Effectiveness and Safety of a Shorter Treatment Regimen in a Setting with a High Burden of Multidrug-Resistant Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Study Population
2.4. Diagnosis, Treatment and Monitoring
2.5. Data Collection
2.6. Definitions
2.7. Analysis and Statistics
3. Results
3.1. Baseline Charachteristics and Cohort Description
3.2. Treatment Outcome
3.3. Characteristics of Drug Adverse Events
3.4. Predictors of Drug Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
©World Health Organization 2021
Open Access Statement
References
- World Health Organisation (WHO). Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Dadu, A.; Hovhannesyan, A.; Ahmedov, S.; Van Der Werf, M.J.; Dara, M. Drug-resistant tuberculosis in eastern Europe and central Asia: A time-series analysis of routine surveillance data. Lancet Infect. Dis. 2019, 3099, 1–9. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control (ECDC). Tuberculosis Surveillance and Monitoring in Europe 2020–2018 Data; WHO Regional Office for Europe, ECCD: Stockholm, Sweden, 2020. [Google Scholar]
- World Health Organization (WHO). Companion Handbook; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Shringarpure, K.S.; Isaakidis, P.; Sagili, K.D.; Baxi, R.K.; Das, M.; Daftary, A. “When treatment is more challenging than the disease”: A qualitative study of MDR-TB patient retention. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Padilla, E.; Marquer, C.; Kalon, S.; Qayyum, S.; Hayrapetyan, A.; Varaine, F.; Bastard, M.; Bonnet, M. Reasons for defaulting from drug-resistant tuberculosis treatment in Armenia: A quantitative and qualitative study. Int. J. Tuberc. Lung Dis. 2014, 18, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, A.; Maug, A.K.J.; Salim, A.H.; Das, P.K.; Sarker, M.R.; Daru, P.; Rieder, H.L. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 2010, 182, 684–692. [Google Scholar] [CrossRef]
- Aung, K.J.M.; Van Deun, A.; Declercq, E.; Sarker, M.R.; Das, P.K.; Hossain, M.A.; Rieder, H.L. Successful “9-month Bangladesh regimen” for multidrugresistant tuberculosis among over 500 consecutive patients. Int. J. Tuberc. Lung Dis. 2014, 18, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Trébucq, A.; DeCroo, T.; Van Deun, A.; Piubello, A.; Chiang, C.-Y.; Koura, K.G.; Schwoebel, V. Short-Course Regimen for Multidrug-Resistant Tuberculosis: A Decade of Evidence. J. Clin. Med. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.A.; Salim, M.H.; Du Cros, P.; Casas, E.C.; Khamraev, A.; Sikhondze, W.; Benedetti, A.; Bastos, M.; Lan, Z.; Jaramillo, E.; et al. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: Individual patient data and aggregate data meta-analyses. Eur. Respir. J. 2017, 50, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Trébucq, A.; Schwoebel, V.; Kashongwe, Z.; Bakayoko, A.; Kuaban, C.; Noeske, J.; Hassane, S.; Souleymane, B.; Piubello, A.; Ciza, F.; et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int. J. Tuberc. Lung Dis. 2018, 22, 17–25. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Treatment Guidelines for Drug-Resistant Tuberculosis. 2016 Updated; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- du Cros, P.; Khamraev, A.; Tigay, Z.; Abdrasuliev, T.; Greig, J.; Cooke, G.; Herboczek, K.; Pylypenko, T.; Berry, C.; Ronnachit, A.; et al. Outcomes with a shorter multidrug-resistant tuberculosis regimen from Karakalpakstan, Uzbekistan. ERJ. Open. Res. 2020, 7. [Google Scholar] [CrossRef]
- Ministry of Health of Republic of Uzbekistan. Clinical Protocol of Treatment of Drug-Resistant Forms of Tuberculosis Using New Tuberculosis Drugs and Shorter Treatment Regimens [in Russian]; Ministry of Health of Republic of Uzbekistan: Tashkent, Uzbekistan, 2018. [Google Scholar]
- Sotgiu, G.; Tiberi, S.; Centis, R.; D’Ambrosio, L.; Fuentes, Z.; Zumla, A.; Migliori, G.B. Applicability of the shorter ‘Bangladesh regimen’ in high multidrug-resistant tuberculosis settings. Int. J. Infect. Dis. 2017, 56, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Horter, S.; Achar, J.; Gray, N.; Parpieva, N.; Tigay, Z.; Singh, J.; Stringer, B. Patient and health-care worker perspectives on the short-course regimen for treatment of drug-resistant tuberculosis in Karakalpakstan, Uzbekistan. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Active Tuberculosis Drug-Safety Monitoring and Management; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Challenge TB. Introduction of Active Tuberculosis Drug-Safety Monitoring and Management (aDSM) for New Drugs and Regimens. 2015. Available online: https://www.challengetb.org/publications/tools/pmdt/Introduction_of_active_TB_drug-safety_monitoring_and_management_for_new_drugs_and_regimens.pdf (accessed on 11 April 2021).
- World Health Organization (WHO). WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Ministry of Health of Uzbekistan Republican Specialized Scientific Practical Medical Center of Phthisiology and Pulmonology. Manual of Pharmacovigilance in Tuberculosis Control [in Russian]; Ministry of Health of Uzbekistan: Tashkent, Uzbekistan, 2018. [Google Scholar]
- Division of AIDS National Institute of Allergy and Infectious Diseases National Institutes of Health US Department of Health and Human Services. Division of AIDS (DAIDS). Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1 July 2017. Available online: https://rsc.niaid.nih.gov/ (accessed on 19 December 2020).
- World Health Organization (WHO). Nutrition Landscape Information System (NLiS) Country Profile Indicators: Interpretation Guide, 2nd ed.; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Andersen, P.K.; Gill, R.D. Cox’s regression model for counting processes: A large sample study. An. Stat. 1982, 10, 1100–1112. [Google Scholar] [CrossRef]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; pp. 281–285. [Google Scholar] [CrossRef] [Green Version]
- Nunn, A.J.; Phillips, P.P.; Meredith, S.K.; Chiang, C.-Y.; Conradie, F.; Dalai, D.; Van Deun, A.; Dat, P.-T.; Lan, N.; Master, I.; et al. A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med. 2019, 380, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Piubello, A.; Souleymane, M.B.; Hassane-Harouna, S.; Yacouba, A.; Lempens, P.; Assao-Neino, M.M.; Maman-Lawan, I.; Attaher, S.; Moustapha, B.; Soumana, A.; et al. Management of multidrug-resistant tuberculosis with shorter treatment regimen in Niger: Nationwide programmatic achievements. Respir. Med. 2020, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anh, L.T.N.; Kumar, A.M.V.; Ramaswamy, G.; Htun, T.; Thi, T.T.H.; Nguyen, G.H.; Quelapio, M.; Gebhard, A.; Nguyen, H.B.; Nguyen, N.V. High levels of treatment success and zero relapse in multidrug-resistant tuberculosis patients receiving a levofloxacin-based shorter treatment regimen in Vietnam. Trop. Med. Infect. Dis. 2020, 5, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassane-Harouna, S.; Cherif, G.-F.; Ortuno-Gutierrez, N.; Cisse, D.; Camara, L.M.; Diallo, D.D. Better programmatic outcome with shorter regimen for the treatment of multidrug-resistant tuberculosis (MDR-TB) in Guinea: A retrospective cohort study. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Ulmasova, D.J.; Uzakova, G.; Tillyashayhov, M.N.; Turaev, L.; Van Gemert, W.; Hoffmann, H.; Zignol, M.; Kremer, K.; Gombogaram, T.; Gadoev, J.; et al. Multidrug-resistant tuberculosis in Uzbekistan: Results of a nationwide survey, 2010 to 2011. Eurosurveillance 2013, 18. [Google Scholar] [CrossRef]
- Abidi, S.; Achar, J.; Neino, M.M.A.; Bang, D.; Benedetti, A.; Brode, S.; Campbell, J.R.; Casas, E.C.; Conradie, F.; Dravniece, G.; et al. Standardised shorter regimens versus individualised longer regimens for multidrug-resistant TB. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Van Deun, A.; DeCroo, T.; Kuaban, C.; Noeske, J.; Piubello, A.; Aung, K.J.M.; Rieder, H.L. Gatifloxacin is superior to levofloxacin and moxifloxacin in shorter treatment regimens for multidrug-resistant TB. Int. J. Tuberc. Lung. Dis. 2019, 23, 965–971. [Google Scholar] [CrossRef]
- Ciza, F.; Gils, T.; Sawadogo, M.; Decroo, T.; Roggi, A.; Piubello, A.; Ortuño-Gutiérrez, N. Course of Adverse Events during Short Treatment Regimen in Patients with Rifampicin-Resistant Tuberculosis in Burundi. J. Clin. Med. 2020, 9, 1873. [Google Scholar] [CrossRef]
- Kalandarova, L.; Tillashaikhov, M.; Parpieva, N.; Saidova, S.; Gadoev, J.; Alikhanova, N.; Zachariah, R.; Mukhtarov, D.; Alidjanov, S.; Harries, A.D. Treatment outcomes and adverse reactions in patients with multidrug-resistant tuberculosis managed by ambulatory or hospitalized care from 2010–2011 in Tashkent, Uzbekistan. Public Health Panor. 2016, 02, 21–29. [Google Scholar]
- Harouna, S.H.; Ortuno-Gutierrez, N.; Souleymane, M.B.; Kizito, W.; Morou, S.; Boukary, I.; Zolfo, M.; Benedetti, G.; Piubello, A. Short-course treatment outcomes and adverse events in adults and children-adolescents with MDR-TB in Niger. Int. J. Tuberc. Lung. Dis. 2019, 23, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Sagwa, E.; Mantel-Teeuwisse, A.; Ruswa, N. Occurrence and clinical management of moderate-to-severe adverse events during drug-resistant tuberculosis treatment: A retrospective cohort study. J. Pharm. Policy. Pract. 2014, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merid, M.W.; Gezie, L.D.; Kassa, G.M.; Muluneh, A.G.; Akalu, T.Y.; Yenit, M.K. Incidence and predictors of major adverse drug events among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara regional state public hospitals; Ethiopia: A retrospective cohort study. BMC Infect. Dis. 2019, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Javaid, A.; Sulaiman, S.A.S.; Afridi, A.K.; Zainab; Khan, A.H. Occurrence, Management, and Risk Factors for Adverse Drug Reactions in Multidrug Resistant Tuberculosis Patients. Am. J. Ther. 2018, 25, 533–540. [Google Scholar] [CrossRef]
- Buziashvili, M.; Davtyan, H.; Sereda, Y.; Denisiuk, O.; Gozalov, O.; Lomtadze, N.; Hovhannesyan, A. Incidence rate and time to serious adverse events among rifampicin resistant tuberculosis patients in Georgia treated with new and repurposed anti-tuberculosis drugs, 2016–2018. Monaldi Arch. Chest Dis. 2021, 91, 1649. [Google Scholar] [CrossRef]
- Avaliani, T.; Sereda, Y.; Davtyan, H.; Tukvadze, N.; Togonidze, T.; Kiria, N.; Denisiuk, O.; Gozalov, O.; Ahmedov, S.; Hovhannesyan, A. Effectiveness and safety of fully oral modified shorter treatment regimen for multidrug-resistant tuberculosis in Georgia, 2019–2020. Monaldi Arch. Chest Dis. 2021, 91, 1679. [Google Scholar] [CrossRef]

| Characteristics | N | % |
|---|---|---|
| Sex | ||
| Male | 67 | 70.5 |
| Female | 28 | 29.5 |
| Age | ||
| <40 years | 33 | 34.7 |
| ≥40 years | 62 | 65.3 |
| Category | ||
| New | 46 | 48.4 |
| Retreated | 49 | 51.6 |
| HIV status | ||
| Negative | 84 | 88.4 |
| Positive | 11 | 11.6 |
| Antiretroviral treatment (among HIV pos) | ||
| Yes | 6 | 54.5 |
| No | 5 | 45.5 |
| Body-mass index | ||
| ≥18.5 kg/m2 | 58 | 74.4 |
| <18.5 km/m2 | 21 | 26.9 |
| missing | 16 | |
| Clinical presentation | ||
| Disseminated PTB | 6 | 6.3 |
| Focal PTB | 11 | 11.6 |
| Infiltrative PTB | 62 | 65.3 |
| Pulmonary tuberculoma | 3 | 3.2 |
| Cavernous PTB | 4 | 4.2 |
| Fibrous-cavernous PTB | 7 | 7.4 |
| Tuberculous pleurisy | 2 | 2.1 |
| Any complication | ||
| No | 72 | 75.8 |
| Yes | 23 | 24.2 |
| Any comorbidity | ||
| No | 39 | 41.1 |
| Yes | 56 | 58.9 |
| Diabetes | ||
| Yes | 18 | 28.1 |
| No | 46 | 71.9 |
| Missing | 31 | |
| Hepatitis | ||
| Yes | 19 | 29.7 |
| No | 45 | 70.3 |
| Missing | 31 | |
| Anemia | ||
| Yes | 33 | 51.6 |
| No | 31 | 48.4 |
| Missing | 31 | |
| Year of treatment start | ||
| 2018 | 48 | 50.5 |
| 2019 | 47 | 49.5 |
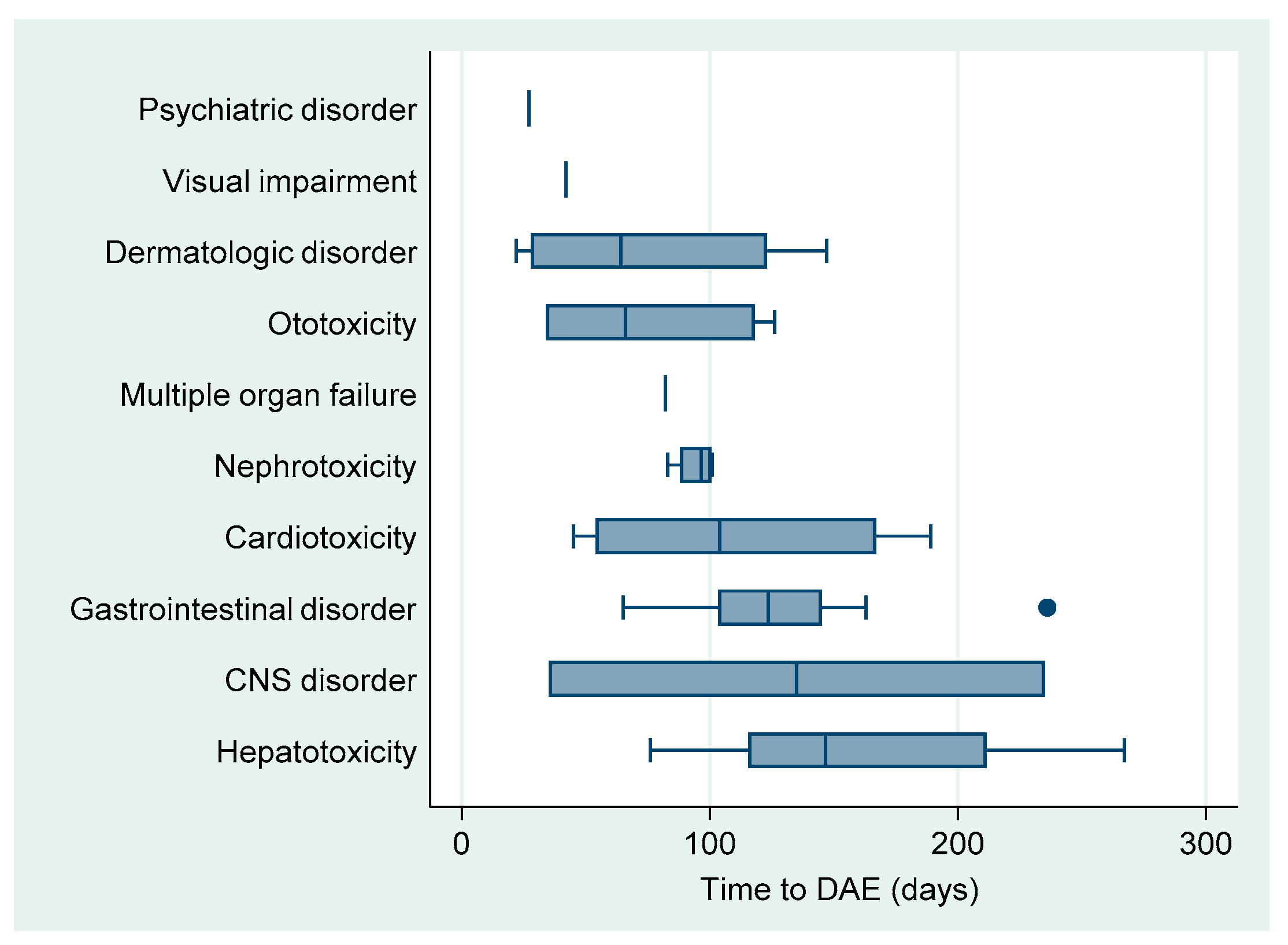
| Drug Adverse Event Type | Total | Grade | Rate Per 100 PM | Median Time to DAE 1 | (IQR) 1 | (Range) 1 | |||
|---|---|---|---|---|---|---|---|---|---|
| N | (% of all) | I | II | III–IV | |||||
| Cardiotoxicity | 4 | (8.5) | 0 | 2 | 2 | 0.524 | 104 | (54–167) | (45–189) |
| Central nervous system disorder | 2 | (4.3) | 0 | 0 | 2 | 0.262 | 135 | (35–235) | (35–235) |
| Dermatologic disorder | 7 | (14.9) | 0 | 3 | 4 | 0.916 | 64 | (28–123) | (22–147) |
| Gastrointestinal disorder | 12 | (25.5) | 2 | 4 | 6 | 1.571 | 123 | (103–145) | (65–236) |
| Hepatotoxicity | 8 | (17.0) | 1 | 4 | 3 | 1.047 | 146 | (115–211) | (76–267) |
| Multiple organ failure | 1 | (2.1) | 0 | 0 | 1 | 0.131 | 82 | ||
| Nephrotoxicity | 4 | (8.5) | 0 | 2 | 2 | 0.524 | 96 | (88–100) | (83–101) |
| Ototoxicity | 7 | (14.9) | 0 | 1 | 6 | 0.916 | 66 | (34–118) | (34–126) |
| Psychiatric disorder | 1 | (2.1) | 0 | 0 | 1 | 0.131 | 27 | ||
| Visual impairment | 1 | (2.1) | 0 | 0 | 1 | 0.131 | 42 | ||
| Total | 47 | (100.0) | 3 | 16 | 28 | 6.152 | 101 | (64–139) | (22–267) |
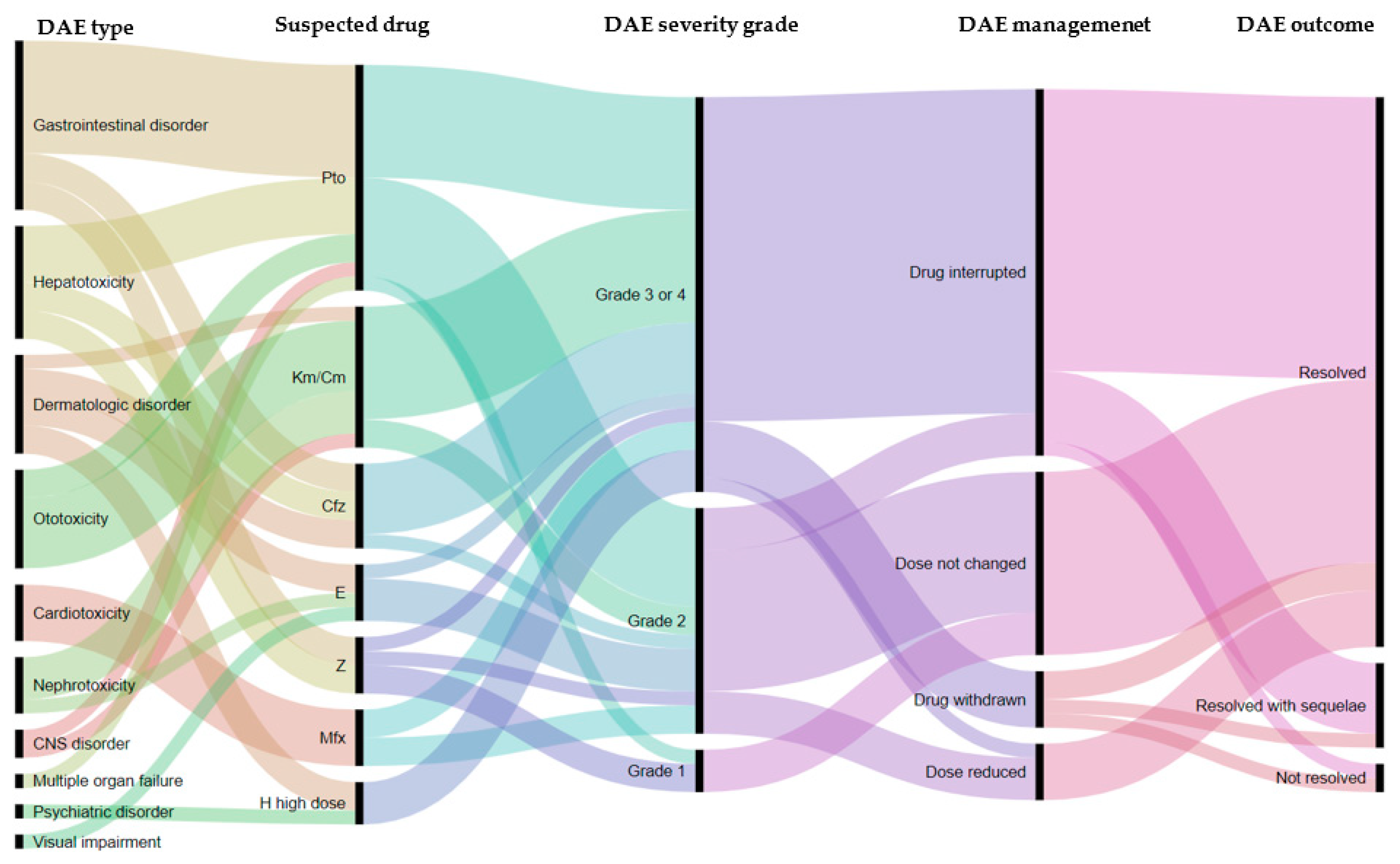
| Suspected Drug | Number DAEs | Number Patients with DAE | Toxicity (%) |
|---|---|---|---|
| Moxifloxacin | 4 | 3 | (3.2) |
| Kanamycin/capreomycin | 10 | 10 | (10.5) |
| Clofazimine | 6 | 4 | (4.2) |
| Protionamide | 16 | 15 | (15.8) |
| Pyrazinamide | 4 | 4 | (4.2) |
| Ethambutol | 4 | 4 | (4.2) |
| High dose isoniazid | 3 | 3 | (3.2) |
| Type of DAE | N | Management of DAE | Outcome of DAE | |||||
|---|---|---|---|---|---|---|---|---|
| Dose Not Changed | Dose Reduced | Drug Interrupted | Drug(s) Withdrawal | Resolved | Resolved with Sequelae | Not Resolved | ||
| Cardiotoxicity | 4 | 1 | 1 | 2 | 0 | 4 | 0 | 0 |
| CNS disorder | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 |
| Dermatologic disorder | 7 | 0 | 1 | 6 | 0 | 7 | 0 | 0 |
| Gastro-intestinal disorder | 12 | 5 | 1 | 5 | 1 | 12 | 0 | 0 |
| Hepatotoxicity | 8 | 4 | 1 | 3 | 0 | 8 | 0 | 0 |
| Multiple organ failure | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Nephrotoxicity | 4 | 2 | 0 | 1 | 1 | 4 | 0 | 0 |
| Ototoxicity | 7 | 1 | 0 | 5 | 1 | 1 | 4 | 2 |
| Psychiatric disorder | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Visual impairment | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Total | 47 | 13 | 4 | 26 | 4 | 39 | 6 | 2 |
| Characteristics | N | DAE (n) | Follow-Up Time (100 * Month) | Rate (100 p/m) | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | aHR | 95%CI | LRT p Value | |||||
| Sex | ||||||||||
| Male | 67 | 28 | 5.253 | 5.33 | 0.69 | (0.40–1.20) | 0.187 | 0.64 | (0.37–1.09) | 0.102 |
| Female | 28 | 19 | 2.392 | 7.94 | 1.00 | 1.00 | ||||
| Age group | ||||||||||
| <40 years | 33 | 15 | 2.890 | 5.19 | 1.00 | 1.00 | ||||
| ≥40 years | 62 | 32 | 4.755 | 6.73 | 1.24 | (0.72–2.13) | 0.445 | 1.21 | (0.71–2.08) | 0.483 |
| TB treatment history | ||||||||||
| New | 46 | 22 | 3.814 | 5.77 | 1.00 | |||||
| Retreated | 49 | 25 | 3.831 | 6.53 | 1.15 | (0.68–1.99) | 0.592 | |||
| HIV | ||||||||||
| Negative | 84 | 38 | 6.600 | 5.76 | 1.00 | 1.00 | ||||
| Positive | 11 | 9 | 1.045 | 8.61 | 1.79 | (1.05–3.07) | 0.032 | 1.81 | (1.07–3.06) | 0.026 |
| ART (among HIV pos) | ||||||||||
| yes | 6 | 5 | 0.671 | 7.45 | ||||||
| no | 5 | 4 | 0.374 | 10.70 | ||||||
| Body-mass index | ||||||||||
| ≥18.5 kg/m2 | 58 | 24 | 4.795 | 5.01 | 1.00 | |||||
| <18.5 km/m2 | 21 | 15 | 1.586 | 9.46 | 1.82 | (0.98–3.37) | 0.057 | |||
| missing | 16 | |||||||||
| Any complication | ||||||||||
| No | 72 | 39 | 6.023 | 6.48 | 1.00 | |||||
| Yes | 23 | 8 | 1.543 | 5.18 | 0.75 | (0.33–1.68) | 0.481 | |||
| Any comorbidity | ||||||||||
| No | 39 | 14 | 3.061 | 4.57 | 1.00 | |||||
| Yes | 56 | 33 | 4.484 | 7.36 | 1.61 | (0.90–2.88) | 0.111 | |||
| Diabetes | ||||||||||
| Yes | 18 | 10 | 1.400 | 7.14 | 1.03 | (0.50–2.14) | 0.921 | |||
| No | 46 | 25 | 3.718 | 6.72 | 1.00 | |||||
| Missing | 31 | |||||||||
| Hepatitis | ||||||||||
| Yes | 19 | 11 | 1.546 | 7.12 | 1.11 | (0.54–2.31) | 0.771 | |||
| No | 45 | 24 | 3.573 | 6.72 | 1 | |||||
| Missing | 31 | |||||||||
| Anemia | ||||||||||
| Yes | 33 | 13 | 2.583 | 5.03 | 0.62 | (0.31–1.22) | 0.615 | |||
| No | 31 | 22 | 2.535 | 8.68 | 1 | |||||
| Missing | 31 | |||||||||
| Alcohol use | ||||||||||
| Yes | 18 | 8 | 1.326 | 6.03 | 0.96 | (0.46–2.00) | 0.919 | |||
| No | 71 | 37 | 5.785 | 6.40 | 1 | |||||
| Missing | 6 | |||||||||
| Year of registration | ||||||||||
| 2018 | 48 | 20 | 3.961 | 5.05 | 1 | |||||
| 2019 | 47 | 27 | 3.763 | 7.17 | 1.36 | (0.78–2.35) | 0.276 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 World Health Organization. Licensee MDPI, Basel, Switzerland. This is an open access article distributed under the terms of the Creative Commons Attribution IGO License (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any reproduction of this article there should not be any suggestion that WHO or this article endorse any specific organisation or products. The use of the WHO logo is not permitted. This notice should be preserved along with the article’s original URL.
Share and Cite
Trubnikov, A.; Hovhannesyan, A.; Akopyan, K.; Ciobanu, A.; Sadirova, D.; Kalandarova, L.; Parpieva, N.; Gadoev, J. Effectiveness and Safety of a Shorter Treatment Regimen in a Setting with a High Burden of Multidrug-Resistant Tuberculosis. Int. J. Environ. Res. Public Health 2021, 18, 4121. https://doi.org/10.3390/ijerph18084121
Trubnikov A, Hovhannesyan A, Akopyan K, Ciobanu A, Sadirova D, Kalandarova L, Parpieva N, Gadoev J. Effectiveness and Safety of a Shorter Treatment Regimen in a Setting with a High Burden of Multidrug-Resistant Tuberculosis. International Journal of Environmental Research and Public Health. 2021; 18(8):4121. https://doi.org/10.3390/ijerph18084121
Chicago/Turabian StyleTrubnikov, Aleksandr, Arax Hovhannesyan, Kristina Akopyan, Ana Ciobanu, Dilbar Sadirova, Lola Kalandarova, Nargiza Parpieva, and Jamshid Gadoev. 2021. "Effectiveness and Safety of a Shorter Treatment Regimen in a Setting with a High Burden of Multidrug-Resistant Tuberculosis" International Journal of Environmental Research and Public Health 18, no. 8: 4121. https://doi.org/10.3390/ijerph18084121
APA StyleTrubnikov, A., Hovhannesyan, A., Akopyan, K., Ciobanu, A., Sadirova, D., Kalandarova, L., Parpieva, N., & Gadoev, J. (2021). Effectiveness and Safety of a Shorter Treatment Regimen in a Setting with a High Burden of Multidrug-Resistant Tuberculosis. International Journal of Environmental Research and Public Health, 18(8), 4121. https://doi.org/10.3390/ijerph18084121










