Parent–Infant Skin-to-Skin Contact and Stress Regulation: A Systematic Review of the Literature
Abstract
1. Introduction
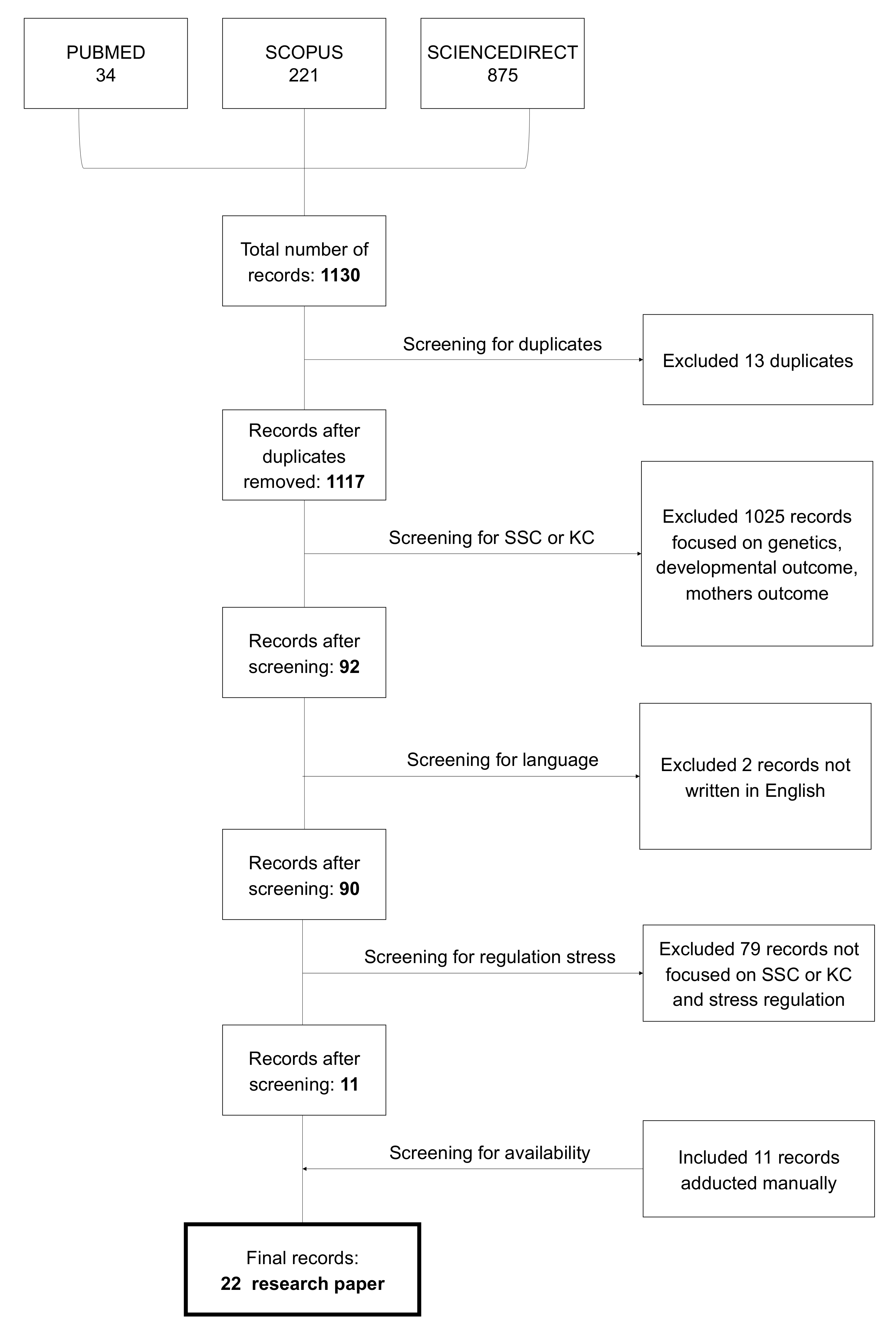
2. Materials and Methods
3. Results
3.1. Heart Rate Variability
3.2. Maternal Stress
3.3. Cortisol
3.4. Oxytocin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldman, R. The development of regulatory functions from birth to 5 years: Insight from premature infants. Child Dev. 2009, 80, 544–561. [Google Scholar] [CrossRef] [PubMed]
- Bergamn, L.; Linley, L.; Fawcus, S. Randomized controlled trial of skin-to-skin contact from birth versus conventional incu-bator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatr. 2004, 93, 779–785. [Google Scholar] [CrossRef]
- Morgan, B.E.; Horn, A.R.; Bergman, N.J. Should Neonates Sleep Alone? Biol. Psychiatry 2011, 70, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W.; Furman, S.A. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal persective. Infant. Child Dev. 2011, 20, 106–118. [Google Scholar] [CrossRef]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef]
- Haraldsdottir, K.; Watson, A.M.; Goss, K.N.; Beshish, A.G.; Pegelow, D.F.; Palta, M.; Tetri, L.H.; Barton, G.P.; Brix, M.D.; Centanni, R.M.; et al. Impaired autonomic function in adolescents born preterm. Physiol. Rep. 2018, 6, e13620. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.A. Early relationships as regulators of infant physiology and behaviour. Acta Paediatr. 1994, 397, 9–18. [Google Scholar] [CrossRef]
- Bergman, N.J. Birth practices: Maternal-neonate separation as a source of toxic stress. Birth Defects Res. 2019, 111, 1087–1109. [Google Scholar] [CrossRef]
- Widström, A.; Brimdyr, K.; Svensson, K.; Cadwell, K.; Nissen, E. Skin-to-skin contact the first hour after birth, underlying implications and clinical practice. Acta Paediatr. 2019, 108, 1192–1204. [Google Scholar] [CrossRef]
- Harlow, H.F. The Nature of Love. Am. Psychol. 1958, 13, 673. [Google Scholar] [CrossRef]
- Harlow, H.F. Mice, monkeys, men, and motives. Psychol. Rev. 1953, 60, 23–32. [Google Scholar] [CrossRef]
- Harlow, H.F.; Suomi, S.J. Social Recovery by Isolation-Reared Monkeys. Proc. Natl. Acad. Sci. USA 1971, 68, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Harlow, H.F.; Zimmermann, R.R. Affectional Response in the Infant Monkey: Orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science 1959, 130, 421–432. [Google Scholar] [CrossRef]
- Suomi, S.J. Early determinants of behaviour: Evidence from primate studies. Br. Med. Bull. 1997, 53, 170–184. [Google Scholar] [CrossRef]
- Abdulghani, N.; Edvardsson, K.; Amir, L.H. Worldwide prevalence of mother-infant skin-to-skin contact after vaginal birth: A systematic review. PLoS ONE 2018, 13, e0205696. [Google Scholar] [CrossRef]
- Arnon, S.; Diamant, C.; Bauer, S.; Regev, R.; Sirota, G.; Litmanovitz, I. Maternal singing during kangaroo care led to auto-nomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 2014, 103, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.R.; Anderson, G.C.; Bergman, N.; Dowswell, T. Early Skin-to-Skin Contact for Mothers and Their Healthy New-Born Infants. Cochrane Database of Systematic Reviews. 2012. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003519.pub4/full (accessed on 21 January 2021).
- Feldman, R.; Rosenthal, Z.; Eidelman, A.I. Maternal-pre-term skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry 2014, 75, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Smotherman, W.P.; Hunt, L.E.; McGinnis, L.M.; Levine, S. Mother-infant separation in group-living rhesus macaques: A hormonal analysis. Dev. Psychobiol. 1979, 12, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Acolet, D.; Modi, N.; Giannakoulopoulos, X.; Bond, C.; Weg, W.; Clow, A.; Glover, V. Changes in plasma cortisol and catechola-mine concentrations in response to massage in preterm infants. Arch. Dis. Child. 1993, 68, 29–31. [Google Scholar] [CrossRef]
- Feldman, R.; Singer, M.; Zagoory, O. Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 2010, 13, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Beijers, R.; Riksen-Walraven, M.; de Weerth, C. Cortisol reactivity in young infants. Psychoneuroendocrinology 2010, 35, 329–338. [Google Scholar] [CrossRef]
- Herman, J.P. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 2013, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Gitau, R.; Modi, N.; Gianakoulopoulos, X.; Bond, C.; Glover, V.; Stevenson, J. Acute effects of maternal skin-to-skin contact and massage on saliva cortisol in preterm babies. J. Reprod. Infant Psychol. 2002, 20, 83–88. [Google Scholar] [CrossRef]
- Pados, B.F. Physiology of Stress and Use of Skin-to-Skin Care as a Stress-Reducing Intervention in the NICU. Nurs. Women’s Heal. 2019, 23, 59–70. [Google Scholar] [CrossRef]
- World Health Organization. Mental Health and Psychosocial Considerations during the COVID-19 Outbreak. 18 March 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331490/WHO-2019-nCoV-MentalHealth-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 21 January 2021).
- Nussbaumer-Streit, B.; Mayr, V.; Dobrescu, A.I.; Chapman, A.; Persad, E.; Klerings, I.; Wagner, G.; Siebert, U.; Ledinger, D.; Zachariah, C.; et al. Quarantine alone or in combination with other public health measures to control COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020, 4, 013574. [Google Scholar] [CrossRef]
- Hertenstein, M.J.; Keltner, D.; App, B.; Bulleit, B.A.; Jaskolka, A.R. Touch communicates distinct emotions. Emotion 2006, 6, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S. Touch Starvation is a Consequence of COVID-19′s Physical Distancing. Texas Medical Centre Retrieved. Available online: https://www.tmc.edu/news/2020/05/touch-starvation (accessed on 21 January 2021).
- Mortenson Burnside, I. Touch is talking. Am. J. Nurs. 1973, 73, 2060–2063. [Google Scholar]
- Field, T.; Diego, M. Vagal activity, early growth and emotional development. Infant Behav. Dev. 2008, 31, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal theory: Phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 2001, 42, 123–146. [Google Scholar] [CrossRef]
- Thayer, J.F.; Sternberg, E. Beyond Heart Rate Variability: Vagal Regulation of Allostatic Systems. Ann. N. Y. Acad. Sci. 2006, 1088, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Butruille, L.; Blouin, A.; De Jonckheere, J.; Mur, S.; Margez, T.; Rakza, T.; Storme, L. Impact of skin-to-skin contact on the autonomic nervous system in the pre-term infant and his mother. Infant. Behav. Dev. 2017, 49, 83–86. [Google Scholar] [CrossRef]
- Carozza, S.; Leong, V. The role of caregiver touch in early neurodevelopment and parent- infant interactional synchrony. PsyArXiv Prepr. 2020. [Google Scholar] [CrossRef]
- Cho, E.S.; Kim, S.J.; Kwon, M.S.; Cho, H.; Kim, E.H.; Jun, E.M.; Lee, S. The effects of the kangaroo care in the neonatal inten-sive care unit on the physiological functions of preterm infants, maternal-infant attachment and maternal stress. J. Pediatr. Nurs. 2016, 31, 430–438. [Google Scholar] [CrossRef]
- Cleveland, L.; Hill, C.M.; Pulse, W.S.; DiCioccio, H.C.; Field, T.; White-Traut, R. Systematic Review of Skin to Skin Contact for full term, healthy newborns. J. Obstet. Gynecol. Neonatal. Nurs. 2017, 46, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Ludington-Hoe, S.M.; Hussain, N.; Cusson, R.M.; Walsh, S.; Vazquez, V.; Briere, C.-E.; Vittner, D. Parental oxytocin responses during skin-to-skin contact in pre-term infants. Early Hum. Dev. 2015, 91, 401–406. [Google Scholar] [CrossRef]
- Coşkun, D.; Günay, U. The Effects of Kangaroo Care Applied by Turkish Mothers who Have Premature Babies and Cannot Breastfeed on Their Stress Levels and Amount of Milk Production. J. Pediatr. Nurs. 2020, 50, e26–e32. [Google Scholar] [CrossRef]
- El-Farrash, R.A.; Shinkar, D.M.; Ragab, D.A.; Salem, R.M.; Saad, W.E.; Farag, A.S.; Salama, D.H.; Sakr, M.F. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: A randomized controlled trial. Pediatr. Res. 2019, 87, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.S.; Jones, N.A.; Mize, K.D.; Platt, M. Parent training with Kangaroo Care Impacts Infant Neurophysiological De-velopment and Mother- Infant Neuroendocrine Activity. Infant. Behav. Dev. 2020, 58, 101416. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.M.; Brown, R. Autonomic Nervous System Function after a skin to skin contact intervention in infants with con-genital heart disease. J. Cardiovasc. Nurs. 2017, 32, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.M.; Chen, C.-Y.; Stein, P.; Brown, R.; Heathcock, J.C. Neonatal Skin-to-Skin Contact: Implications for Learning and Autonomic Nervous System Function in Infants With Congenital Heart Disease. Biol. Res. Nurs. 2019, 21, 296–306. [Google Scholar] [CrossRef]
- Jones, H.; Santamaria, N. Physiological benefits to parents from undertaking skin to skin contact with their neonate, in a ne-onatal intensive special care unit. Scand. J. Caring Sci. 2017, 32, 1012–1017. [Google Scholar] [CrossRef]
- Kommers, D.R.; Joshi, R.; van Pul, C.; Atallah, L.; Feijs, L.; Oei, G.; Oetomo, S.B.; Andriessen, P. Features of Heart Rate Variability Capture Regulatory Changes During Kangaroo Care in Preterm Infants. J. Pediatr. 2017, 182, 92–98.e1. [Google Scholar] [CrossRef]
- Kommers, D.; Joshi, R.; Van Pul, C.; Feijs, L.; Oei, G.; Oetomo, S.B.; Andriessen, P. Unlike Kangaroo care, mechanically simulated Kangaroo care does not change heart rate variability in preterm neonates. Early Hum. Dev. 2018, 121, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kommers, D.; Broeren, M.; Oei, G.; Feijs, L.; Andriessen, P.; Bambang Oetomo, S. Oxytocin levels in the saliva of preterm in-fant twins during Kangaroo care. Biol. Psychol. 2018, 137, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Kommers, D.R.; Joshi, R.; Van Pul, C.; Feijs, L.; Oetomo, S.B.; Andriessen, P. Changes in autonomic regulation due to Kangaroo care remain unaffected by using a swaddling device. Acta Paediatr. 2018, 108, 258–265. [Google Scholar] [CrossRef]
- Lisanti, A.J.; Demianczyk, A.C.; Costarino, A.; Vogiatzi, M.G.; Hoffman, R.; Quinn, R.; Chittams, J.L.; Medoff-Cooper, B. Skin-to-Skin Care Is a Safe and Effective Comfort Measure for Infants Before and After Neonatal Cardiac Surgery. Pediatr. Crit. Care Med. 2020, 21, e834–e841. [Google Scholar] [CrossRef] [PubMed]
- Mirnia, K.; Bostanabad, M.A.; Asadollahi, M.; Razzaghi, M.H.; Assadollahi, M. Paternal Skin-to-Skin Care and its Effect on Cortisol Levels of the Infants. Iran. J. Pediatr. 2017, 27, e8151. [Google Scholar] [CrossRef]
- Moberg, K.U.; Handlin, L.; Petersson, M. Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact—With a particular focus on the oxytocinergic system. Infant Behav. Dev. 2020, 61, 101482. [Google Scholar] [CrossRef] [PubMed]
- Mörelius, E.; Örtenstrand, A.; Theodorsson, E.; Frostell, A. A randomised trial of continuous skin-to-skin contact after pre-term birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum. Dev. 2015, 91, 63–70. [Google Scholar] [CrossRef]
- Pados, B.F.; Hess, F. Systematic Review of the Effects of Skin-to-Skin Care on Short-Term Physiologic Stress Outcomes in Pre-term Infants in the Neonatal Intensive Care Unit. Adv. Neonatal. Care 2020, 20, 48–58. [Google Scholar] [CrossRef]
- Varela, N.; Tessier, R.; Tarabulsy, G.; Pierce, T. Cortisol and blood pressure levels decreased in fathers during the first hour of skin-to-skin contact with their premature babies. Acta Paediatr. 2018, 107, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Vittner, D.; Butler, S.; Smith, K.; Makris, N.; Brownell, E.; Samra, H.; McGrath, J. Parent Engagement Correlates With Parent and Preterm Infant Oxytocin Release During Skin-to-Skin Contact. Adv. Neonatal. Care 2019, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef]
- Saul, J.P.; Berger, R.D.; Albrecht, P.; Stein, S.P.; Chen, M.H.; Cohen, R.J. Transfer function analysis of the circulation: Unique insights into cardiovascular regulation. Am. J. Physiol. Circ. Physiol. 1991, 261, H1231–H1245. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Singer, D. Heart rate variability: Standards of measurement physiological interpretation and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Feldman, R.; Eidelman, A.I.; Sirota, L.; Weller, A. Comparison of Skin-to-Skin (Kangaroo) and Traditional Care: Parenting Outcomes and Preterm Infant Development. Pediatrics 2002, 110, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tamakoshi, K.; Matsushima, M.; Kawabe, T. Comparison of salivary cortisol, heart rate, and oxygen satu- ration between early skin-to-skin contact with different initiation and duration times in healthy, full-term infants. Early Hum. Dev. 2011, 87, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R. Maternal-infant contact and child development: Insights from the kangaroo care intervention. In Low-Cost Approaches to Promote Physical and Mental Health: Theory, Research, and Practice; L’Abate, L., Ed.; Springer Science and Business Media: New York, NY, USA, 2007; pp. 323–351. [Google Scholar]
- Clark-Gambelunghe, M.B.; Clark, D.A. Sensory Development. Pediatr. Clin. North Am. 2015, 62, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Ludington-Hoe, S.M.; Walsh, S. Randomized cross over trial of kangaroo care to reduce biobehavioral pain re-sponses in preterm infants: A pilot study. Biol. Res. Nurs. 2011, 13, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Handlin, L.; Jonas, W.; Petersson, M.; Ejdebäck, M.; Ransjö-Arvidson, A.B.; Nissen, E.; Uvnäs-Moberg, K. Effects of sucking and skin-to-skin contact on maternal ACTH and cortisol levels during the second day postpartum-influence of epidural analgesia and oxytocin in the perinatal period. Breastfeed Med. 2009, 4, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, T.G.; Edwards, L. Chronic stress and the HPA axis. Standard 2010, 9, 1–12. [Google Scholar]
- Arockiasamy, V.; Holsti, L.; Albersheim, S. Fathers’ Experiences in the Neonatal Intensive Care Unit: A Search for Control. Pediatrics 2008, 121, e215–e222. [Google Scholar] [CrossRef]
- Lundqvist, P.; Westas, L.H.; Hallström, I. From distance toward proximity: Fathers lived experience of caring for their pre-term infants. Pediatr. Nurs. 2007, 22, 490–497. [Google Scholar] [CrossRef]
- Blomqvist, Y.T.; Rubertsson, C.; Kylberg, E.; Jöreskog, K.; Nyqvist, K.H. Kangaroo Mother Care helps fathers of preterm in-fants gain confidence in the paternal role. J. Adv. Nurs. 2012, 68, 1988–1996. [Google Scholar] [CrossRef]
- Cong, X.; Cusson, R.M.; Walsh, S.; Hussain, N.; Ludington-Hoe, S.M.; Zhang, D. Effects of skin-to-skin contact on auto-nomic pain responses in preterm infants. J. Pain 2012, 13, 636–645. [Google Scholar] [CrossRef]
- Lee, J.; Bang, K.S. The effects of kangaroo care on maternal self-esteem and pre- mature infants’ physiological stability. Korean Women Health 2011, 17, 454–462. [Google Scholar] [CrossRef][Green Version]
- Begum, E.A.; Bonno, M.; Ohtani, N.; Yamashita, S.; Tanaka, S.; Yamamoto, H.; Kawai, M.; Komada, Y. Cerebral oxygenation responses during kangaroo care in low birth weight infants. BMC Pediatr. 2008, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, N.; Amir, L.H.; Edvardsson, K. Observational study found that skin-to-skin contact was not common after vaginal birth in Saudi Arabia. Acta Paediatr. 2020, 109, 1681–1682. [Google Scholar] [CrossRef]
- Central Disease Control and Prevention (CDC). H1N1 Flu (Swine Flu) and Feeding your Baby: What Parents Should Know. 2009. Available online: https://www.cdc.gov/h1n1flu/infantfeeding.htm (accessed on 25 June 2020).
- The American Academy of Pediatrics Updates Guidance on Care of Newborn to Mothers COVID-19. Available online: https://services.aap.org/en/news-room/news-releases/aap/2020/the-american-academy-of-pediatrics-updates-guidance-on-care-of-newborns-to-mothers-with-covid-19/ (accessed on 21 January 2021).
- Propositions de la Socieéteé Francaise de Neéonatalogie & de la Socieéte Francaise de Peédiatrie Concernant les Nouveau-Neés dans le Contexte D’eépidemie aà Covid-19. Available online: https://f4ed7074-25ed-461c (accessed on 21 January 2021).
- World Health Organization. New Research Highlights Risks of Separating Newborns from Mothers during COVID-19 Pandemic. Available online: https://www.who.int/news/item/16-03-2021-new-research-highlights-risks-of-separating-newborns-from-mothers-during-covid-19-pandemic (accessed on 21 January 2021).
- Stuebe, A. Should Infants Be Separated from Mothers with COVID-19? First, Do No Harm. Breastfeed. Med. 2020, 15, 351–352. [Google Scholar] [CrossRef]
- Royal College of Midwives; Royal College of Obstetricians and Gynaecologists; Royal College of Paediatrics and Child Health. Coronavirus (COVID-19) Infection in Pregnancy: Information for Health Care Professionals. Version 5. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-03-28-covid19-pregnancy-guidance.pdf (accessed on 31 March 2020).
- Morelius, E.; Theodorsson, E.; Nelson, N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unse-lected group of mothers and infants in neonatal intensive care. Pediatrics 2005, 116, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Gribble, K. Promoting attachment in foster parents: What we can learn from the experience of parents of premature infants. Adopt. Fostering 2016, 40, 113–127. [Google Scholar] [CrossRef]
- DaVanzo, R.; Merewood, A.; Manzoni, P. Skin-to-Skin Contact at Birth in the COVID-19 Era: In Need of Help! Am. J. Perinatol. 2020, 37, S1–S4. [Google Scholar] [CrossRef] [PubMed]
| Study | Scope of the Study | Participants | Findings | Physiological Outcomes |
|---|---|---|---|---|
| Butruille, 2017 [36] | Influence of skin-to-skin contact (SSC) on the parasympathetic activity evaluated by heart rate variability (HRV) | Twenty-two infants and their mothers | SSC had a favourable impact on maternal and premature infant parasympathetic activities with a pronounced neonate response when baseline HRV values were lower | HRV |
| Carozza, 2020 [37] | Brief review of how contact influences the development of infant somatosensory, autonomic, and immune systems | Contact is an essential pathway for establishing and maintaining behavioural, physiological, and neural levels of parent–child interaction synchrony | Stress | |
| Cho, 2016 [38] | Explore the effects of kangaroo care (KC) on physiological functions of preterm infants and maternal stress. | Twenty infants were assigned to the experimental group and 20 to the control group | KC had positive effects on stabilizing infant physiological functions such as respiration rate and reducing maternal stress. | HRV and maternal stress |
| Cleveland, 2017 [39] | Explain the effect of SSC on full-term newborns | Recommendations of SSC for healthy newborns | ||
| Cong, 2015 [40] | Investigate how oxytocin modulates parental stress and anxiety during maternal and paternal SSC | Twenty-eight stable preterm infants and their parents | Both maternal and paternal oxytocin levels were significantly increased from baseline during the SSC. Both maternal and paternal cortisol levels decreased significantly from baseline during SSC. | Oxytocin and cortisol |
| Coskun, 2019 [41] | Investigate the effects of KC on the mother’s stress and amount of milk production | Eighty-four preterm newborns and their mothers | KC is effective in stimulating breast milk production and lowering maternal stress levels. | Maternal stress |
| El Farrash, 2019 [42] | Assess the effect of KC and its duration on the neurobehavioral system and salivary cortisol | One hundred and twenty stable preterm neonates | KC improved higher scores for regulation, non-optimal reflexes, and movement quality and lower scores for handling, excitement, and cortisol compared with the control group | Cortisol |
| Hardin, 2020 [43] | Examine EEG patterns along with basal oxytocin and cortisol reactivity in infants related to KC | Thirty-three mother–infant dyads at neonatal and three-month periods | KC increased oxytocin levels and decreased cortisol reactivity. | Oxytocin and cortisol |
| Harrison, 2017 [44] | Examine SSC’s effects on autonomic nervous system (ANS) functions | Eighteen infants and their mothers | HRV measures suggested improvements to the ANS functions after SSC | HRV |
| Harrison, 2019 [45] | Investigate the effects of SSC on learning and autonomic functions in three-month-old infants | Ten infants with congenital heart disease (CHD) who received neonatal SSC, 16 typically developing (TD) infants, and 10 infants with CCHD without SSC | The findings suggest improvements in cognitive and autonomic development in three-month-old CCHD infants who received neonatal SSC. | HRV |
| Jones et al. 2017 [46] | Explore the effect of SSC between parents and their neonates on parents’ heart rate (HR) | Twenty-six parents and their babies | A statistically significant difference between the parents’ initial HR and HR after SSC | HRV |
| Kommers, 2017 [47] | Explore whether HRV could be a measure to track regulatory changes during KC | Eleven preterm infants | A statistically significant difference in HRV between periods of KC and pre-KC | HRV |
| Kommers, 2018 [48] | Explore if a mattress that mimics breathing motion and heartbeat sounds can have the same effects as KC in preterm infants, as measured by HRV | Twenty preterm infants | HRV decreased during KC and after KC. No non-mattress effects were reported. | HRV |
| Kommers, 2018 [49] | Explore whether KC influences the salivary oxytocin concentration in preterm infants | Eleven twin pairs | Preterm infant twins’ oxytocin concentrations decreased during KC | Oxytocin |
| Kommers et al., 2019 [50] | Vital signs and HRV were analysed during KC with and without the use of a swaddling device to identify any potential changes | Twenty preterm infants | KC decreased heart rate, respiratory rate, and HRV. No changes were found regarding the swaddling device | HRV |
| Lisanti, 2020 [51] | Estimate SSC’s effect on mothers’ bio-behavioural stress measures (anxiety and salivary cortisol) before and after neonatal cardiac surgery | Thirty women and their infants | Significant reductions in self-reported anxiety and salivary cortisol scores were identified as a result of SSC. | Cortisol, HRV |
| Mirnia et al., 2017 [52] | Investigate the effect of SSC by fathers on salivary cortisol of infants | Forty-five premature infants and their fathers | SSC decreased levels of cortisol in babies. | Cortisol |
| Moberg, 2020 [53] | Describe the oxytocinergic system and the cutaneous sensory pathways activated by SSC | Decreased stress levels could be considered due to oxytocin’s ability to reduce the amygdala’s activity, the HPA-axis, and the sympathetic nervous system. | Oxytocin | |
| Morelius, 2015 [54] | Investigate the effects of SSC on salivary cortisol, parental stress, depression, and breastfeeding | Thirty-seven families | SSC reduces infant cortisol reactivity in response to treatment and improves concordance between mother and infant salivary cortisol levels. | Cortisol and parental stress |
| Pados, 2019 [26] | Describe the physiological stress mechanisms that contribute to infant mortality and morbidity in the NICU and the physiological mechanisms by which SSC acts on the stress response system | Importance of SSC to mother–infant stress regulation | Stress | |
| Pados et al., 2020 [55] | Investigate if SSC is an intervention used to reduce stress in the NICU | Research supports that SSC improves short-term cardiorespiratory stress outcomes compared with incubator care. | Stress, cortisol HRV, oxytocin | |
| Varela, 2017 [56] | Explore paternal physiological stress during SSC with their babies | Forty-nine fathers | Fathers who practiced SSC showed a significant reduction in physiological stress outcomes. | Cortisol |
| Vitnner, 2018 [57] | Examine the relationship between parental involvement and salivary oxytocin and cortisol levels for parents participating in SSC intervention | Thirty-two stable preterm infants and their mothers and fathers | Significant relationships exist between parental engagement and salivary oxytocin and cortisol levels. | Oxytocin and cortisol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionio, C.; Ciuffo, G.; Landoni, M. Parent–Infant Skin-to-Skin Contact and Stress Regulation: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 4695. https://doi.org/10.3390/ijerph18094695
Ionio C, Ciuffo G, Landoni M. Parent–Infant Skin-to-Skin Contact and Stress Regulation: A Systematic Review of the Literature. International Journal of Environmental Research and Public Health. 2021; 18(9):4695. https://doi.org/10.3390/ijerph18094695
Chicago/Turabian StyleIonio, Chiara, Giulia Ciuffo, and Marta Landoni. 2021. "Parent–Infant Skin-to-Skin Contact and Stress Regulation: A Systematic Review of the Literature" International Journal of Environmental Research and Public Health 18, no. 9: 4695. https://doi.org/10.3390/ijerph18094695
APA StyleIonio, C., Ciuffo, G., & Landoni, M. (2021). Parent–Infant Skin-to-Skin Contact and Stress Regulation: A Systematic Review of the Literature. International Journal of Environmental Research and Public Health, 18(9), 4695. https://doi.org/10.3390/ijerph18094695







