Colorectal Cancer among Resettlers from the Former Soviet Union and in the General German Population: Clinical and Pathological Characteristics and Trends
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Saarland | Münster | ||
|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | |
| Constant | −5.79 | <0.001 | −6.75 | <0.001 |
| Calendar year | 0.02 | <0.001 | 0.11 | <0.001 |
| Age group | <0.001 | <0.001 | ||
| 55–59 | −1.19 | −1.44 | ||
| 60–64 | −0.73 | −0.99 | ||
| 65–69 | −0.41 | −0.62 | ||
| 70–74 | −0.16 | −0.34 | ||
| 75–79 | 0.03 | −0.10 | ||
| 80–84 | 0.18 | 0.00 | ||
| 85+ | Ref. | Ref. | ||
| Colonoscopy | <0.001 | <0.001 | ||
| No (calendar year < 2002) | Ref. | Ref. | ||
| Yes (calendar year ≥ 2002) | 0.64 | 1.46 | ||
| Colonoscopy X calendar year | <0.001 | <0.001 | ||
| No | Ref. | Ref. | ||
| Yes | −0.05 | −0.13 | ||
| Variable. | Saarland | Münster | ||
|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | |
| Constant | −27.42 | 0.976 | −28.19 | 0.972 |
| Calendar year | −0.01 | 0.028 | 0.02 | <0.001 |
| Age group | <0.001 | <0.001 | ||
| 0–4 | −20.09 | −20.15 | ||
| 5–9 | −20.18 | −6.42 | ||
| 10–14 | −20.24 | −4.99 | ||
| 15–19 | −4.71 | −4.21 | ||
| 20–24 | −4.13 | −4.13 | ||
| 25–29 | −3.87 | −3.58 | ||
| 30–34 | −3.14 | −2.77 | ||
| 35–39 | −2.11 | −2.10 | ||
| 40–44 | −1.45 | −1.45 | ||
| 45–49 | −0.74 | −0.70 | ||
| 50–54 | Ref. | Ref. | ||
| Variable | Young-Onset CRC | Non-Young-Onset CRC | ||
|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | |
| Constant | −0.22 | 0.593 | −0.37 | 0.414 |
| Calendar year | 0.02 | 0.429 | −0.05 | 0.341 |
| Sex | 0.455 | 0.024 | ||
| Male | Ref. | Ref. | ||
| Female | −0.19 | 0.31 | ||
| Colonoscopy | 0.113 | |||
| No (calendar year < 2002) | Ref. | |||
| Yes (calendar year ≥ 2002) | −0.97 | |||
| Colonoscopy X calendar year | 0.072 | |||
| No | Ref. | |||
| Yes | 0.11 | |||
References
- Koch-Institut, R. Krebs in Deutschland Für 2015/2016; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar]
- Brenner, H.; Schrotz-King, P.; Holleczek, B.; Katalinic, A.; Hoffmeister, M. Declining bowel cancer incidence and mortality in Germany: An analysis of time trends in the first ten years after the introduction of screening colonoscopy. Dtsch. Ärzteblatt Int. 2016, 113, 101. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Ahnen, D.J.; Wade, S.W.; Jones, W.F.; Sifri, R.; Mendoza Silveiras, J.; Greenamyer, J.; Guiffre, S.; Axilbund, J.; Spiegel, A.; You, Y.N. The Increasing Incidence of Young-Onset Colorectal Cancer: A Call to Action. Mayo Clin. Proc. 2014, 89, 216–224. [Google Scholar] [CrossRef]
- Ballester, V.; Rashtak, S.; Boardman, L. Clinical and molecular features of young-onset colorectal cancer. World J. Gastroenterol. 2016, 22, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Ahnen, D.J. Colorectal Cancer in the Young. Curr. Gastroenterol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Druesne-Pecollo, N.; Touvier, M.; Latino-Martel, P. Do alcoholic beverages, obesity and other nutritional factors modify the risk of familial colorectal cancer? A systematic review. Crit. Rev. Oncol. Hematol. 2017, 119, 94–112. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; DesMeules, M. Energy intake, physical activity, energy balance, and cancer: Epidemiologic evidence. Methods Mol. Biol. 2009, 472, 191–215. [Google Scholar] [CrossRef]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef]
- Yuhara, H.; Steinmaus, C.; Cohen, S.E.; Corley, D.A.; Tei, Y.; Buffler, P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 2011, 106, 1911–1921, quiz 1922. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Prev. Biomark. 2015, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S. Is colorectal cancer screening necessary before 50 years of age? Intest. Res. 2017, 15, 550–551. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.; Gillman, M.W.; Harper, D.M.; Kemper, A.R.; Krist, A.H. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016, 315, 2564–2575. [Google Scholar] [PubMed]
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Brenner, H.; Altenhofen, L.; Stock, C.; Hoffmeister, M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin. Gastroenterol. Hepatol. 2015, 13, 717–723. [Google Scholar] [CrossRef]
- Bundesausschuss, G. Beschluss des Gemeinsamen Bundesausschusses über eine Richtlinie für Organisierte Krebsfrüherkennungsprogramme und eine Änderung der Krebsfrüherkennungs-Richtlinie. Decision of the Federal Joint Committee on a Directive of Organized Cancer Screening Programs and an Amendment to the Cancer Screening Directive; Federal Joint Committee: Berlin, Germany, 2018. [Google Scholar]
- Bundesverwaltungsamt. (Spät-)Aussiedler und Ihre Angehörigen Zeitreihe 1950–2017; Herkunftsstaaten: Cologne, Germany, 2017. [Google Scholar]
- Bundesamt, S. Bevölkerung und Erwerbstätigkeit; Statistisches Bundesamt: Wiesbaden, Germany, 2018. [Google Scholar]
- Kaucher, S.; Deckert, A.; Becher, H.; Winkler, V. Migration pattern and mortality of ethnic German migrants from the former Soviet Union: A cohort study in Germany. J. BMJ Open 2017, 7, e019213. [Google Scholar] [CrossRef] [PubMed]
- Kaucher, S.; Kajüter, H.; Becher, H.; Winkler, V. Cancer Incidence and Mortality among Ethnic German Migrants From the Former Soviet Union. Front. Oncol. 2018, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Winkler, V.; Kaucher, S.; Deckert, A.; Leier, V.; Holleczek, B.; Meisinger, C.; Razum, O.; Becher, H. Aussiedler Mortality (AMOR): Cohort studies on ethnic German migrants from the Former Soviet Union. BMJ Open 2019, 9, e024865. [Google Scholar] [CrossRef]
- Berrino, F.; Brown, C.; Moller, T.; Sobin, L.; Faivre, J. ENCR Recommendations—Condensed TNM for Coding the Extent of Disease. Lyon Eur. Netw. Cancer Regist. 2002, 2–5. [Google Scholar]
- Pace, M.; Lanzieri, G.; Glickman, M.; Zupanič, T. Revision of the European Standard Population: Report of Eurostat’s Task Force; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health inter-ventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355, Corrigendum to 2020, 49, 1414. [Google Scholar]
- McCullagh, P.; Nelder, J. Generalized Linear Models, 2nd ed.; Chapman and Hall: New York, NY, USA, 1989. [Google Scholar]
- Liang, J.; Kalady, M.F.; Church, J. Young age of onset colorectal cancers. Int. J. Colorectal Dis. 2015, 30, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Young, J.P.; Win, A.K.; Rosty, C.; Flight, I.; Roder, D.; Young, G.P.; Frank, O.; Suthers, G.K.; Hewett, P.J.; Ruszkiewicz, A. Rising incidence of early-onset colorectal cancer in A ustralia over two decades: Report and review. J. Gastroenterol. Hepatol. 2015, 30, 6–13. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Russo, A.; Franceschi, S.; La Vecchia, C.; Dal Maso, L.; Montella, M.; Conti, E.; Giacosa, A.; Falcini, F.; Negri, E. Body size and colorectal-cancer risk. Int. J. Cancer 1998, 78, 161–165. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Schlesinger, S.; Fedirko, V.; Jenab, M.; Bueno-de-Mesquita, B.; Freisling, H.; Romieu, I.; Pischon, T.; Kaaks, R.; Gunter, M.J. Metabolic mediators of the association between adult weight gain and colorectal cancer: Data from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am. J. Epidemiol. 2017, 185, 751–764. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Livingston, E.H.; Cifford, K.Y. Colorectal cancer in the young. Am. J. Surg. 2004, 187, 343–348. [Google Scholar] [CrossRef]
- Richman, S.; Adlard, J. Left and right sided large bowel cancer. BMJ 2002, 324, 931–932. [Google Scholar] [CrossRef]
- Kang, H.; O’Connell, J.B.; Maggard, M.A.; Sack, J.; Ko, C.Y. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis. Colon Rectum 2005, 48, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Razum, O.; Coebergh, J.-W. Cancer risk diversity in non-western migrants to Europe: An overview of the literature. Eur. J. Cancer 2010, 46, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Becher, H.; Winkler, V.J. Estimating the standardized incidence ratio (SIR) with incomplete follow-up data. BMC Med. Res. Methodol. 2017, 17, 55. [Google Scholar] [CrossRef]
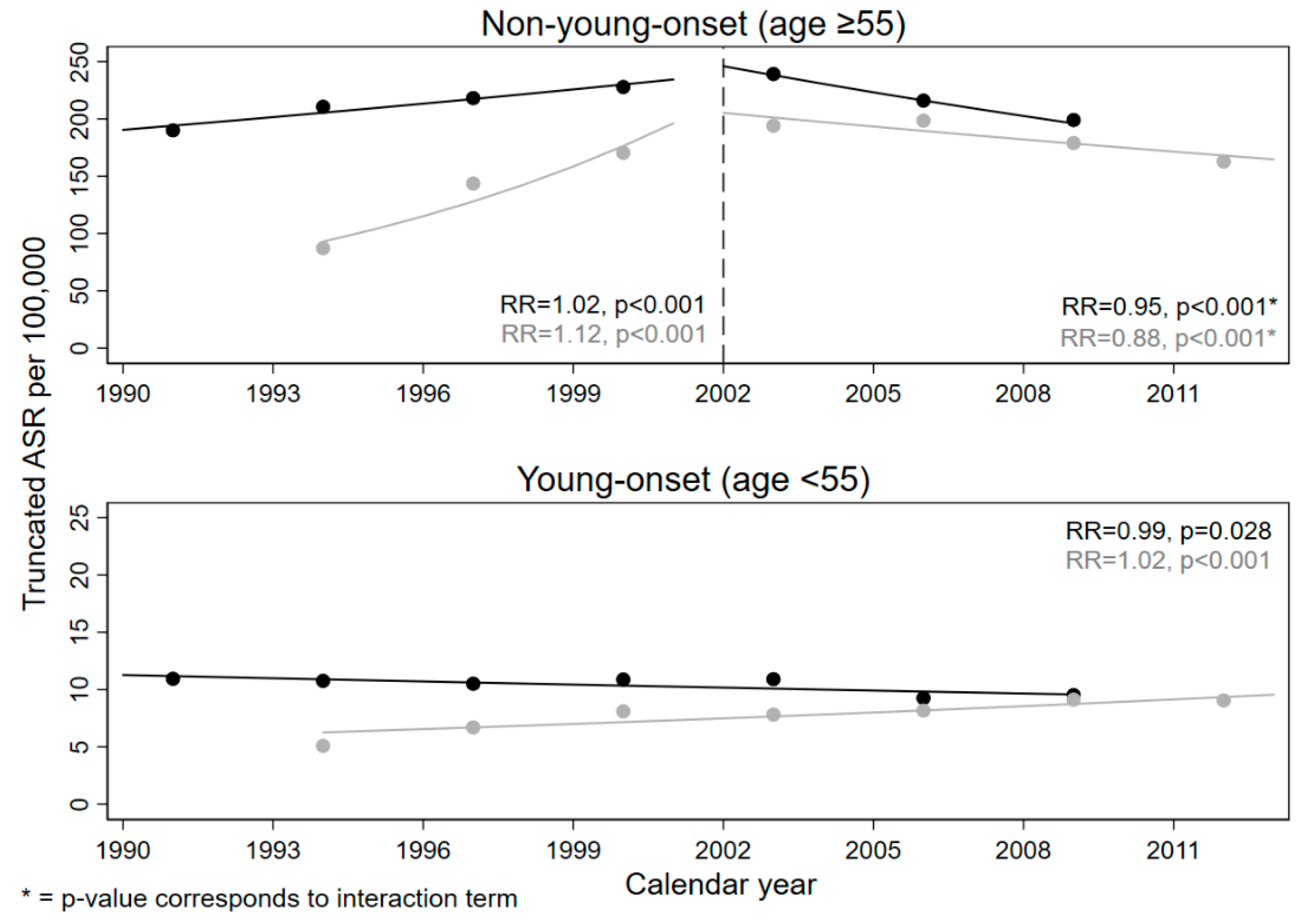
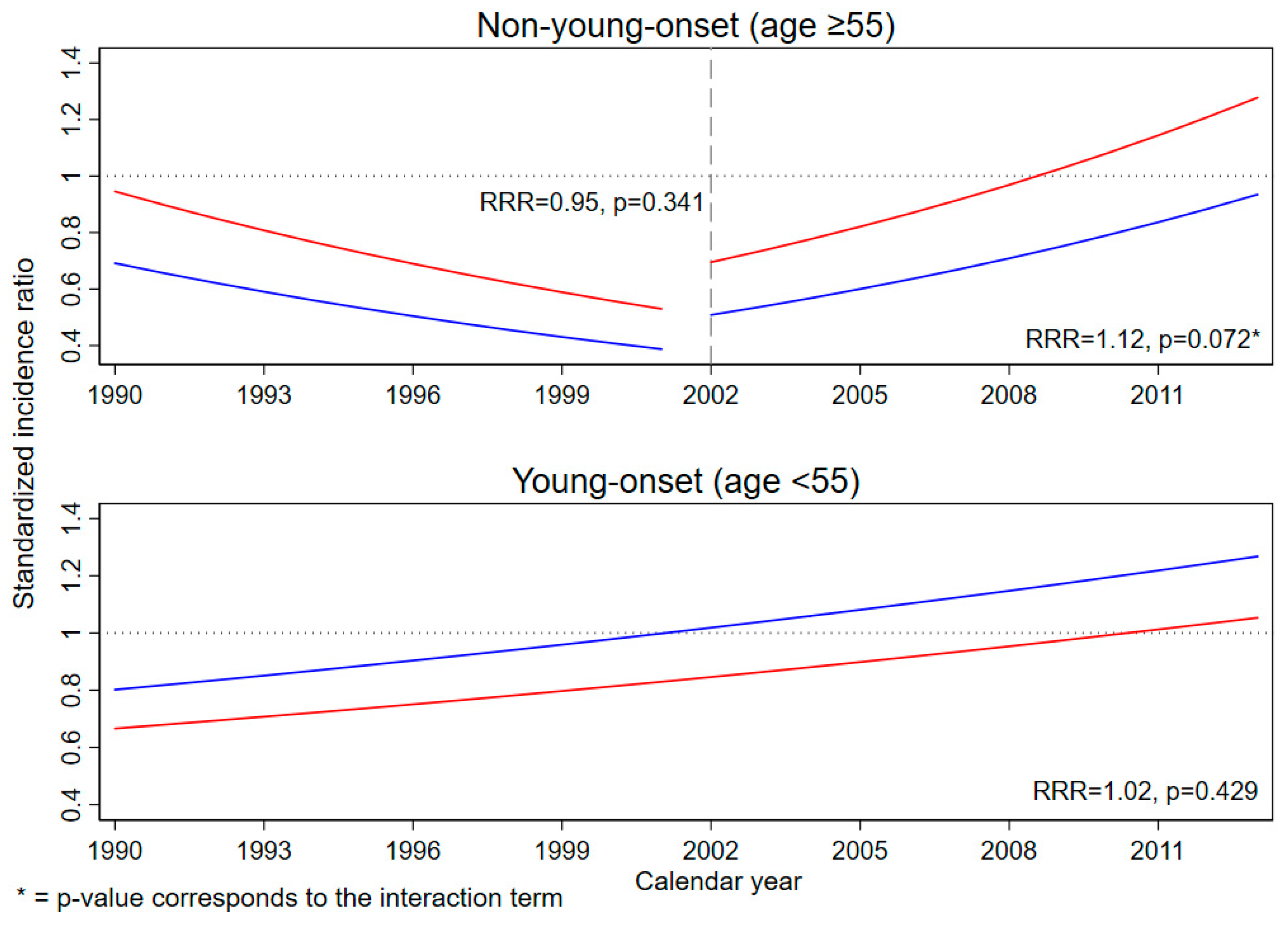
| Characteristics | General Population | Resettler | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total | 48,980 | 100.0 | 229 | 100.0 | |
| Region | Saarland | 17,405 | 35.5 | 76 | 33.2 |
| Münster | 31,575 | 64.5 | 153 | 66.8 | |
| Time period | 1990–2001 | 19,466 | 39.7 | 52 | 22.7 |
| 2002–2013 (colonoscopy) | 29,514 | 60.3 | 177 | 77.3 | |
| Young-onset | yes (age < 55) | 4906 | 10.0 | 51 | 22.3 |
| no (age ≥ 55) | 44,074 | 90.0 | 178 | 77.7 | |
| Sex | Female | 25,349 | 51.8 | 106 | 46.3 |
| Male | 23,631 | 48.2 | 123 | 53.7 | |
| Anatomic location | Right colon | 13,123 | 26.8 | 55 | 24.0 |
| Left colon | 12,756 | 26.0 | 83 | 36.3 | |
| Rectum | 15,810 | 32.3 | 66 | 28.8 | |
| Other/unknown | 7291 | 14.9 | 25 | 10.9 | |
| Histologic Type | Mucinous adenocarcinoma | 8463 | 17.3 | 34 | 14.9 |
| Signet-ring cell carcinoma | 311 | 0.6 | 1 | 0.4 | |
| Other adenocarcinoma subtypes | 36,344 | 74.2 | 183 | 79.9 | |
| Other/unknown | 3862 | 7.9 | 11 | 4.8 | |
| Tumor grade | Low | 34,663 | 70.8 | 174 | 76.0 |
| High | 10,499 | 21.4 | 44 | 19.2 | |
| Unknown | 3818 | 7.8 | 11 | 4.8 | |
| Tumor stage | Local | 18,469 | 37.7 | 93 | 40.6 |
| Advanced | 17,404 | 35.5 | 85 | 37.1 | |
| Unknown | 13,107 | 26.8 | 51 | 22.3 | |
| Characteristics | Total | Saarland | Münster | ||||
|---|---|---|---|---|---|---|---|
| Obs. | SIR (95% CI) | Obs. | SIR (95% CI) | Obs. | SIR (95% CI) | ||
| Total (1990–2013) | 229 | 0.78 (0.68–0.89) | 76 | 0.73 (0.57–0.91) | 153 | 0.81 (0.68–0.94) | |
| Time period | 1990–2001 | 52 | 0.61 (0.46–0.80) | 31 | 0.72 (0.49–1.02) | 21 | 0.50 (0.31–0.76) |
| 2002–2013 (colonoscopy) | 177 | 0.85 (0.73–0.98) | 45 | 0.74 (0.54–0.98) | 132 | 0.89 (0.75–1.06) | |
| Young-onset | Yes (age < 55) | 51 | 0.99 (0.74–1.31) | 14 | 0.80 (0.44–1.34) | 37 | 1.10 (0.77–1.51) |
| No (age ≥ 55) | 178 | 0.73 (0.63–0.85) | 62 | 0.72 (0.55–0.92) | 116 | 0.74 (0.61–0.89) | |
| Sex | Female | 123 | 0.85 (0.71–1.02) | 47 | 0.97 (0.71–1.29) | 76 | 0.80 (0.63–0.99) |
| Male | 106 | 0.70 (0.58–0.85) | 29 | 0.52 (0.35–0.75) | 77 | 0.81 (0.64–1.02) | |
| Anatomical location | Right colon | 55 | 0.69 (0.52–0.91) | 15 | 0.65 (0.36–1.06) | 40 | 0.72 (0.51–0.98) |
| Left colon | 83 | 1.08 (0.86–1.33) | 29 | 1.08 (0.73–1.55) | 54 | 1.07 (0.80–1.39) | |
| Rectum | 66 | 0.68 (0.53–0.87) | 16 | 0.45 (0.26–0.73) | 50 | 0.82 (0.61–1.08) | |
| Others (incl. % unknown) | 25 (20) | 0.60 (0.39–0.89) | 16 (31.3) | 0.86 (0.49–1.40) | 9 (0) | 0.39 (0.18–0.75) | |
| Histologic type | Mucinous adenocarcinoma | 34 | 0.67 (0.47–0.94) | 14 | 0.82 (0.45–1.38) | 20 | 0.60 (0.36–0.92) |
| Signet-ring cell carcinoma | 1 | 0.45 (0.01–2.49) | 1 | 1.13 (0.03–6.31) | 0 | 0.00 (0.00–2.73) | |
| Other adenocarcinomas | 183 | 0.83 (0.71–0.96) | 59 | 0.73 (0.56–0.94) | 124 | 0.88 (0.74–1.05) | |
| Others | 11 | 0.53 (0.26–0.95) | 2 | 0.35 (0.04–1.26) | 9 | 0.60 (0.27–1.14) | |
| Tumor grade | Low grade | 174 | 0.83 (0.71–0.96) | 61 | 0.82 (0.62–1.05) | 113 | 0.83 (0.68–0.99) |
| High grade | 44 | 0.68 (0.50–0.92) | 13 | 0.58 (0.31–0.98) | 31 | 0.74 (0.50–1.05) | |
| Unknown | 11 | 0.57 (0.29–1.03) | 2 | 0.29 (0.03–1.04) | 9 | 0.73 (0.34–1.40) | |
| Tumor stage | Local stage | 93 | 0.82 (0.66–1.01) | 26 | 0.67 (0.44–0.98) | 67 | 0.90 (0.70–1.15) |
| Advanced stage | 85 | 0.79 (0.63–0.98) | 32 | 0.86 (0.59–1.22) | 53 | 0.76 (0.57–0.99) | |
| Unknown | 39 | 0.64 (0.45–0.87) | 18 | 0.49 (0.25–0.88) | 28 | 0.72 (0.48–1.05) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahanani, M.R.; Kaucher, S.; Kajüter, H.; Holleczek, B.; Becher, H.; Winkler, V. Colorectal Cancer among Resettlers from the Former Soviet Union and in the General German Population: Clinical and Pathological Characteristics and Trends. Int. J. Environ. Res. Public Health 2021, 18, 4547. https://doi.org/10.3390/ijerph18094547
Mahanani MR, Kaucher S, Kajüter H, Holleczek B, Becher H, Winkler V. Colorectal Cancer among Resettlers from the Former Soviet Union and in the General German Population: Clinical and Pathological Characteristics and Trends. International Journal of Environmental Research and Public Health. 2021; 18(9):4547. https://doi.org/10.3390/ijerph18094547
Chicago/Turabian StyleMahanani, Melani Ratih, Simone Kaucher, Hiltraud Kajüter, Bernd Holleczek, Heiko Becher, and Volker Winkler. 2021. "Colorectal Cancer among Resettlers from the Former Soviet Union and in the General German Population: Clinical and Pathological Characteristics and Trends" International Journal of Environmental Research and Public Health 18, no. 9: 4547. https://doi.org/10.3390/ijerph18094547
APA StyleMahanani, M. R., Kaucher, S., Kajüter, H., Holleczek, B., Becher, H., & Winkler, V. (2021). Colorectal Cancer among Resettlers from the Former Soviet Union and in the General German Population: Clinical and Pathological Characteristics and Trends. International Journal of Environmental Research and Public Health, 18(9), 4547. https://doi.org/10.3390/ijerph18094547








