Relationship between Self-Reported Sleep Duration and Risk of Anemia: Data from the Korea National Health and Nutrition Examination Survey 2016–2017
Abstract
1. Introduction
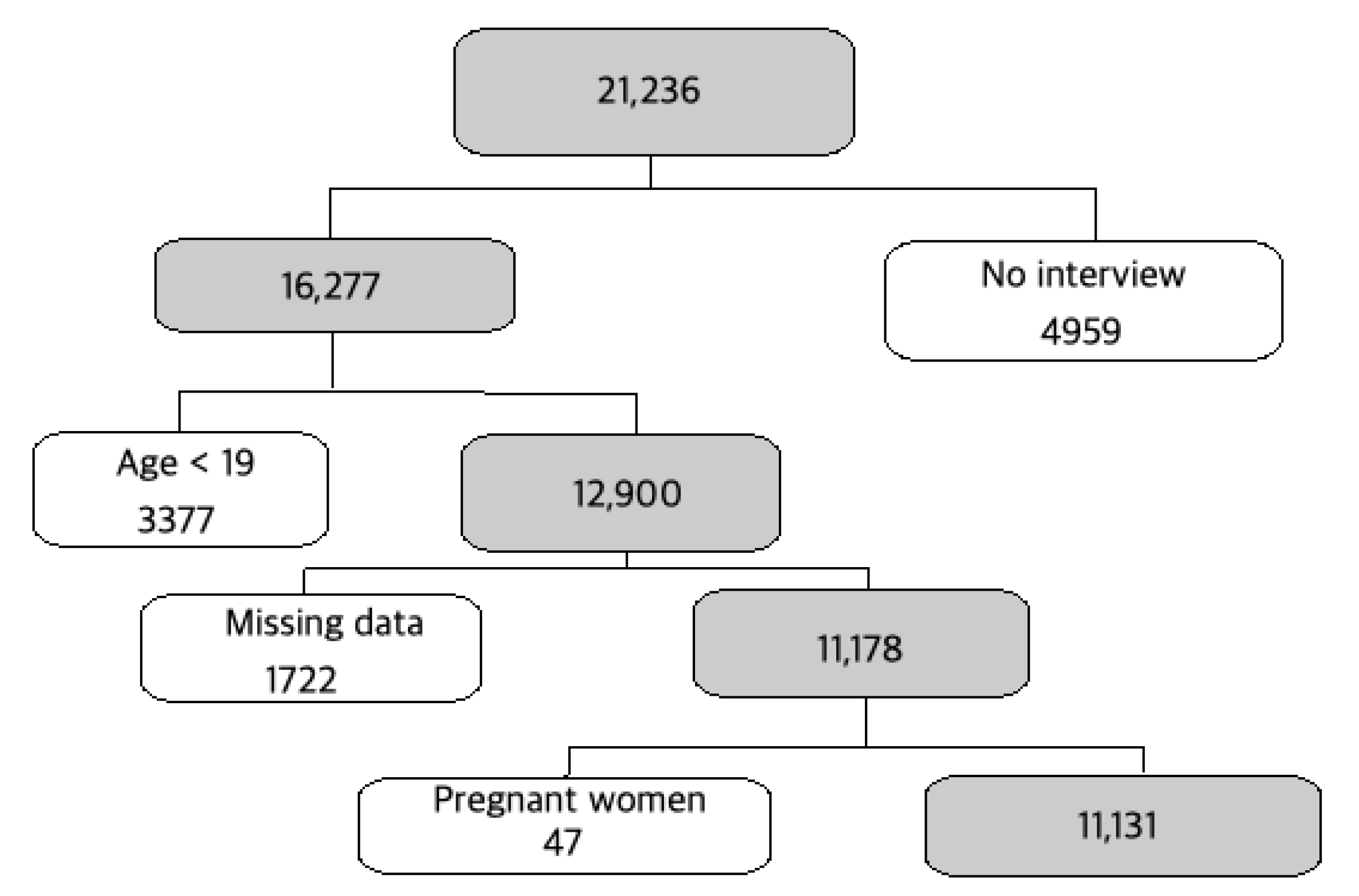
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.2.1. Sleep-Duration Assessment
2.2.2. Anemia Assessment
2.2.3. Sociodemographic and Health Behavior Variables
2.2.4. Health Status Variables
2.2.5. Biological Variables
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.Y.; Deng, W.; Zhang, R.Y.; Huang, J.P.; Wang, X.F.; Qian, D.G.; Xu, J.; Jin, L.; Wang, X.F. Anemia, Physical Function, and Mortality in Long-Lived Individuals Aged 95 and Older: A Population-Based Study. J. Am. Geriatr. Soc. 2015, 63, 2202–2204. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Mendes de Leon, C.; Artz, A.; Tang, Y.; Shah, R.; Evans, D. A Population-Based Study of Hemoglobin, Race, and Mortality in Elderly Persons. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 873–878. [Google Scholar] [CrossRef]
- Penninx, B.W.; Pahor, M.; Woodman, R.C.; Guralnik, J.M. Anemia in Old Age is Associated with Increased Mortality and Hospitalization. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 474–479. [Google Scholar] [CrossRef]
- Culleton, B.F.; Manns, B.J.; Zhang, J.; Tonelli, M.; Klarenbach, S.; Hemmelgarn, B.R. Impact of Anemia on Hospitalization and Mortality in Older Adults. Blood 2006, 107, 3841–3846. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Smith, G.L.; Radford, M.J.; Foody, J.M.; Krumholz, H.M. The Prognostic Importance of Anemia in Patients with Heart Failure. Am. J. Med. 2003, 114, 112–119. [Google Scholar] [CrossRef]
- De Lima, J.J.G.; Gowdak, L.H.W.; David-Neto, E.; Bortolotto, L.A. Diabetes, Cardiovascular Disease, and Cardiovascular Risk in Patients with Chronic Kidney Disease. High. Blood Press Cardiovasc. Prev. 2021, 28, 159–165. [Google Scholar] [CrossRef]
- Dhar, R.; Zazulia, A.R.; Videen, T.O.; Zipfel, G.J.; Derdeyn, C.P.; Diringer, M.N. Red Blood Cell Transfusion Increases Cerebral Oxygen Delivery in Anemic Patients with Subarachnoid Hemorrhage. Stroke 2009, 40, 3039–3044. [Google Scholar] [CrossRef]
- Del Fabbro, P.; Luthi, J.C.; Carrera, E.; Michel, P.; Burnier, M.; Burnand, B. Anemia and Chronic Kidney Disease are Potential Risk Factors for Mortality in Stroke Patients: A Historic Cohort Study. BMC Nephrol. 2010, 11, 27. [Google Scholar] [CrossRef]
- Milionis, H.; Papavasileiou, V.; Eskandari, A.; D’Ambrogio-Remillard, S.; Ntaios, G.; Michel, P. Anemia on admission predicts short- and long-term outcomes in patients with acute ischemic stroke. Int. J. Stroke 2015, 10, 224–230. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Li, Y.; Chen, P.; Chen, L. Anemia increases the mortality risk in patients with stroke: A meta-analysis of cohort studies. Sci. Rep. 2016, 6, 26636. [Google Scholar] [CrossRef]
- Martinez-Gomez, D.; Eisenmann, J.C.; Gomez-Martinez, S.; Hill, E.E.; Zapatera, B.; Veiga, O.; Marcos, A. AFINOS Study Group. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011, 12, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007, 30, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.N.; Kim, J.S.; Crainiceanu, C.; O’Hearn, D.; Punjabi, N.M. Habitual Sleep Duration and all-Cause Mortality in a General Community Sample. Sleep 2016, 39, 1903–1909. [Google Scholar] [CrossRef]
- Minarik, P.A. Sleep disturbance in midlife women. J. Obstet. Gynecol. Neonatal Nurs. 2009, 38, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.K.; Hatch, E.E.; Wise, L.A. Sleep and Female Reproduction. Curr. Opin. Obstet. Gynecol. 2019, 31, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Q.; Hu, W.; Han, X.; Gan, J.; Zheng, X.; Wang, X.; Wu, S. Night Sleep Duration and Risk of Incident Anemia in a Chinese Population: A Prospective Cohort Study. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Jackowsha, M.; Kumari, M.; Steptoe, A. Sleep and Biomarkers in the English Longitudinal Study of Ageing: Associations with C-Reactive Protein, Fibrinogen, Dehydroepiandrosterone Sulfate and Hemoglobin. Psychoneuro Endocrinol. 2013, 38, 1484–1493. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Le, C.H. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003-2012). PLoS ONE 2016, 11, e0166635. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Q.; Li, Y.; Zhao, F.; Chang, H.; Lyu, J. Longitudinal Study of the Relationship between Sleep Duration and Hypertension in Chinese Adult Residents (CHNS 2004–2011). Sleep Med. 2019, 58, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Simonetti, M.; Marconi, E.; Brignoli, O.; Cancian, M.; Masotti, A.; Pegoraro, V.; Heiman, F.; Cricelli, C.; Lapi, F. Gender Differences in Determinants of Iron-Deficiency Anemia: A Population-Based Study Conducted in Four European Countries. Ann. Hematol. 2019, 98, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. The Sex Difference in Haemoglobin Levels in Adults—Mechanisms, Causes, and Consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef]
- Moschovis, P.P.; Wiens, M.O.; Arlington, L.; Antsygina, O.; Hayden, D.; Dzik, W.; Kiwanuka, J.P.; Christiani, D.C.; Hibberd, P.L. Individual, Maternal and Household Risk Factors for Anaemia among Young Children in SubSaharan Africa: A Cross-Sectional Study. BMJ Open 2018, 8, e019654. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Zha, P. Trends in Socioeconomic Inequalities and Prevalence of Anemia among Children and Nonpregnant Women in Low- and Middle-Income Countries. JAMA Netw. Open. 2018, 1, e182899. [Google Scholar] [CrossRef] [PubMed]
- Mirowsky, J.; Ross, C.E. Education, cumulative advantage, and health. Ageing Int. 2005, 30, 27–62. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, E.C.; Lee, K.S.; Lee, Y.; Shim, S.; Kim, J.; Chon, D.; Lee, S.G. Association of sleep duration with rheumatoid arthritis in Korean adults: Analysis of seven years of aggregated data from the Korea National Health and Nutrition Examination Survey (KNHANES). BMJ Open 2016, 6, e011420. [Google Scholar] [CrossRef]
- Vulser, H.; Wiernik, E.; Hoertel, N.; Thomas, F.; Pannier, B.; Czernichow, S.; Hanon, O.; Simon, T.; Simon, J.; Danchin, N.; et al. Association between depression and anemia in otherwise healthy adults. Acta Psychiatr. Scand. 2016, 134, 150–160. [Google Scholar] [CrossRef]
- Grandner, M.A.; Buxton, O.M.; Jackson, N.; Sands-Lincoln, M.; Pandey, A.; Jean-Louis, G. Extreme sleep durations and increased C-reactive protein: Effects of sex and ethnoracial group. Sleep 2013, 36, 769E–779E. [Google Scholar] [CrossRef]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated Inflammatory Markers in Response to Prolonged Sleep Restriction are Associated with Increased Pain Experience in Healthy Volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef]
- Peirano, P.D.; Algarín, C.R.; Chamorro, R.A.; Reyes, S.C.; Durán, S.A.; Garrido, M.I.; Lozoff, B. Sleep Alterations and Iron Deficiency Anemia in Infancy. Sleep Med. 2010, 11, 637–642. [Google Scholar] [CrossRef][Green Version]
- Miller, M.A.; Kandala, N.B.; Kivimaki, M.; Kumari, M.; Brunner, E.J.; Lowe, G.D.; Marmot, M.G.; Cappuccio, F.P. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep 2009, 32, 857–864. [Google Scholar]
- Matthews, K.A.; Zheng, H.; Kravitz, H.M.; Sowers, M.; Bromberger, J.T.; Buysse, D.J.; Owens, J.F.; Sanders, M.; Hall, M. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women: Study of Women’s Health across the Nation sleep study. Sleep 2010, 33, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.N.; Snyder, P.J.; Stephens-Shields, A.J.; Artz, A.S.; Bhasin, S.; Cohen, H.J.; Farrar, J.T.; Gill, T.M.; Zeldow, B.; Cella, D.; et al. Association of Testosterone Levels With Anemia in Older Men: A Controlled Clinical Trial. JAMA Intern. Med. 2017, 177, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Regulation of erythropoietin production. J. Physiol. 2011, 598, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Shahani, S.; Braga-Basaria, M.; Maggio, M.; Basaria, S. Androgens and erythropoiesis: Past and present. J. Endocrinol. Investig. 2009, 32, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, H.M.; Kazlauskaite, R.; Joffe, H. Sleep, Health, and Metabolism in Midlife Women and Menopause: Food for Thought. Obstet. Gynecol. Clin. N. Am. 2018, 45, 679–694. [Google Scholar] [CrossRef]
- Bolge, S.C.; Balkrishnan, R.; Kannan, H.; Seal, B.; Drake, C.L. Burden associated with chronic sleep maintenance insomnia characterized by nighttime awakenings among women with menopausal symptoms. Menopause 2010, 17, 80–86. [Google Scholar] [CrossRef]
- Suarez, E.C. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for gender disparity. Brain Behav. Immun. 2008, 22, 960–968. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Cohen, B.E.; Neylan, T.C.; Whooley, M.A. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: Findings from the Heart and Soul Study. J. Psychiatr. Res. 2013, 47, 1228–1235. [Google Scholar] [CrossRef]
- Prather, A.A.; Puterman, E.; Epel, E.S.; Dhabhar, F.S. Poor sleep quality potentiates stress-induced cytokine reactivity in postmenopausal women with high visceral abdominal adiposity. Brain Behav. Immun. 2014, 35, 155–162. [Google Scholar] [CrossRef] [PubMed]
| Variable | Sleep Duration (hours) | p-Value * | ||||
|---|---|---|---|---|---|---|
| <5 h | 5 h ~ <6 h | 6 h ~ <8 h | 8 h ~ <9 h | ≥9 h | ||
| Questionnaire-Based Data | (n = 492) | (n = 1129) | (n = 6147) | (n = 2226) | (n = 1137) | |
| Age, years | 47.12 ± 1.04 ab | 47.76 ± 0.59 ab | 46.28 ± 0.3 a | 46.88 ± 0.48 a | 48.88 ± 0.82 bc | 0.002 |
| male, sex, n (%) | 206 (47.7) ab | 461 (46.5) a | 2821(52.7) b | 950(47.4) ab | 476(45.7) a | <0.001 |
| Family income, n (%) , | ||||||
| Low | 134 (23.5) | 220 (16.0) | 940 (12.5) | 435 (16.1) | 380 (28.4) | <0.001 |
| Lower middle | 123 (24.6) | 269 (23.0) | 1433 (22.1) | 579 (24.5) | 284 (23.5) | |
| Upper middle | 135 (30.4) | 308 (28.9) | 1760 (30.8) | 602 (29.0) | 268 (26.4) | |
| High | 99 (21.5) | 331 (32.1) | 1997 (34.6) | 604 (30.4) | 201 (21.6) | |
| High school or above , n (%) | 316 (72.8) ab | 804 (78.0) b | 4603 (81.3) b | 1514 (76.1) ab | 630 (66.3) a | <0.001 |
| Current smoker , n (%) | 103 (24.0) a | 217 (21.4) a | 1158 (22.8) b | 363 (19.6) a | 197 (21.6) a | 0.043 |
| Frequency of alcohol intake/month, n (%) | ||||||
| Never | 103 (21.7) | 182 (15.4) | 895 (13.4) | 336 (14.4) | 221 (18.6) | 0.021 |
| Less than once | 108 (27.9) | 314 (31.4) | 1768 (30.9) | 637 (31.9) | 299 (30.6) | |
| Two– four times | 86 (21.0) | 260 (26.8) | 1456 (28.8) | 497 (28.0) | 204 (23.4) | |
| Over four times | 115 (29.4) | 248 (26.4) | 1417 (26.9) | 472 (25.7) | 244 (27.3) | |
| Aerobic regular exercise, n (%) | 211 (45.9) ab | 512 (49.0) a | 2849 (49.0) a | 941 (46.4) ab | 388 (39.5) b | <0.001 |
| High stress or above , n (%) | 47 (10.5) a | 56 (5.0) bc | 273 (4.3) c | 93 (4.6) b | 54 (5.4) b | <0.001 |
| Depression, n (%) | 37 (7.5) a | 50 (4.0) ab | 241 (3.5) b | 95 (3.9) b | 63 (5.1) ab | <0.001 |
| Hypertension, n (%) | 143 (24.5) a | 284 (20.1) ab | 1325 (17.4) b | 576 (19.6) ab | 352 (24.7) a | <0.001 |
| Diabetes mellitus, n (%) | 62 (10.0) a | 99 (7.5) ab | 551 (7.2) b | 223 (7.8) ab | 148 (9.8) a | 0.026 |
| Dyslipidemia, n (%) | 107 (20.2) | 215 (15.5) | 1059 (14.9) | 420 (15.7) | 198(14.7) | 0.071 |
| Stroke, n (%) | 7 (0.8) a | 18 (1.5) a | 113 (1.4) a | 63 (2.0) a | 44 (3.4) b | <0.001 |
| MI or Angina, n (%) | 12 (1.4) ab | 19 (1.1) a | 105 (1.3) ab | 55 (2.0) b | 31 (2.3) b | 0.010 |
| Cancer, n (%) | 12 (1.7) | 17 (1.3) | 128 (1.7) | 47 (1.8) | 30 (2.1) | 0.667 |
| Exam-Based Data | ||||||
| BMI (kg/m2) | 24.70 ± 0.22 a | 24.28 ± 0.11 b | 23.95 ± 0.06 b | 23.80 ± 0.10 bc | 23.58 ± 0.13 c | <0.001 |
| HbA1c (%) | 5.80 ± 0.06 a | 5.69 ± 0.03 b | 5.61 ± 0.01 b | 5.63 ± 0.02 b | 5.66 ± 0.03 ab | 0.002 |
| Total cholesterol (mg/dL) | 197.36 ± 2.16 | 194.71 ± 1.15 | 193.29 ± 0.60 | 192.80 ± 0.88 | 191.77 ± 1.36 | 0.159 |
| HDL-cholesterol | 51.67 ± 0.65 | 51.35 ± 0.48 | 51.26 ± 0.21 | 50.92 ± 0.29 | 50.65 ± 0.43 | 0.522 |
| Triglycerides | 154.23 ± 10.72 | 139.81 ± 4.45 | 138.73 ± 2.86 | 138.73 ± 2.86 | 138.91 ± 3.31 | 0.708 |
| LDL-cholesterol | 121.59 ± 4.53 | 121.52 ± 3.02 | 118.59 ± 1.35 | 118.50 ± 2.36 | 120.88 ± 3.20 | 0.837 |
| Hemoglobin (g/dL) | 14.16 ± 0.10 a | 14.12 ± 0.06 a | 14.29 ± 0.03 b | 14.16 ± 0.04 a | 14.06 ± 0.06 a | <0.001 |
| hs-CRP (mg/L) | 1.27 ± 0.10 | 1.16 ± 0.06 | 1.14 ± 0.03 | 1.21 ± 0.05 | 1.29 ± 0.07 | 0.162 |
| Variables | Anemia (n = 1001) | Without Anemia (n = 10,130) | p-Value * |
|---|---|---|---|
| Questionnaire-Based Data | |||
| Age, years | 51.55 ± 0.7 | 46.48 ± 0.23 | <0.001 |
| male, sex, n (%) | 234 (20.0) | 4680 (52.7) | <0.001 |
| Family income level , n (%) | <0.001 | ||
| Low | 268 (22.2) | 1841 (15.0) | |
| Lower middle | 261 (25.8) | 2427 (22.7) | |
| Upper middle | 239 (25.4) | 2834 (30.2) | |
| High | 232 (26.6) | 3000 (32.1) | |
| High school or above , n (%) | 638 (70.6) | 7229 (78.8) | <0.001 |
| Current smoker , n (%) | 65 (6.8) | 1973 (23.3) | <0.001 |
| Frequency of alcohol intake per month, n (%) | <0.001 | ||
| Never | 227 (25.6) | 1510 (13.8) | |
| Less than once | 288 (37.4) | 2838 (30.5) | |
| Two– four times | 184 (23.5) | 2319 (27.9) | |
| Over four times | 122 (13.5) | 2374 (27.8) | |
| Aerobic regular exercise, n (%) | 380(41.2) | 4521(48.0) | 0.001 |
| High stress or above , n (%) | 53 (5.8) | 440 (5.0) | 0.015 |
| Depression, n (%) | 56 (5.2) | 430 (3.9) | 0.038 |
| Hypertension, n (%) | 308 (25.8) | 2405 (18.9) | <0.001 |
| Diabetes mellitus, n (%) | 175 (15.1) | 905 (7.1) | <0.001 |
| Dyslipidemia, n (%) | 180(16.8) | 1819 (15.2) | 0.218 |
| Stroke, n (%) | 33 (2.7) | 212 (1.6) | 0.018 |
| MI or Angina pectoris, n (%) | 47 (3.6) | 273(2.0) | 0.001 |
| Cancer , n (%) | 48 (4.2) | 186 (1.5) | <0.001 |
| Exam-Based Data | |||
| BMI (kg/m2) | 22.85 ± 0.12 | 24.04 ± 0.05 | <0.001 |
| HbA1c (%) | 5.76 ± 0.03 | 5.62 ± 0.01 | <0.001 |
| Total cholesterol (mg/dL) | 181.56 ± 1.34 | 194.38 ± 0.49 | <0.001 |
| HDL-cholesterol (mg/dL) | 51.91 ± 0.51 | 51.10 ± 0.16 | 0.119 |
| Triglycerides (mg/dL) | 102.61 ± 2.33 | 142.90 ± 1.88 | <0.001 |
| LDL-cholesterol (mg/dL) | 110.92 ± 4.41 | 119.53 ± 1.04 | 0.059 |
| Hemoglobin (g/dL) | 11.14 ± 0.04 | 14.48 ± 0.02 | <0.001 |
| hs-CRP (mg/L) | 1.29 ± 0.08 | 1.17 ± 0.02 | 0.139 |
| Sleep Duration (Hours) | |||||
|---|---|---|---|---|---|
| Total | <5 h | 5 h ~ <6 h | 6 h ~ <8 h | 8 h ~ <9 h | ≥9 h |
| Model 1 a | 1.37 (0.96–1.96) | 1.25 (0.75–1.59) | reference | 1.04 (0.85–1.28) | 1.29 (1.00–1.65) |
| Model 2 b | 1.25 (0.87–1.43) | 1.11 (0.86–1.43) | reference | 0.97 (0.79–1.19) | 1.14 (0.89–1.46) |
| Model 3 c | 1.12 (0.94–2.13) | 1.08 (0.82–1.43) | reference | 0.95 (0.75–1.19) | 1.07 (0.80–1.43) |
| Model 4 d | 1.60 (1.05–2.39) | 1.14 (0.86–1.51) | reference | 0.91 (0.72–1.15) | 1.01 (0.76–1.36) |
| Women | |||||
| Model 1 a | 1.14 (0.75–1.74) | 1.13 (0.86–1.48) | reference | 0.85 (0.68–1.07) | 0.94 (0.69–1.28) |
| Model 2 b | 1.14 (0.75–1.74) | 1.12 (0.85–1.47) | reference | 0.85 (0.68–1.01) | 0.93 (0.69–1.27) |
| Model 3 c | 1.37 (0.85–2.19) | 1.08 (0.80–1.470 | reference | 0.82 (0.64–1.06) | 0.85 (0.60–1.20) |
| Model 4 d | 1.51 (0.93–2.44) | 1.10 (0.81–1.49) | reference | 0.81(0.63–1.05) | 0.84 (0.60–1.19) |
| Men | |||||
| Model 1 a | 2.01 (1.05–3.84) | 1.23 (0.67–2.25) | reference | 1.57 (1.07–2.31) | 2.48 (1.63–3.81) |
| Model 2 b | 1.75 (0.90–3.37) | 1.23 (0.66–2.28) | reference | 1.22 (0.81–1.85) | 1.34 (0.84–2.11) |
| Model 3 c | 1.52 (0.72–3.20) | 1.29 (0.68–2.47) | reference | 1.14 (0.72–1.82) | 1.15 (0.58–1.93) |
| Model 4 d | 1.51 (0.72–3.15) | 1.49(0.78–2.87) | reference | 1.16 (0.72–1.89) | 1.06 (0.62–1.79) |
| Total | Sleep Duration (Hours) | ||||
|---|---|---|---|---|---|
| <5 h | 5 h ~ <6 h | 6 h ~ <8 h | 8 h ~ <9 h | ≥9 h | |
| Premenopausal State | |||||
| Model 1 a | 1.31 (0.72–2.38) | 1.32 (0.92–1.88) | reference | 0.89 (0.68–1.19) | 0.53 (0.35–0.83) |
| Model 2 b | 1.34 (0.72–2.47) | 1.27 (0.89–1.83) | reference | 0.92 (0.69–1.21) | 0.59 (0.38–0.92) |
| Model 3 c | 1.62 (0.87–3.05) | 1.28 (0.88–1.85) | reference | 0.86 (0.64–1.16) | 0.61 (0.38–0.96) |
| Model 4 d | 1.87 (1.01–3.49) | 1.31(0.89–1.91) | reference | 0.85 (0.63–1.45) | 0.61 (0.38–0.96) |
| Postmenopausal State | |||||
| Model 1 a | 1.13 (0.69–1.91) | 0.96 (0.59–1.57) | reference | 0.82 (0.55–1.24) | 2.02 (1.33–3.08) |
| Model 2 b | 0.96 (0.55–1.68) | 0.93 (0.56–1.52) | reference | 0.76 (0.51–1.15) | 1.51 (0.99–2.43) |
| Model 3 c | 1.12 (0.54–2.31) | 0.76 (0.42–1.39) | reference | 0.71 (0.41–1.21) | 1.49 (0.82–2.71) |
| Model 4 d | 1.10 (0.53–2.32) | 0.80 (0.43–1.49) | reference | 0.69 (0.40–1.19) | 1.51 (0.82–2.79) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, M.-Y.; Kim, J.-h.; Kang, J.-S. Relationship between Self-Reported Sleep Duration and Risk of Anemia: Data from the Korea National Health and Nutrition Examination Survey 2016–2017. Int. J. Environ. Res. Public Health 2021, 18, 4721. https://doi.org/10.3390/ijerph18094721
Chun M-Y, Kim J-h, Kang J-S. Relationship between Self-Reported Sleep Duration and Risk of Anemia: Data from the Korea National Health and Nutrition Examination Survey 2016–2017. International Journal of Environmental Research and Public Health. 2021; 18(9):4721. https://doi.org/10.3390/ijerph18094721
Chicago/Turabian StyleChun, Min-Young, Jeong-hoon Kim, and Ju-Seop Kang. 2021. "Relationship between Self-Reported Sleep Duration and Risk of Anemia: Data from the Korea National Health and Nutrition Examination Survey 2016–2017" International Journal of Environmental Research and Public Health 18, no. 9: 4721. https://doi.org/10.3390/ijerph18094721
APA StyleChun, M.-Y., Kim, J.-h., & Kang, J.-S. (2021). Relationship between Self-Reported Sleep Duration and Risk of Anemia: Data from the Korea National Health and Nutrition Examination Survey 2016–2017. International Journal of Environmental Research and Public Health, 18(9), 4721. https://doi.org/10.3390/ijerph18094721







