Effects of a Mixture of Ivy Leaf Extract and Coptidis rhizome on Patients with Chronic Bronchitis and Bronchiectasis
Abstract
1. Introduction
2. Materials and Methods
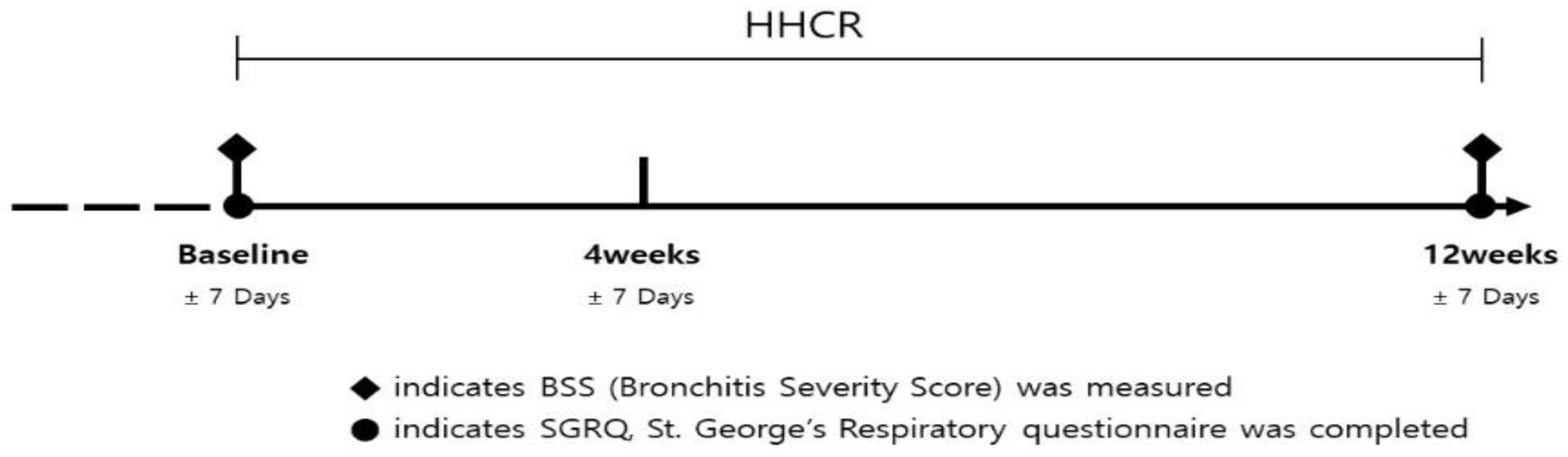
2.1. Study Design
2.2. Patient Participation
2.3. Efficacy Outcomes and General Measures
2.4. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
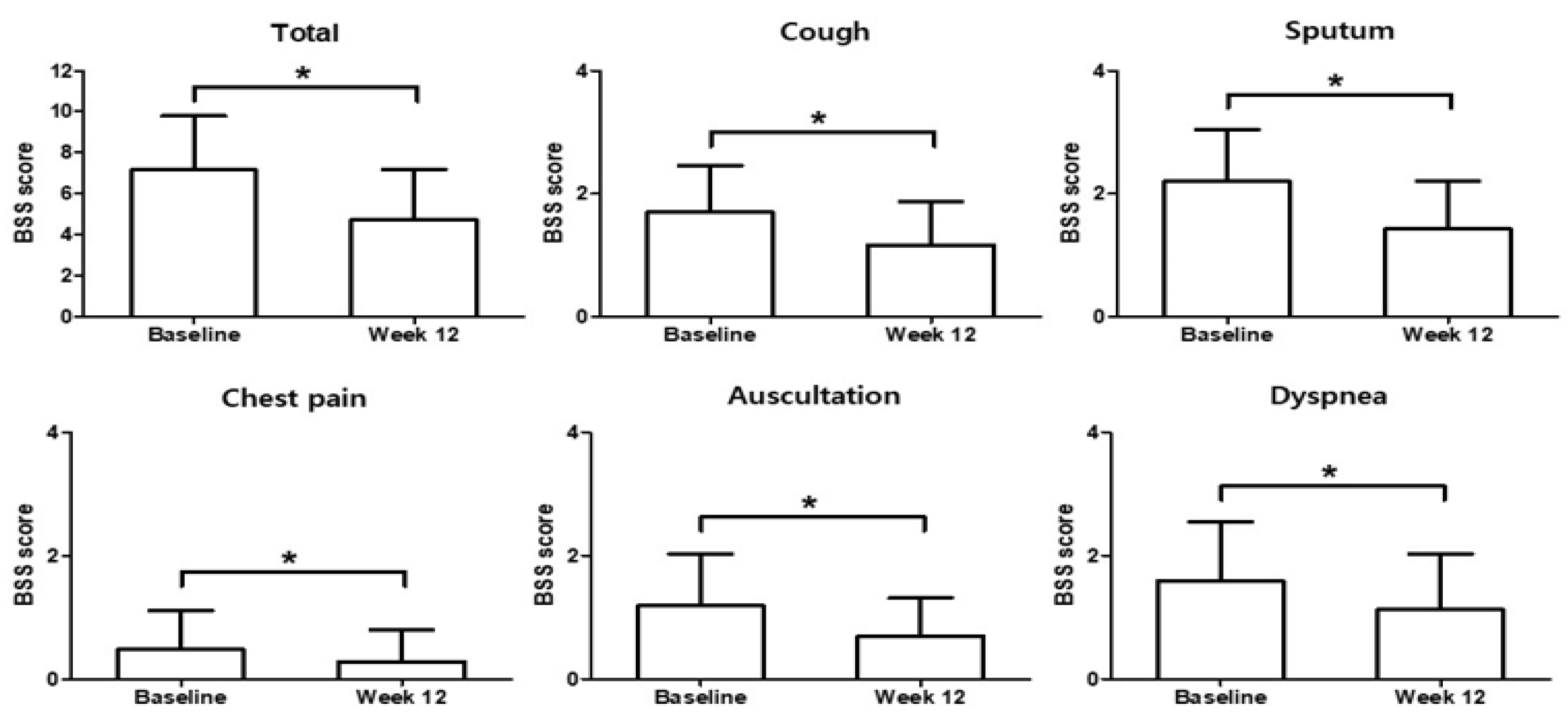
3.2. Primary Endpoint
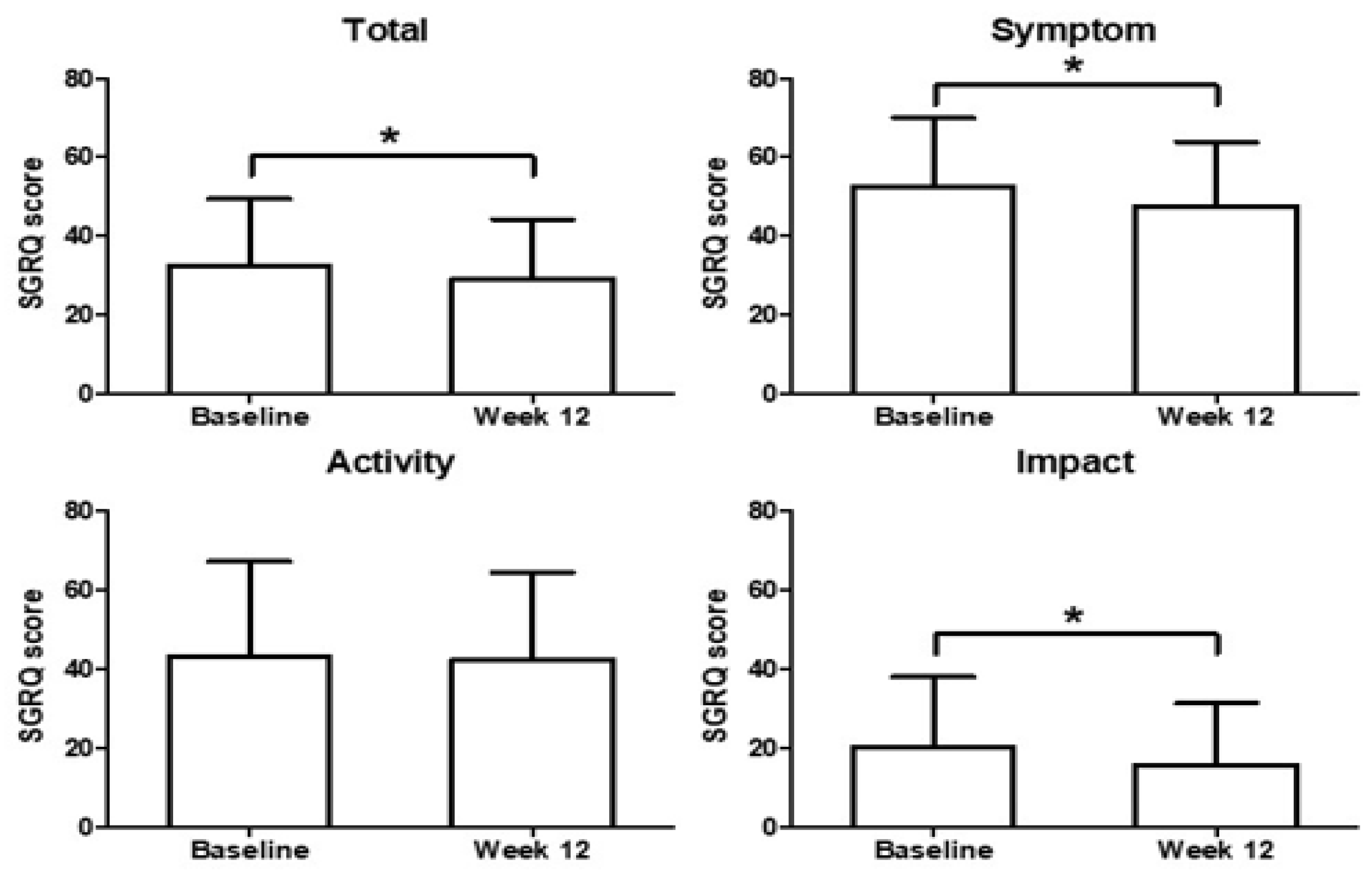
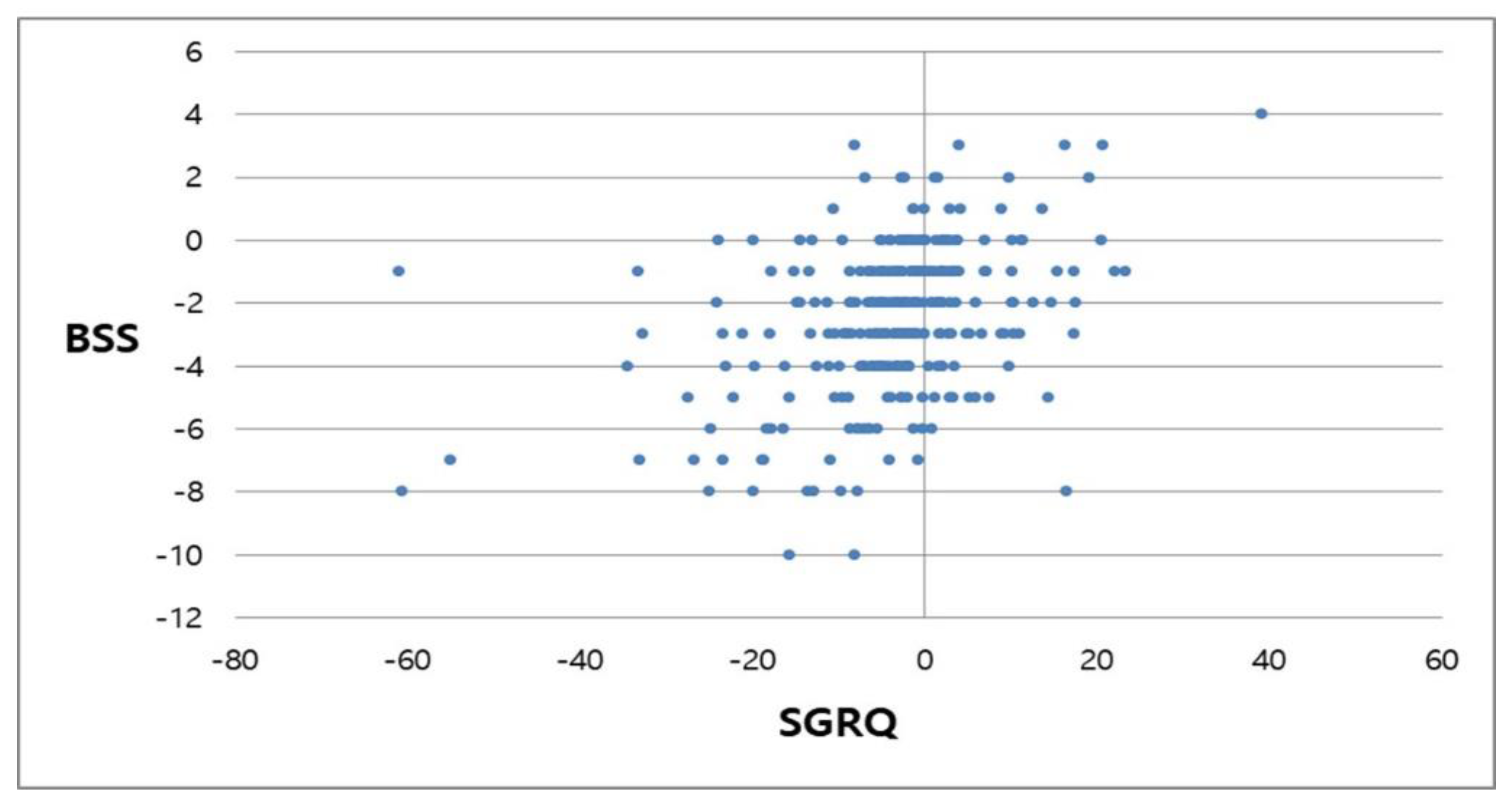
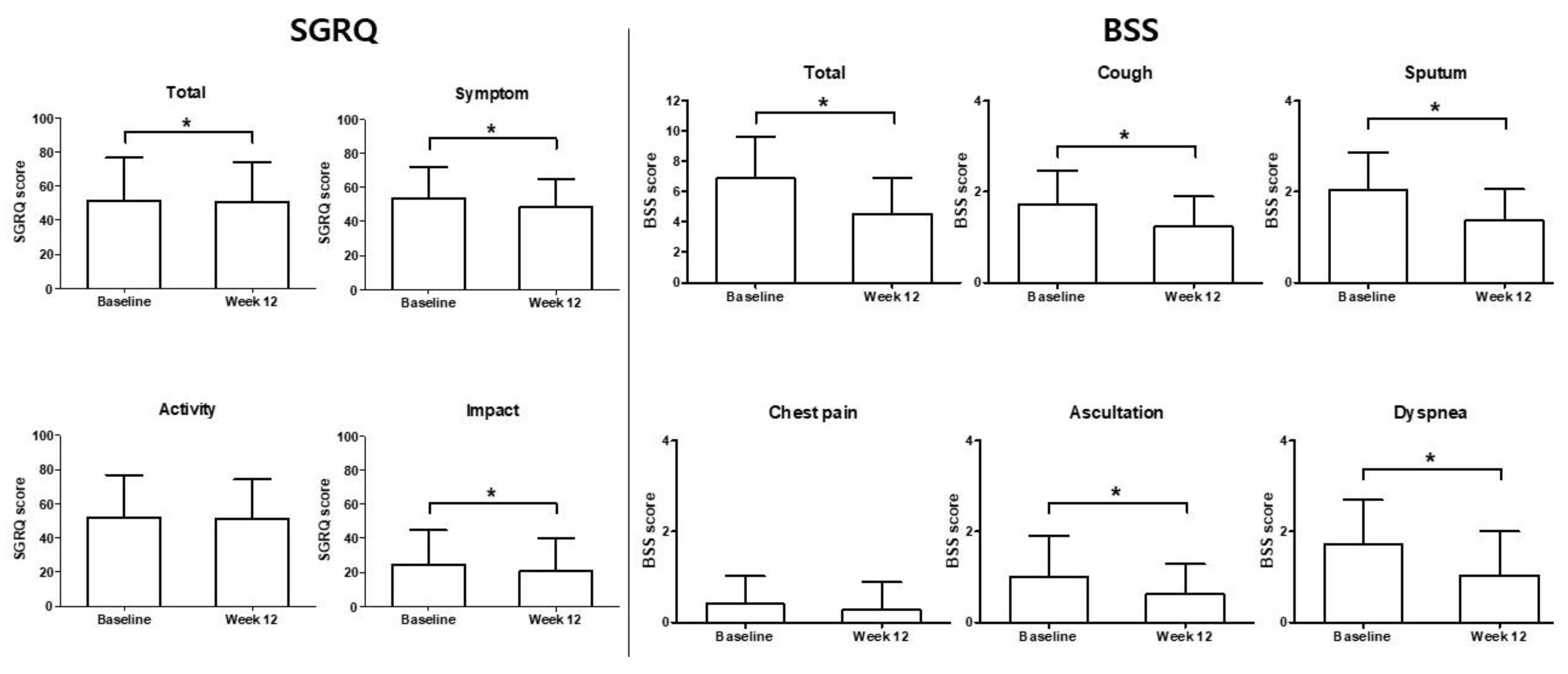
3.3. Secondary Endpoint
3.4. Changes in SGRQ Score and BSS in Patients Concomitantly Treated with a Bronchodilator
3.5. Safety and Tolerability
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef]
- Snijders, D.; Fernandez Dominguez, B.; Calgaro, S.; Bertozzi, I.; Escribano Montaner, A.; Perilongo, G.; Barbato, A. Mucociliary clearance techniques for treating non-cystic fibrosis bronchiectasis: Is there evidence? Int. J. Immunopathol. Pharmacol. 2015, 28, 150–159. [Google Scholar] [CrossRef]
- Kong, D.L.L.; Teng, S.; Sun, T.; Deng, Z.; Li, Y. Research on export current situation and countermeasure of Coptis chinensis from Shizhu county. Morder. Chin. Med. 2013, 15, 193–225. [Google Scholar]
- Choi, Y.Y.; Kim, M.H.; Cho, I.H.; Kim, J.H.; Hong, J.; Lee, T.H.; Yang, W.M. Inhibitory effect of Coptis chinensis on inflammation in LPS-induced endotoxemia. J. Ethnopharmacol. 2013, 149, 506–512. [Google Scholar] [CrossRef]
- Kim, K.R.K.N.; Park, Y.; Kim, J.H.; Lee, E.J.; Kim, K.S. Berberine suppresses interleukin-1beta-Induced MUC5AC gene expression in human airway epithelial cells. J. Rhinol. 2011, 18, 116–121. [Google Scholar]
- Sánchez-Mendoza, M.E.; Castillo-Henkel, C.; Navarrete, A. Relaxant action mechanism of berberine identified as the active principle of Argemone ochroleuca Sweet in guinea-pig tracheal smooth muscle. J. Pharm. Pharmacol. 2008, 60, 229–236. [Google Scholar] [CrossRef]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute anti-inflammatory activity of four saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine Int. J. Phytother. Phytopharm. 2005, 12, 440–444. [Google Scholar] [CrossRef]
- Wang, J.; Ran, Q.; Zeng, H.R.; Wang, L.; Hu, C.J.; Huang, Q.W. Cellular stress response mechanisms of Rhizoma coptidis: A systematic review. Chin. Med. 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Song, K.J.; Shin, Y.J.; Lee, K.R.; Lee, E.J.; Suh, Y.S.; Kim, K.S. Expectorant and antitussive effect of Hedera helix and Rhizoma coptidis extracts mixture. Yonsei Med. J. 2015, 56, 819–824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pauwels, R.A.; Buist, A.S.; Ma, P.; Jenkins, C.R.; Hurd, S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): Executive summary. Respir. Care 2001, 46, 798–825. [Google Scholar] [PubMed]
- Matthys, H.; Kamin, W. Positioning of the Bronchitis Severity Score (BSS) for standardised use in clinical studies. Curr. Med. Res. Opin. 2013, 29, 1383–1390. [Google Scholar] [CrossRef]
- Kardos, P.; Lehrl, S.; Kamin, W.; Matthys, H. Assessment of the effect of pharmacotherapy in common cold/acute bronchitis— The Bronchitis Severity Scale (BSS). Pneumologie 2014, 68, 542–546. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Holmes, P.W. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir. Med. 2006, 100, 2183–2189. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Smith, M.P.; McHugh, B.J.; Doherty, C.; Govan, J.R.; Hill, A.T. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2012, 186, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, R.E.; Wells, A.U.; Copley, S.J.; Desai, S.R.; Howling, S.J.; Cole, P.J.; Wilson, R.; Hansell, D.M. A comparison of serial computed tomography and functional change in bronchiectasis. Eur. Respir. J. 2002, 20, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The bronchiectasis severity index. An international derivation and validation study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef]
- Aliberti, S.; Lonni, S.; Dore, S.; McDonnell, M.J.; Goeminne, P.C.; Dimakou, K.; Fardon, T.C.; Rutherford, R.; Pesci, A.; Restrepo, M.I.; et al. Clinical phenotypes in adult patients with bronchiectasis. Eur. Respir. J. 2016, 47, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Masters, I.B.; Chang, A.B. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: What influences lung function stability? Chest 2010, 138, 158–164. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Aliberti, S.; Polverino, E.; Vendrell, M.; Crichton, M.; Loebinger, M.; Dimakou, K.; Clifton, I.; Van Der Eerden, M.; Rohde, G.; et al. The EMBARC European Bronchiectasis Registry: Protocol for an international observational study. ERJ Open Res. 2016, 2. [Google Scholar] [CrossRef]
- Raghu, G.; King, T.E.; Behr, J.; Brown, K.K.; du Bois, R.M.; Leconte, I.; Roux, S.; Swigris, J. Quality of life and dyspnoea in patients treated with bosentan for idiopathic pulmonary fibrosis (BUILD-1). Eur. Respir. J. 2010, 35, 118–123. [Google Scholar] [CrossRef]
- Antoniu, S.A. UPLIFT Study: The effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin, D.P.; Celli, B.; Senn, S.: A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Umar, T.; Guo, M.; Deng, G. Hederacoside-C Inhibition of Staphylococcus aureus-Induced Mastitis via TLR2 & TLR4 and Their Downstream Signaling NF-κB and MAPKs Pathways In Vivo and In Vitro. Inflammation 2020, 43, 579–594. [Google Scholar]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Umar, T.; Guo, M.; Deng, G. Anti-inflammatory effects of Hederacoside-C on Staphylococcus aureus induced inflammation via TLRs and their downstream signal pathway in vivo and in vitro. Microbial. Pathog. 2019, 137, 103767. [Google Scholar] [CrossRef]
- Rubin, B.K. Mucolytics, expectorants, and mucokinetic medications. Respir. Care. 2007, 52, 859–865. [Google Scholar]
| Total (n = 304) | |
|---|---|
| Sex, n (%) | |
| Male | 236 (77.63) |
| Female | 68 (22.37) |
| Age (year) | |
| Mean ± SD | 67.88 ± 8.94 |
| Median | 69.00 |
| Min, Max | 37.00, 88.00 |
| Height (cm) | |
| Mean ± SD | 163.27 ± 7.70 |
| Median | 164.00 |
| Weight (kg) | |
| Mean ± SD | 60.93 ± 10.31 |
| Median | 60.35 |
| Min, Max | 37.00, 90.00 |
| BMI (kg/m2) | |
| Mean ± SD | 22.83 ± 3.33 |
| Median | 22.75 |
| Min, Max | 13.68, 31.60 |
| Drug Name | Total (n = 304) |
|---|---|
| Use of any concomitant drug (n (%)) | |
| ICS alone | |
| Fluticasone furoate | 3 (0.99) |
| Budesonide | 1 (0.33) |
| Ciclesonide | 10 (3.29) |
| ICS-LABA | |
| Beclometasone/Formoterol | 7 (2.30) |
| Fluticasone/Formoterol | 6 (1.97) |
| Fluticasone/Salmeterol | 6 (1.97) |
| Budesonide/Formoterol | 20 (6.58) |
| Fluticasone/Vilanterol | 16 (5.26) |
| LABA | |
| Tulobuterol | 1 (0.33) |
| LAMA | |
| Aclidinium bromide | 9 (2.96) |
| Umeclidinium bromide | 1 (0.33) |
| Tiotropium bromide | 33 (10.86) |
| LABA-LAMA | |
| Olodaterol/Tiotropium bromide | 27 (8.88) |
| Indacaterol/Glycopyrronium bromide | 23 (7.57) |
| Vilanterol/Umeclidinium bromide | 23 (7.57) |
| Mucolytics and antioxidant | |
| Erdosteine | 40 (13.16) |
| Carbocysteine | 1 (0.33) |
| Ambroxol | 3 (0.99) |
| Bromhexine | 3 (0.99) |
| Acetylcystein | 16 (5.26) |
| Others | |
| Doxofylline | 33 (10.86) |
| Theophylline | 6 (1.97) |
| Roflumilast | 8 (2.63) |
| Pranlukast | 26 (8.55) |
| Montelukast | 15 (4.93) |
| Theobromine | 11 (3.62) |
| Total (n = 304) | |
|---|---|
| Any AEs | 28 (9.21%) |
| Respiratory | 16 |
| Gastrointestinal | 7 |
| Nervous system | 3 |
| Others | 2 |
| Any SAE | 3 |
| Respiratory | 2 |
| Others | 1 |
| Any ADR | 4 |
| Respiratory | 1 |
| Gastrointestinal | 2 |
| Nervous system | 1 |
| Any SADR | 0 |
| Hospitalizations | 0 |
| MACE/Fatal AEs | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, G.; Kim, Y.-I.; Park, S.J.; Lee, S.Y.; Kim, J.W.; Yoon, S.H.; Lee, K.S.; Byun, M.K.; Kim, H.-R.; Chung, J. Effects of a Mixture of Ivy Leaf Extract and Coptidis rhizome on Patients with Chronic Bronchitis and Bronchiectasis. Int. J. Environ. Res. Public Health 2021, 18, 4024. https://doi.org/10.3390/ijerph18084024
Hong G, Kim Y-I, Park SJ, Lee SY, Kim JW, Yoon SH, Lee KS, Byun MK, Kim H-R, Chung J. Effects of a Mixture of Ivy Leaf Extract and Coptidis rhizome on Patients with Chronic Bronchitis and Bronchiectasis. International Journal of Environmental Research and Public Health. 2021; 18(8):4024. https://doi.org/10.3390/ijerph18084024
Chicago/Turabian StyleHong, Goohyeon, Yu-Il Kim, Seoung Ju Park, Sung Yong Lee, Jin Woo Kim, Seong Hoon Yoon, Keu Sung Lee, Min Kwang Byun, Hak-Ryul Kim, and Jaeho Chung. 2021. "Effects of a Mixture of Ivy Leaf Extract and Coptidis rhizome on Patients with Chronic Bronchitis and Bronchiectasis" International Journal of Environmental Research and Public Health 18, no. 8: 4024. https://doi.org/10.3390/ijerph18084024
APA StyleHong, G., Kim, Y.-I., Park, S. J., Lee, S. Y., Kim, J. W., Yoon, S. H., Lee, K. S., Byun, M. K., Kim, H.-R., & Chung, J. (2021). Effects of a Mixture of Ivy Leaf Extract and Coptidis rhizome on Patients with Chronic Bronchitis and Bronchiectasis. International Journal of Environmental Research and Public Health, 18(8), 4024. https://doi.org/10.3390/ijerph18084024






