Risk Factors for Postpartum Hemorrhage in a Thai–Myanmar Border Community Hospital: A Nested Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
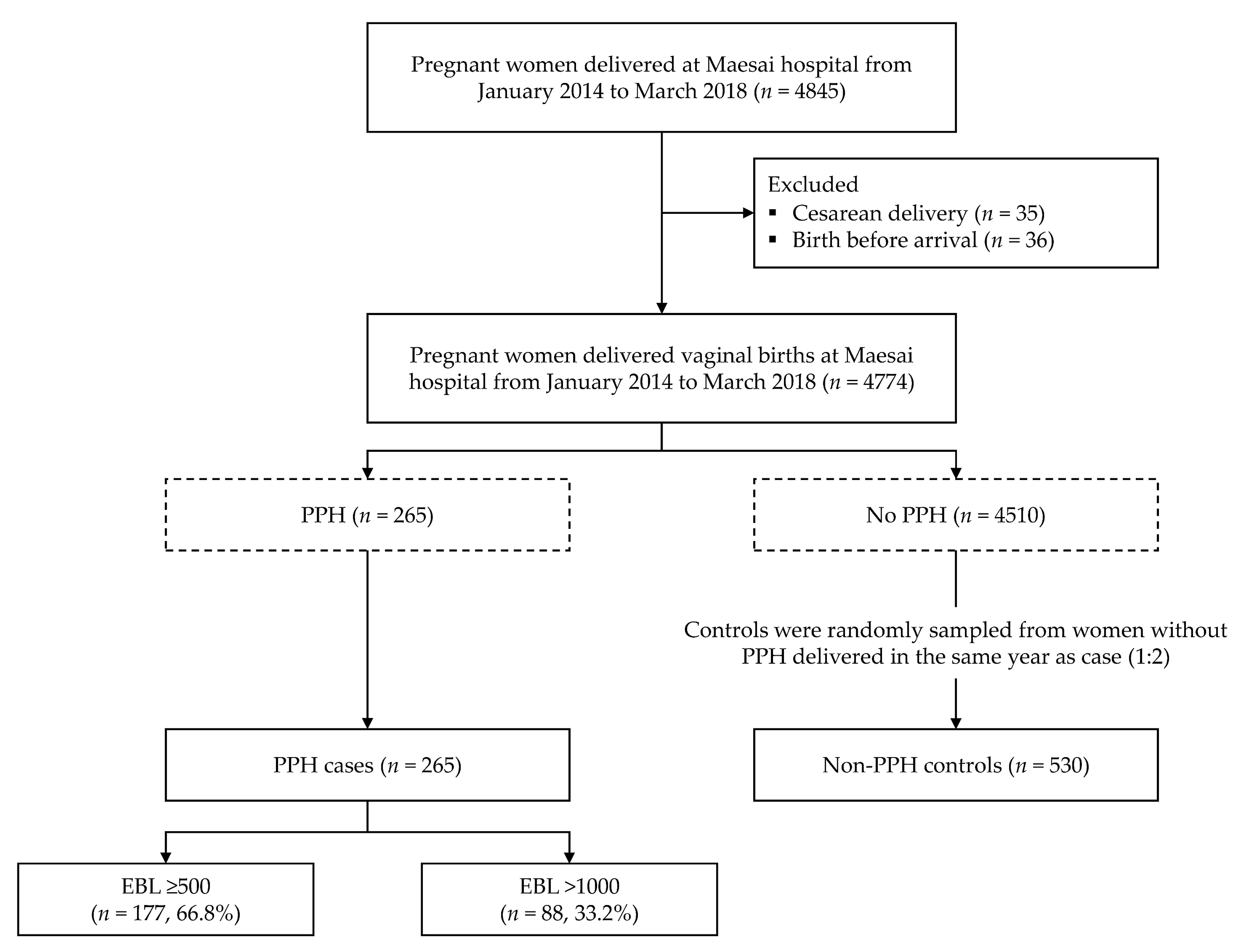
2.2. Cases and Controls
2.3. Data Collection
2.4. Definitions of Terms
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global Causes of Maternal Death: A WHO Systematic Analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [Green Version]
- Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet. Gynecol. 2017, 130, e168–e186. [Google Scholar] [CrossRef]
- Dahlke, J.D.; Mendez-Figueroa, H.; Maggio, L.; Hauspurg, A.K.; Sperling, J.D.; Chauhan, S.P.; Rouse, D.J. Prevention and Management of Postpartum Hemorrhage: A Comparison of 4 National Guidelines. Am. J. Obstet. Gynecol. 2015, 213, 76.e1–76.e10. [Google Scholar] [CrossRef]
- Carroli, G.; Cuesta, C.; Abalos, E.; Gulmezoglu, A.M. Epidemiology of Postpartum Haemorrhage: A Systematic Review. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Prapawichar, P.; Ratinthorn, A.; Utriyaprasit, K.; Viwatwongkasem, C. Maternal and Health Service Predictors of Postpartum Hemorrhage across 14 District, General and Regional Hospitals in Thailand. BMC Pregnancy Childbirth 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talungchit, P.; Liabsuetrakul, T. Clinical Audit of Postpartum Hemorrhage at District-Level and Referral-Level Hospitals in Southern Thailand. J. Med. Assoc. Thai. 2012, 95, 1244. [Google Scholar]
- Abedzadeh-Kalahroudi, M. Prevention of Postpartum Hemorrhage: Our Options. Nurs. Midwifery Stud. 2015, 4, e29641. [Google Scholar] [CrossRef] [Green Version]
- Tort, J.; Rozenberg, P.; Traoré, M.; Fournier, P.; Dumont, A. Factors Associated with Postpartum Hemorrhage Maternal Death in Referral Hospitals in Senegal and Mali: A Cross-Sectional Epidemiological Survey. BMC Pregnancy Childbirth 2015, 15, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyfløt, L.T.; Sandven, I.; Stray-Pedersen, B.; Pettersen, S.; Al-Zirqi, I.; Rosenberg, M.; Jacobsen, A.F.; Vangen, S. Risk Factors for Severe Postpartum Hemorrhage: A Case-Control Study. BMC Pregnancy Childbirth 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, A.S.; Manji, K.P. Risk Factors and Outcomes of Fetal Macrosomia in a Tertiary Centre in Tanzania: A Case-Control Study. BMC Pregnancy Childbirth 2016, 16, 243. [Google Scholar] [CrossRef] [Green Version]
- Lertbunnaphong, T.; Leetheeragul, J.; Thitadilok, W. Risk Factors of Primary Postpartum Hemorrhage in Siriraj Hospital. Siriraj Med. J. 2010, 62, 195–198. [Google Scholar]
- Magann, E.F.; Evans, S.; Hutchinson, M.; Collins, R.; Lanneau, G.; Morrison, J.C. Postpartum Hemorrhage after Cesarean Delivery: An Analysis of Risk Factors. South. Med. J. 2005, 98, 681–685. [Google Scholar] [CrossRef]
- Shields, L.E.; Wiesner, S.; Fulton, J.; Pelletreau, B. Comprehensive Maternal Hemorrhage Protocols Reduce the Use of Blood Products and Improve Patient Safety. Am. J. Obstet. Gynecol. 2015, 212, 272–280. [Google Scholar] [CrossRef]
- Tunçalp, O.; Souza, J.P.; Gülmezoglu, M. New WHO Recommendations on Prevention and Treatment of Postpartum Hemorrhage. Int. J. Gynaecol. Obstet. 2013, 123, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.; Mhyre, J.M.; Leffert, L.R.; Hoban, R.A.; Yakoob, M.Y.; Bateman, B.T. The Association of Maternal Race and Ethnicity and the Risk of Postpartum Hemorrhage. Anesth. Analg. 2012, 115, 1127–1136. [Google Scholar] [CrossRef]
- Choe, S.-A.; Min, H.-S.; Cho, S.-I. The Income-Based Disparities in Preeclampsia and Postpartum Hemorrhage: A Study of the Korean National Health Insurance Cohort Data from 2002 to 2013. SpringerPlus 2016, 5, 895. [Google Scholar] [CrossRef] [Green Version]
- Liabsuetrakul, T.; Palanukunwong, K.; Chinduereh, A.; Oumudee, N. Evaluation of a Multifaceted Postpartum Hemorrhage-Management Intervention in Community Hospitals in Southern Thailand. Int. J. Gynaecol. Obstet. 2017, 139, 39–44. [Google Scholar] [CrossRef]
- Pothisiri, W. Postpartum Care in Thailand: Experience, Practice and Policy. Ph.D. Thesis, London School of Economics and Political Science, London, UK, 2010. [Google Scholar]
- Ende, H.B.; Lozada, M.J.; Chestnut, D.H.; Osmundson, S.S.; Walden, R.L.; Shotwell, M.S.; Bauchat, J.R. Risk Factors for Atonic Postpartum Hemorrhage: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2021, 137, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Lao, T.T.; Sahota, D.S.; Cheng, Y.K.Y.; Law, L.W.; Leung, T.Y. Advanced Maternal Age and Postpartum Hemorrhage—Risk Factor or Red Herring? J. Matern.-Fetal Neonatal Med. 2014, 27, 243–246. [Google Scholar] [CrossRef]
- Sittiparn, W.; Siwadune, T. Risk Score for Prediction of Postpartum Hemorrhages in Normal Labor at Chonburi Hospital. J. Med. Assoc. Thai. 2017, 100, 382. [Google Scholar] [PubMed]
- Rueangchainikhom, W.; Srisuwan, S.; Prommas, S.; Sarapak, S. Risk Factors for Primary Postpartum Hemorrhage in Bhumibol Adulyadej Hospital. J. Med. Assoc. Thai. 2009, 92, 1586. [Google Scholar]
- Tuporn, N.; Ratanasiri, A.; Nutravong, T.; Boonprasert, K.; Pikul, T.N. Risk Scoring System for the Prediction of Postpartum Blood Loss over 300 ML at Chiang Rai Regional Hospital. Siriraj Med. J. 2019, 71, 10–116. [Google Scholar] [CrossRef]
- Phinyo, P.; Patumanond, J. Indicators for Extended Length of Stay in the Emergency Service Unit of a Thai Community Hospital: A Multi-Level Analysis. Epidemiol. Biostat. Public Health 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Van Stralen, K.J.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Case-Control Studies—An Efficient Observational Study Design. Nephron Clin. Pract. 2010, 114, c1–c4. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Pearce, N. Case–Control Studies: Basic Concepts. Int. J. Epidemiol. 2012, 41, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Mgaya, A.H.; Massawe, S.N.; Kidanto, H.L.; Mgaya, H.N. Grand Multiparity: Is It Still a Risk in Pregnancy? BMC Pregnancy Childbirth 2013, 13, 241. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Iron Deficiency Anemia. Assessment, Prevention, and Control. A Guide for Programme Managers; WHO: Geneva, Switzerland, 2001; pp. 47–62. [Google Scholar]
- Cheng, Y.W.; Caughey, A.B. Defining and Managing Normal and Abnormal Second Stage of Labor. Obstet. Gynecol. Clin. N. Am. 2017, 44, 547–566. [Google Scholar] [CrossRef]
- Herman, A. Complicated Third Stage of Labor: Time to Switch on the Scanner. Ultrasound Obstet. Gynecol. 2000, 15, 89–95. [Google Scholar] [CrossRef]
- Heinze, G.; Puhr, R. Bias-Reduced and Separation-Proof Conditional Logistic Regression with Small or Sparse Data Sets. Stat. Med. 2010, 29, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, J.; Dai, L.; Li, M.; Miao, L.; Liang, J.; Wang, Y. Trends in Maternal Mortality Due to Obstetric Hemorrhage in Urban and Rural China, 1996–2005. J. Perinat. Med. 2011, 39, 35–41. [Google Scholar] [CrossRef]
- Kruk, M.E.; Leslie, H.H.; Verguet, S.; Mbaruku, G.M.; Adanu, R.M.K.; Langer, A. Quality of Basic Maternal Care Functions in Health Facilities of Five African Countries: An Analysis of National Health System Surveys. Lancet Glob. Health 2016, 4, e845–e855. [Google Scholar] [CrossRef] [Green Version]
- Bateman, B.T.; Berman, M.F.; Riley, L.E.; Leffert, L.R. The Epidemiology of Postpartum Hemorrhage in a Large, Nationwide Sample of Deliveries. Anesth. Analg. 2010, 110, 1368–1373. [Google Scholar] [CrossRef]
- Durmaz, A.; Komurcu, N. Relationship Between Maternal Characteristics and Postpartum Hemorrhage: A Meta-Analysis Study. J. Nurs. Res. JNR 2018, 26, 362–372. [Google Scholar] [CrossRef]
- Combs, C.A.; Murphy, E.L.; Laros, R.K. Factors Associated with Postpartum Hemorrhage with Vaginal Birth. Obstet. Gynecol. 1991, 77, 69–76. [Google Scholar] [PubMed]
- Mavrides, E.; Allard, S.; Chandraharan, E.; Collins, P.; Green, L.; Hunt, B.J.; Riris, S.; Thomson, A.J. on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and Management of Postpartum Haemorrhage. BJOG Int. J. Obstet. Gynaecol. 2017, 124, e106–e149. [Google Scholar] [CrossRef]
- Kunstadter, P. Ethnicity, Socioeconomic Characteristics and Knowledge, Beliefs and Attitudes about HIV among Yunnanese Chinese, Hmong, Lahu and Northern Thai in a North-Western Thailand Border District. Cult. Health Sex. 2013, 15, S383–S400. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, A.; Noor, N.; Sullivan, E.; Knight, M. The Impact of Socioeconomic Position on Severe Maternal Morbidity Outcomes among Women in Australia: A National Case-Control Study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1601–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Räisänen, S.; Kramer, M.R.; Gissler, M.; Saari, J.; Heinonen, S. Unemployment at Municipality Level Is Associated with an Increased Risk of Small for Gestational Age Births—A Multilevel Analysis of All Singleton Births during 2005–2010 in Finland. Int. J. Equity Health 2014, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Harvey, S.A.; Lim, E.; Gandhi, K.R.; Miyamura, J.; Nakagawa, K. Racial-Ethnic Disparities in Postpartum Hemorrhage in Native Hawaiians, Pacific Islanders, and Asians. Hawaii J Med. Public Health 2017, 76, 128–132. [Google Scholar] [PubMed]
- Wetta, L.A.; Szychowski, J.M.; Seals, S.; Mancuso, M.S.; Biggio, J.R.; Tita, A.T. Risk Factors for Uterine Atony/Postpartum Hemorrhage Requiring Treatment after Vaginal Delivery. Am. J. Obstet. Gynecol. 2013, 209, 51.e1–51.e6. [Google Scholar] [CrossRef] [Green Version]
- Al-Zirqi, I.; Vangen, S.; Forsen, L.; Stray-Pedersen, B. Prevalence and Risk Factors of Severe Obstetric Haemorrhage. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Gregory, K.D.; Korst, L.M. Age and Racial/Ethnic Differences in Maternal, Fetal, and Placental Conditions in Laboring Patients. Am. J. Obstet. Gynecol. 2003, 188, 1602–1606, discussion 1606–1608. [Google Scholar] [CrossRef] [PubMed]
- Biguzzi, E.; Franchi, F.; Acaia, B.; Ossola, W.; Nava, U.; Paraboschi, E.M.; Asselta, R.; Peyvandi, F. Genetic Background and Risk of Postpartum Haemorrhage: Results from an Italian Cohort of 3219 Women. Haemoph. Off. J. World Fed. Hemoph. 2014, 20, e377–e383. [Google Scholar] [CrossRef] [PubMed]
- Salomon, O.; Steinberg, D.M.; Pshithizki, M.; Zalel, Y.; Lerner, A.; Rosenberg, N.; Achiron, R. The Influence of Prothrombotic Polymorphisms and Obstetrical and Medical Variables on the Length of Secondary Postpartum Hemorrhage. J. Womens Health 2005, 14, 306–310. [Google Scholar] [CrossRef]
- FitzGerald, C.; Hurst, S. Implicit Bias in Healthcare Professionals: A Systematic Review. BMC Med. Ethics 2017, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Geller, S.E.; Adams, M.G.; Kelly, P.J.; Kodkany, B.S.; Derman, R.J. Postpartum Hemorrhage in Resource-Poor Settings. Int. J. Gynaecol. Obstet. 2006, 92, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Mairiga, A. How Birth Interval and Antenatal Care Affects Postpartum Haemorrhage Prevention in Maiduguri, Nigeria. J. Appl. Pharm. Sci. 2013, 3, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Lurie, S.; Steinberg, N.; Tannus, S.; Golan, A.; Sadan, O. Mode of Delivery in a Subsequent Pregnancy Following Previous Instrumental Delivery. J. Perinat. Med. 2013, 41, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Lutomski, J.E.; Byrne, B.M.; Devane, D.; Greene, R.A. Increasing Trends in Atonic Postpartum Haemorrhage in Ireland: An 11-Year Population-Based Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 306–314. [Google Scholar] [CrossRef]
- Al-Zirqi, I.; Vangen, S.; Forsén, L.; Stray-Pedersen, B. Effects of Onset of Labor and Mode of Delivery on Severe Postpartum Hemorrhage. Am. J. Obstet. Gynecol. 2009, 201, 273.e1–273.e9. [Google Scholar] [CrossRef]
- Rossen, J.; Okland, I.; Nilsen, O.B.; Eggebø, T.M. Is There an Increase of Postpartum Hemorrhage, and Is Severe Hemorrhage Associated with More Frequent Use of Obstetric Interventions? Acta Obstet. Gynecol. Scand. 2010, 89, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Mansy, A. Does Labor Augmentation with Oxytocin Increase the Risk of Postpartum Hemorrhage? A Randomized Controlled Trial. Clin. Mother Child. Health 2017, 14. [Google Scholar] [CrossRef]
- Phaneuf, S.; Rodríguez Liñares, B.; TambyRaja, R.L.; MacKenzie, I.Z.; López Bernal, A. Loss of Myometrial Oxytocin Receptors during Oxytocin-Induced and Oxytocin-Augmented Labour. J. Reprod. Fertil. 2000, 120, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.C.; Sebire, N.J.; Harris, J.P.; Regan, L.; Robinson, S. Risk Factors for Macrosomia and Its Clinical Consequences: A Study of 350,311 Pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, 9–14. [Google Scholar] [CrossRef]
- Fong, A.; Wu, E.; Pan, D.; Chung, J.H.; Ogunyemi, D.A. Temporal Trends and Morbidities of Vacuum, Forceps, and Combined Use of Both. J. Matern.-Fetal Neonatal Med. 2014, 27, 1886–1891. [Google Scholar] [CrossRef]
- De Leeuw, J.W.; Struijk, P.C.; Vierhout, M.E.; Wallenburg, H.C. Risk Factors for Third Degree Perineal Ruptures during Delivery. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 383–387. [Google Scholar] [CrossRef]
| PPH Cases (n = 265) | No PPH Controls (n = 530) | p-Value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Demographic factors | |||||
| Maternal age, (years, mean ± SD) | 26.3 | ±6.3 | 26.1 | ±5.8 | 0.644 |
| Normal age pregnancy (20–34) | 205 | (77.4) | 447 | (84.3) | 0.040 |
| Teenage pregnancy (<20) | 28 | (10.6) | 44 | (8.3) | |
| Elderly pregnancy (≥35) | 32 | (12.1) | 39 | (7.4) | |
| Nationality | |||||
| Thai | 45 | (17.0) | 138 | (26.0) | <0.001 |
| Burmese | 115 | (43.4) | 295 | (55.7) | |
| Minority/tribes | 105 | (39.6) | 97 | (18.3) | |
| Obstetrics factors | |||||
| Parity | |||||
| Non-nulliparous (primiparous and multiparous) | 135 | (50.9) | 363 | (68.5) | <0.001 |
| Nulliparous | 130 | (49.1) | 167 | (31.5) | |
| Antenatal care history | |||||
| Have ANC history | 256 | (96.6) | 512 | (96.6) | 1.000 |
| No ANC history | 9 | (3.4) | 18 | (3.4) | |
| Adequacy of ANC | |||||
| Adequate (≥5 visits) | 191 | (72.1) | 433 | (81.7) | 0.002 |
| Inadequate (<5 visits) | 74 | (27.9) | 97 | (18.3) | |
| Gestational age | |||||
| Preterm (<37) | 14 | (5.3) | 42 | (7.9) | 0.311 |
| Term (37–41) | 251 | (94.7) | 486 | (91.9) | |
| Post term (>41) | 0 | (0) | 1 | (0.2) | |
| Presence of the following maternal risk factors | |||||
| Maternal anemia | 45 | (17.0) | 80 | (15.1) | 0.535 |
| Maternal HIV | 0 | (0) | 6 | (1.1) | 0.186 |
| History of previous PPH | 11 | (4.2) | 1 | (0.2) | <0.001 |
| History of deliver fetal weight >3500 gm | 25 | (9.4) | 25 | (4.7) | 0.013 |
| History of instrumental delivery | 16 | (6.0) | 9 | (1.7) | 0.002 |
| Pregnancy-induced hypertension | 7 | (2.6) | 7 | (1.3) | 0.251 |
| Physical examination | |||||
| BMI at delivery, (kg/m2, mean ± SD) | |||||
| <25.0 | 70 | (26.4) | 157 | (29.6) | <0.001 |
| 25.0–29.9 | 108 | (40.8) | 267 | (50.4) | |
| 30.0–34.9 | 59 | (22.3) | 86 | (16.2) | |
| ≥35.0 | 28 | (10.6) | 20 | (3.8) | |
| Fundal height, (cm, mean ± SD) | 35.2 | ±2.6 | 34.5 | ±2.3 | <0.001 |
| <36.0 | 168 | (63.4) | 447 | (84.3) | <0.001 |
| ≥36.0 | 97 | (36.6) | 83 | (15.7) | |
| Cervical dilation on admission, (cm) | |||||
| ≤3 cm | 173 | (65.3) | 340 | (64.2) | 0.056 |
| 4–7 cm | 78 | (29.4) | 137 | (25.9) | |
| ≥8 cm | 14 | (5.3) | 53 | (10.0) | |
| PPH Cases (n = 265) | No PPH Controls (n = 530) | p-Value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Intrapartum factors | |||||
| Labor augmentation | 72 | (27.2) | 64 | (12.1) | <0.001 |
| Length of 1st stage (hour, median (IQR)) | 8 | (4.5, 13) | 7 | (4, 11.4) | 0.085 |
| Length of 2nd stage in nulliparous (min, median (IQR)) | 10 | (5.5, 20) | 12 | (4, 21) | 0.878 |
| Length of 2nd stage in non-nulliparous (min, median (IQR)) | 13 | (6, 25) | 10 | (5, 20) | 0.190 |
| Prolonged 2nd stage | 9 | (3.4) | 23 | (4.3) | 0.572 |
| Length of 3rd stage (min, median (IQR)) | 7 | (5, 18) | 6 | (4, 9) | <0.001 |
| Prolonged 3rd stage | 28 | (10.6) | 11 | (2.1) | <0.001 |
| Require manual removal of placenta | 35 | (13.2) | 2 | (0.4) | <0.001 |
| Delivery | |||||
| Spontaneous delivery | 228 | (86.0) | 500 | (94.3) | <0.001 |
| Instrumental delivery | 37 | (14.0) | 30 | (5.7) | |
| Episiotomy wound | |||||
| No tear | 200 | (75.5) | 420 | (79.3) | <0.001 |
| First or second degree tear | 52 | (19.6) | 109 | (20.6) | |
| Third or fourth degree tear | 13 | (4.9) | 1 | (0.2) | |
| Fetal weight (gm, mean ± SD) | |||||
| <3500 | 195 | (73.6) | 453 | (85.5) | <0.001 |
| 3500–4000 | 62 | (23.4) | 73 | (13.8) | |
| >4000 | 8 | (3.0) | 4 | (0.8) | |
| Multivariable Analysis | ||||
|---|---|---|---|---|
| EBL ≥ 500 mL | EBL > 1000 mL | |||
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Maternal age | ||||
| Normal age pregnancy (20–34) | Reference | Reference | ||
| Teenage pregnancy (<20) | 0.94 (0.52, 1.70) | 0.845 | 1.06 (0.46, 2.42) | 0.893 |
| Elderly pregnancy (≥35) | 2.36 (1.32, 4.23) | 0.004 | 2.82 (1.37, 5.81) | 0.005 |
| Nationality | ||||
| Thai | Reference | Reference | ||
| Burmese | 1.39 (0.88, 2.19) | 0.156 | 2.31 (1.09, 4.89) | 0.029 |
| Minority/tribes | 3.29 (2.02, 5.36) | <0.001 | 3.83 (1.79, 8.14) | <0.001 |
| Parity | ||||
| Non-nulliparous (primiparous and multiparous) | Reference | Reference | ||
| Nulliparous | 3.17 (2.19, 4.59) | <0.001 | 2.37 (1.40, 4.00) | 0.001 |
| Adequacy of ANC | ||||
| Adequate (≥5 visits) | Reference | Reference | ||
| Inadequate (<5 visits) | 1.66 (1.11, 2.48) | 0.013 | 1.12 (0.65, 1.92) | 0.693 |
| History of previous PPH | ||||
| Absence | Reference | Reference | ||
| Presence | 22.77 (2.82, 184.13) | 0.003 | 11.32 (3.22, 39.77) | <0.001 |
| History of deliver fetal weight > 3500 gm | ||||
| Absence | Reference | Reference | ||
| Presence | 1.44 (0.71, 2.89) | 0.309 | 1.01 (0.40, 2.53) | 0.976 |
| History of instrumental delivery | ||||
| Absence | Reference | Reference | ||
| Presence | 2.39 (0.96, 5.98) | 0.062 | 2.98 (1.14, 7.81) | 0.026 |
| BMI at delivery | ||||
| <25.0 | Reference | Reference | ||
| 25.0–29.9 | 0.83 (0.55, 1.24) | 0.361 | 0.93 (0.51, 1.69) | 0.820 |
| 30.0–34.9 | 1.44 (0.89, 2.36) | 0.141 | 1.62 (0.81, 3.16) | 0.173 |
| ≥35.0 | 2.44 (1.15, 5.14) | 0.019 | 3.05 (1.26, 7.37) | 0.013 |
| Fundal height | ||||
| <36.0 | Reference | Reference | ||
| ≥36.0 | 2.97 (1.99, 4.43) | <0.001 | 1.69 (0.99, 2.87) | 0.052 |
| Multivariable Analysis | ||||
|---|---|---|---|---|
| EBL ≥ 500 mL | EBL > 1000 mL | |||
| Adjusted OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | |
| Labor augmentation | ||||
| No | Reference | Reference | ||
| Yes | 2.34 (1.55, 3.53) | <0.001 | 3.50 (2.06, 5.96) | <0.001 |
| Require manual removal of placenta | ||||
| Absence | Reference | Reference | ||
| Presence | 49.35 (11.66, 208.89) | <0.001 | 26.59 (12.30, 57.50) | <0.001 |
| Delivery | ||||
| Spontaneous delivery | Reference | Reference | ||
| Instrumental delivery | 2.58 (1.48, 4.51) | 0.001 | 1.26 (0.57, 2.76) | 0.560 |
| Episiotomy wound | ||||
| No tear | Reference | Reference | ||
| First or second degree tear | 1.16 (0.78, 1.72) | 0.465 | 1.26 (0.70, 2.28) | 0.434 |
| Third or fourth degree tear | 25.89 (3.28, 204.29) | 0.002 | 1.24 (0.26, 5.95) | 0.789 |
| Fetal weight | ||||
| <3500 | Reference | Reference | ||
| 3500–4000 | 2.11 (1.41, 3.16) | <0.001 | 1.65 (0.92, 2.98) | 0.095 |
| >4000 | 5.92 (1.72, 20.37) | 0.005 | 5.42 (1.48, 19.85) | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thepampan, W.; Eungapithum, N.; Tanasombatkul, K.; Phinyo, P. Risk Factors for Postpartum Hemorrhage in a Thai–Myanmar Border Community Hospital: A Nested Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 4633. https://doi.org/10.3390/ijerph18094633
Thepampan W, Eungapithum N, Tanasombatkul K, Phinyo P. Risk Factors for Postpartum Hemorrhage in a Thai–Myanmar Border Community Hospital: A Nested Case-Control Study. International Journal of Environmental Research and Public Health. 2021; 18(9):4633. https://doi.org/10.3390/ijerph18094633
Chicago/Turabian StyleThepampan, Waraporn, Nuchsara Eungapithum, Krittai Tanasombatkul, and Phichayut Phinyo. 2021. "Risk Factors for Postpartum Hemorrhage in a Thai–Myanmar Border Community Hospital: A Nested Case-Control Study" International Journal of Environmental Research and Public Health 18, no. 9: 4633. https://doi.org/10.3390/ijerph18094633
APA StyleThepampan, W., Eungapithum, N., Tanasombatkul, K., & Phinyo, P. (2021). Risk Factors for Postpartum Hemorrhage in a Thai–Myanmar Border Community Hospital: A Nested Case-Control Study. International Journal of Environmental Research and Public Health, 18(9), 4633. https://doi.org/10.3390/ijerph18094633






