers for Endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, 281–289. [Google Scholar] [CrossRef]
- Huang, R.S.; Gamazon, E.R.; Ziliak, D.; Wen, Y.; Im, H.K.; Zhang, W.; Wing, C.; Duan, S.; Bleibel, W.K.; Cox, N.J.; et al. Population differences in microRNA expression and biological implications. RNA Biol. 2011, 8, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Ameling, S.; Kacprowski, T.; Chilukoti, R.K.; Malsch, C.; Liebscher, V.; Suhre, K.; Pietzner, M.; Friedrich, N.; Homuth, G.; Hammer, E.; et al. Associations of circulating plasma microRNAs with age, body mass index and sex in a population-based study. BMC Med. Genom. 2015, 8, 61. [Google Scholar] [CrossRef]
- Wu, M.-H.; Chen, K.-F.; Lin, S.-C.; Lgu, C.-W.; Tsai, S.-J. Aberrant Expression of Leptin in Human Endometriotic Stromal Cells Is Induced by Elevated Levels of Hypoxia Inducible Factor-1α. Am. J. Pathol. 2007, 170, 590–598. [Google Scholar] [CrossRef]
- Backes, C.; Meese, E.; Keller, A. Specific miRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Ramon, L.A.; Braza-Boïls, A.; Gilabert-Estellés, J.; Gilabert, J.; Espana, F.; Chirivella, M.; Estelles, A. microRNAs expression in endometriosis and their relation to angiogenic factors. Hum. Reprod. 2011, 26, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Wang, C.-C.; Wu, M.-H.; Yang, S.-H.; Li, Y.-H.; Tsai, S.-J. Hypoxia-Induced MicroRNA-20a Expression Increases ERK Phosphorylation and Angiogenic Gene Expression in Endometriotic Stromal Cells. J. Clin. Endocrinol. Metab. 2012, 97, E1515–E1523. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.E.; Rodrigues-Baptista, K.C.; Perini JASoares de Moura, R. Euterpe oleracea extract (Açaí) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLoS ONE. 2016, 11, e0166059. [Google Scholar] [CrossRef] [PubMed]
- Drosdzol-Cop, A.; Skrzypulec-Plinta, V.; Stojko, R. Serum and peritoneal fluid immunological markers in adolescent girls with chronic pelvic pain. Obstet. Gynecol. Surv. 2012, 67, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Drosdzol-Cop, A.; Skrzypulec-Plinta, V. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. J. Obstet. Gynaecol. Res. 2012, 38, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Tapmeier, T.T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.T.; Becker, C.M. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toczek, J.; Jastrzębska-Stojko, Ż.; Stojko, R.; Drosdzol-Cop, A. Endometriosis: New Perspective for the Diagnosis of Certain Cytokines in Women and Adolescent Girls, as Well as the Progression of Disease Outgrowth: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4726. https://doi.org/10.3390/ijerph18094726
Toczek J, Jastrzębska-Stojko Ż, Stojko R, Drosdzol-Cop A. Endometriosis: New Perspective for the Diagnosis of Certain Cytokines in Women and Adolescent Girls, as Well as the Progression of Disease Outgrowth: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(9):4726. https://doi.org/10.3390/ijerph18094726
Chicago/Turabian StyleToczek, Jakub, Żaneta Jastrzębska-Stojko, Rafał Stojko, and Agnieszka Drosdzol-Cop. 2021. "Endometriosis: New Perspective for the Diagnosis of Certain Cytokines in Women and Adolescent Girls, as Well as the Progression of Disease Outgrowth: A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 9: 4726. https://doi.org/10.3390/ijerph18094726
APA StyleToczek, J., Jastrzębska-Stojko, Ż., Stojko, R., & Drosdzol-Cop, A. (2021). Endometriosis: New Perspective for the Diagnosis of Certain Cytokines in Women and Adolescent Girls, as Well as the Progression of Disease Outgrowth: A Systematic Review. International Journal of Environmental Research and Public Health, 18(9), 4726. https://doi.org/10.3390/ijerph18094726






