Association between Triglyceride Glucose Index and Corrected QT Prolongation in Chinese Male Steelworkers
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Baseline Data Collection
2.3. Laboratory Measurements
2.4. Electrocardiography, QT, and QTc Interval Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amiri, P.; Jalali-Farahani, S.; Karimi, M.; Taherian, R.; Kazempour-Ardebili, S.; Hosseini-Esfahani, F.; Mirmiran, P.; Azizi, F. Factors associated with pre-diabetes in Tehranian men and women: A structural equations modeling. PLoS ONE 2017, 12, e0188898. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Bang, H.; Pankow, J.S.; Ballantyne, C.M.; Golden, S.H.; Folsom, A.R.; Chambless, L.E. Atherosclerosis Risk in Communities Investigators. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities Study. Diabetes Care 2005, 28, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B. Prediction of incident diabetes mellitus in middle-aged adults: The Framingham Offspring Study. Arch. Intern. Med. 2007, 167, 1068–1074. [Google Scholar] [CrossRef]
- Tomizawa, M.; Kawanabe, Y.; Shinozaki, F.; Sato, S.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed. Rep. 2014, 2, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Wei, F.; Song, J.; Cao, Z.; Chen, C.; Zhang, K.; Feng, S.; Wang, Y.; Li, W.D. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: A prospective study with 8-year follow-ups in two cohorts. J. Transl. Med. 2019, 17, 403. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Seidler, A.; Moalemi, A.; Pearson, T.A. Normal triglyceride levels and coronary artery disease events: The Baltimore Coronary Observational Long-Term Study. J. Am. Coll. Cardiol. 1998, 31, 1252–1257. [Google Scholar] [CrossRef]
- Shaye, K.; Amir, T.; Shlomo, S.; Yechezkel, S. Fasting glucose levels within the high normal range predict cardiovascular outcome. Am. Heart J. 2012, 164, 111–116. [Google Scholar] [CrossRef]
- Angoorani, P.; Heshmat, R.; Ejtahed, H.S.; Motlagh, M.E.; Ziaodini, H.; Taheri, M.; Aminaee, T.; Goodarzi, A.; Qorbani, M.; Kelishadi, R. Validity of triglyceride-glucose index as an indicator for metabolic syndrome in children and adolescents: The CASPIAN-V study. Eat. Weight Disord. 2018, 23, 877–883. [Google Scholar] [CrossRef]
- Won, K.B.; Park, E.J.; Han, D.; Lee, J.H.; Choi, S.Y.; Chun, E.J.; Park, S.H.; Han, H.W.; Sung, J.; Jung, H.O.; et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc. Diabetol. 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Kazani, M.V.; Armeni, E.; Georgiopoulos, G.; Tampakis, K.; Rizos, D.; Augoulea, A.; Kaparos, G.; Alexandrou, A.; Stamatelopoulos, K. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018, 27, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Íñigo, L.; Navarro-González, D.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martínez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lepeschkin, E.; Surawicz, B. Measurement of the QT interval of the electrocardiogram. Circulation 1952, 6, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Hou, C.J.; Lauer, M.R.; Sung, R.J. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann. Intern. Med. 1995, 122, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J. Measurement of the QT interval and the risk associated with QTc interval prolongation: A review. Am. J. Cardiol. 1993, 72, 23B–25B. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Wolf, S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation 1978, 57, 1074–1077. [Google Scholar] [CrossRef]
- Robbins, J.; Nelson, J.C.; Rautaharju, P.M.; Gottdiener, J.S. The association between the length of the QT interval and mortality in the cardiovascular health study. Am. J. Med. 2003, 115, 689–694. [Google Scholar] [CrossRef]
- Mozos, I.; Filimon, L. QT and Tpeak-Tend intervals in shift workers. J. Electrocardiol. 2013, 46, 60–65. [Google Scholar] [CrossRef]
- Chung, F.M.; Yang, Y.H.; Shieh, T.Y.; Shin, S.J.; Tsai, J.C.; Lee, Y.J. Effect of alcohol consumption on estimated glomerular filtration rate and creatinine clearance rate. Nephrol. Dial. Transplant. 2005, 20, 1610–1616. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2016, 39 (Suppl. 1), S13–S22. [Google Scholar] [CrossRef]
- Lu, Y.C.; Wang, C.P.; Hsu, C.C.; Chiu, C.A.; Yu, T.H.; Hung, W.C.; Lu, L.F.; Chung, F.M.; Tsai, I.T.; Lin, H.C.; et al. Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2013, 29, 551–556. [Google Scholar]
- Lu, Y.C.; Chang, C.C.; Wang, C.P.; Hung, W.C.; Tsai, I.T.; Tang, W.H.; Wu, C.C.; Wei, C.T.; Chung, F.M.; Lee, Y.J.; et al. Circulating fatty acid-binding protein 1 (FABP1) and nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Int. J. Med. Sci. 2020, 17, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [PubMed]
- Naksuk, N.; Hu, T.; Krittanawong, C.; Thongprayoon, C.; Sharma, S.; Park, J.Y.; Rosenbaum, A.N.; Gaba, P.; Killu, A.M.; Sugrue, A.M.; et al. Association of serum magnesium on mortality in patients admitted to the intensive cardiac care unit. Am. J. Med. 2017, 130, e5–e229. [Google Scholar] [CrossRef]
- Salvi, V.; Karnad, D.R.; Panicker, G.K.; Natekar, M.; Hingorani, P.; Kerkar, V.; Ramasamy, A.; de Vries, M.; Zumbrunnen, T.; Kothari, S.; et al. Comparison of 5 methods of QT interval measurements on electrocardiograms from a thorough QT/QTc study: Effect on assay sensitivity and categorical outliers. J. Electrocardiol. 2011, 44, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Hnatkova, K.; Batchvarov, V.; Gang, Y.; Smetana, P.; Camm, A.J. Sample size, power calculations, and their implications for the cost of thorough studies of drug induced QT interval prolongation. Pacing Clin. Electrophysiol. 2004, 27, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.M.; Kors, J.A.; De Bruin, M.L.; van der Hooft, C.S.; Hofman, A.; Heeringa, J.; Deckers, J.W.; Kingma, J.H.; Sturkenboom, M.C.J.M.; Stricker, B.H.C.; et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006, 47, 362–367. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, C.P.; Yu, T.H.; Hung, W.C.; Chung, F.M.; Lu, Y.C.; Hsu, C.C.; Lu, L.F.; Huang, L.L.H.; Lee, Y.J.; et al. Serum total p-cresylsulfate level is associated with abnormal QTc interval in stable angina patients with early stage of renal failure. Clin. Chim. Acta 2014, 437, 25–30. [Google Scholar] [CrossRef]
- Welty, F.K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 840–849. [Google Scholar] [CrossRef]
- Sara, J.D.; Lennon, R.J.; Ackerman, M.J.; Friedman, P.A.; Noseworthy, P.A.; Lerman, A. Coronary microvascular dysfunction is associated with baseline QTc prolongation amongst patients with chest pain and non-obstructive coronary artery disease. J. Electrocardiol. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Kumar, T.; Jha, K.; Sharan, A.; Sakshi, P.; Kumar, S.; Kumari, A. Study of the effect of obesity on QT-interval among adults. J. Fam. Med. Prim. Care 2019, 8, 1626–1629. [Google Scholar]
- Veglio, M.; Borra, M.; Stevens, L.K.; Fuller, J.H.; Perin, P.C. The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia 1999, 42, 68–75. [Google Scholar] [CrossRef]
- Parekh, R.S.; Plantinga, L.C.; Kao, W.H.; Meoni, L.A.; Jaar, B.G.; Fink, N.E.; Powe, N.R.; Coresh, J.; Klag, M.J. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008, 74, 1335–1342. [Google Scholar] [CrossRef]
- Hoffman, B.F.; Guo, S.D.; Feinmark, S.J. Arrhythmias caused by platelet activating factor. J. Cardiovasc. Electrophysiol. 1996, 7, 120–133. [Google Scholar] [CrossRef]
- Du, T.; Yuan, G.; Zhang, M.; Zhou, X.; Sun, X.; Yu, X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014, 13, 146. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, H.S.; Park, Y.M.; Ha, H.S.; Jeong, S.H.; Yang, H.K.; Lee, J.H.; Yim, H.W.; Kang, M.I.; Lee, W.C.; et al. Predicting the development of diabetes using the product of triglycerides and glucose: The chungju metabolic disease cohort (CMC) study. PLoS ONE 2014, 9, e90430. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, H.K.; Ha, H.S.; Lee, J.H.; Kwon, H.S.; Park, Y.M.; Yim, H.W.; Kang, M.I.; Lee, W.C.; Son, H.Y.; et al. Changes in metabolic health status over time and risk of developing type 2 diabetes: A prospective cohort study. Medicine 2015, 94, e1705. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, K.; Yang, H.K.; Kim, M.K.; Yoon, K.H.; Kwon, H.S.; Park, Y.M. Identifying subgroups of obesity using the product of triglycerides and glucose: The Korea National Health and Nutrition Examination Survey, 2008–2010. Clin. Endocrinol. 2014, 82, 213–220. [Google Scholar] [CrossRef]
- Irace, C.; Carallo, C.; Scavelli, F.B.; De Franceschi, M.S.; Esposito, T.; Tripolino, C.; Gnasso, A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int. J. Clin. Pract. 2013, 67, 665–672. [Google Scholar] [CrossRef]
- Kim, M.K.; Ahn, C.W.; Kang, S.; Nam, J.S.; Kim, K.R.; Park, J.S. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc. Diabetol. 2017, 16, 108. [Google Scholar] [CrossRef]
- Jin, J.L.; Cao, Y.X.; Wu, L.G.; You, X.D.; Guo, Y.L.; Wu, N.Q. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J. Thorac. Dis. 2018, 10, 6137–6146. [Google Scholar] [CrossRef]
- Sun, G.Z.; Zhou, Y.; Ye, N.; Wu, S.J.; Sun, Y.X. Independent influence of blood pressure on QTc interval: Results from a general Chinese population. BioMed Res. Int. 2019, 2019, 1656123. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Bertolozzi, I.; Morozzi, G.; Lorenzini, S.; Simpatico, A.; Selvi, E.; Bacarelli, M.R.; Finizola, F.; Vanni, F.; et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart 2017, 103, 1821–1829. [Google Scholar] [CrossRef]
- Thomakos, P.; Liatis, S.; Kalopita, S.; Vlahodimitris, I.; Stathi, C.; Katsilambros, N.; Tentolouris, N.; Makrilakis, K. Cigarette smoking is associated with prolongation of the QTc interval duration in patients with type 2 diabetes mellitus. Int. J. Endocrinol. 2013, 2013, 329189. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.S.; Tseng, P.H.; Tu, C.H.; Chen, C.C.; Liao, W.C.; Lee, Y.C.; Chiu, H.M.; Lin, H.J.; Ho, Y.L.; Yang, W.S.; et al. Nonalcoholic fatty liver disease is associated with QT prolongation in the general population. J. Am. Heart Assoc. 2015, 4, e001820. [Google Scholar] [CrossRef]
- Er, L.K.; Wu, S.; Chou, H.H.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE 2016, 11, e0149731. [Google Scholar] [CrossRef]
- Lytsy, P.; Ingelsson, E.; Lind, L.; Arnlöv, J.; Sundström, J. Interplay of overweight and insulin resistance on hypertension development. J. Hypertens. 2014, 32, 834–839. [Google Scholar] [CrossRef]
- Akande, T.O.; Adeleye, J.O.; Kadiri, S. Insulin resistance in Nigerians with essential hypertension. Afr. Health Sci. 2013, 13, 655–660. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huang, J.; Kan, H.; Castranova, V.; Frisbee, J.C.; Yu, H.G. Defective calcium inactivation causes long QT in obese insulin-resistant rat. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1013–H1022. [Google Scholar] [CrossRef]
- Bellan, M.; Rigamonti, C.; Giacomini, G.M.; Makmur, G.; Marconi, C.; Nicosia, F.; Panero, A.; Benedittis, C.D.; Burlone, M.E.; Minisini, R.; et al. Liver stiffness, not fat liver content, predicts the length of QTc interval in patients with chronic liver disease. Gastroenterol. Res. Pract. 2019, 2019, 6731498. [Google Scholar] [CrossRef]
- Guo, W.; Lu, J.; Qin, P.; Li, X.; Zhu, W.; Wu, J.; Xu, N.; Zhang, Q. The triglyceride-glucose index is associated with the severity of hepatic steatosis and the presence of liver fibrosis in non-alcoholic fatty liver disease: A cross-sectional study in Chinese adults. Lipids Health Dis. 2020, 19, 218. [Google Scholar] [CrossRef]
- Kayali, S.; Demir, F. The effects of cigarette smoking on ventricular repolarization in adolescents. Einstein (Sao Paulo) 2017, 15, 251–255. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Liu, Y.; Sun, G.; Sun, Y.; Guan, Y.; Zhu, G.; Abraham, M.R. Relation of heavy alcohol consumption to QTc interval prolongation. Am. J. Cardiol. 2016, 118, 1201–1206. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Boeckelmann, I.; Hammwhöner, M.; Goette, A. Nicotine, cigarette smoking and cardiac arrhythmia: An overview. Eur. J. Prev. Cardiol. 2012, 19, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.G.; de Castro-Faria-Neto, H.C.; da Silva, C.I.; de Souza e Souza, K.F.; Gonçalves-de-Albuquerque, C.F.; Silva, A.R.; Amorim, L.M.; Freire, A.S.; Santelli, R.E.; Diniz, L.P.; et al. Na/K-ATPase as a target for anticancer drugs: Studies with perillyl alcohol. Mol. Cancer 2015, 14, 105. [Google Scholar] [CrossRef]
- Keiver, K.; Ellis, L.; Anzarut, A.; Weinberg, J. Effect of prenatal ethanol exposure on fetal calcium metabolism. Alcohol. Clin. Exp. Res. 1997, 21, 1612–1618. [Google Scholar] [CrossRef]
- Pietrzykowski, A.Z.; Ortiz-Miranda, S.; Knott, T.K.; Custer, E.; Puig, S.; Lemos, J.R.; Treistman, S.N. Molecular tolerance of voltage-gated calcium channels is evident after short exposures to alcohol in vasopressin-releasing nerve terminals. Alcohol. Clin. Exp. Res. 2013, 37, 933–940. [Google Scholar] [CrossRef]
- Souza-Smith, F.M.; Kerut, E.K.; Breslin, J.W.; Molina, P.E. Mechanisms of acute alcohol intoxication-induced modulation of cyclic mobilization of [Ca2+] in rat mesenteric lymphatic vessels. Lymphat. Res. Biol. 2015, 13, 93–99. [Google Scholar] [CrossRef]
- Garg, A.; Chaturvedi, P.; Gupta, P.C. A review of the systemic adverse effects of areca nut or betel nut. Indian J. Med. Paediatr. Oncol. 2014, 35, 3–9. [Google Scholar] [CrossRef]
- Masulli, M.; Vaccaro, O. Association between cigarette smoking and metabolic syndrome. Diabetes Care 2006, 29, 482–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bajaj, M. Nicotine and insulin resistance: When the smoke clears. Diabetes 2012, 61, 3078–3080. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Torres-Do Rego, A.; Castro Cabezas, M. Alcohol and plasma triglycerides. Curr. Opin. Lipidol. 2013, 24, 321–326. [Google Scholar] [CrossRef]
- Yamada, T.; Hara, K.; Kadowaki, T. Chewing betel quid and the risk of metabolic disease, cardiovascular disease, and all-cause mortality: A meta-analysis. PLoS ONE 2013, 8, e70679. [Google Scholar] [CrossRef]
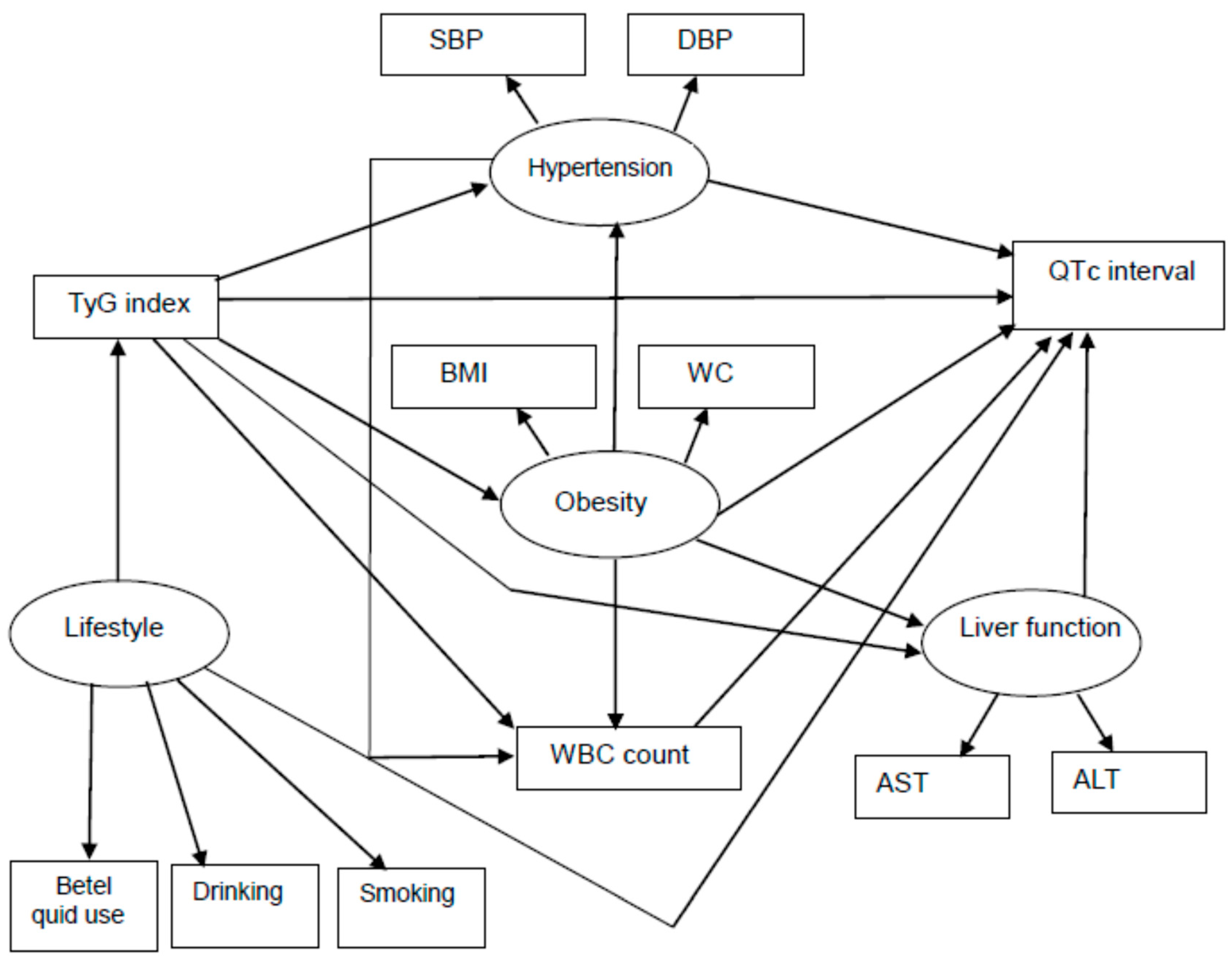
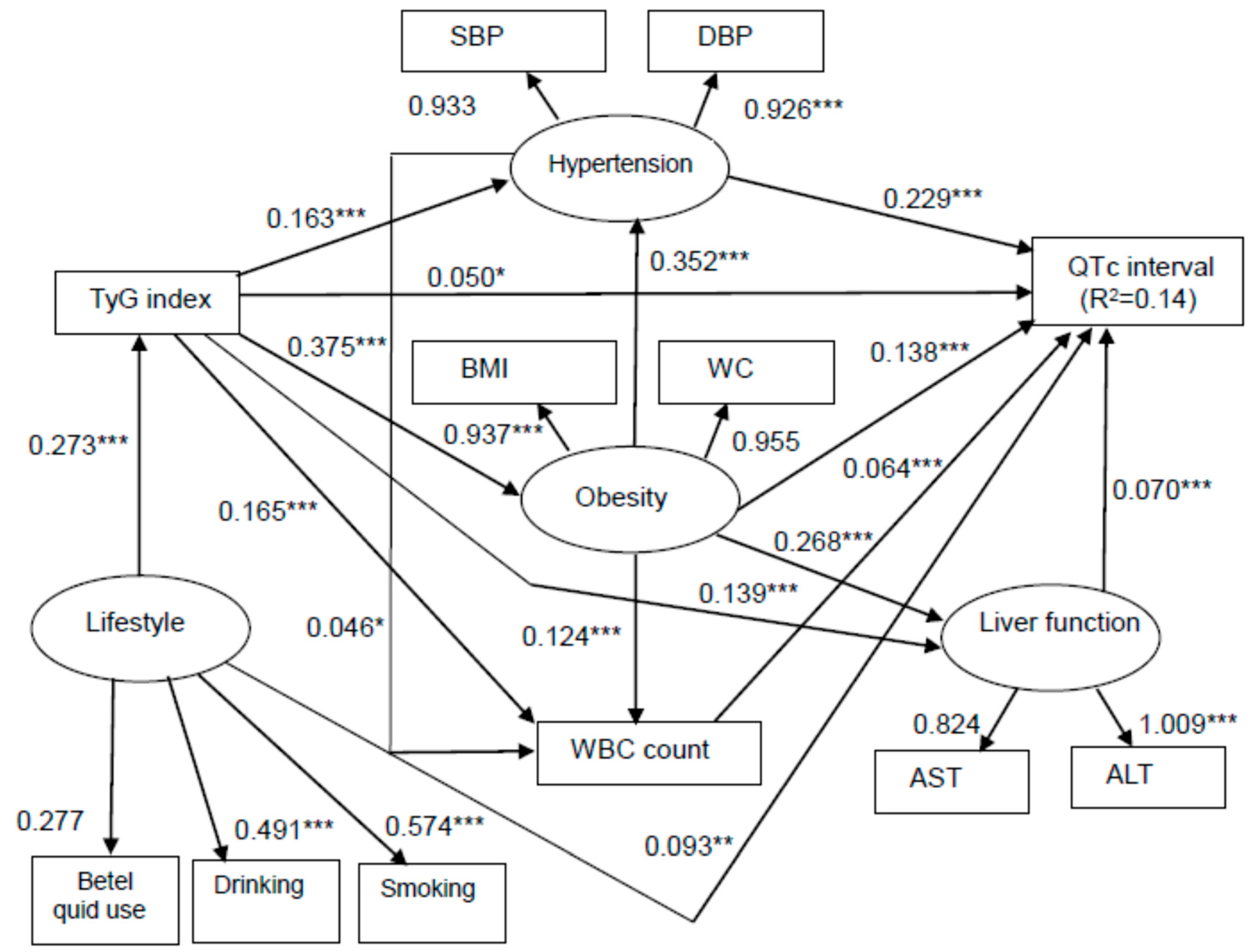
| Variable | First Tertile <4.547 | Second Tertile 4.547–4.810 | Third Tertile >4.810 | p-Value |
|---|---|---|---|---|
| No. | 1060 | 1069 | 1060 | |
| Age (years) (n, %) | ||||
| 544 (51.3) | 417 (39.0) | 320 (30.2) | <0.0001 |
| 376 (35.5) | 484 (45.3) | 547 (51.6) | <0.0001 |
| 123 (11.6) | 151 (14.1) | 173 (16.3) | 0.007 |
| 17 (1.6) | 17 (1.6) | 20 (1.9) | 0.836 |
| Alcohol use (n, %) | 263 (24.8) | 304 (28.4) | 387 (36.5) | <0.0001 |
| Betel quid use (n, %) | 7 (0.7) | 9 (0.8) | 33 (3.1) | <0.0001 |
| Smoking (n, %) | ||||
| 575 (54.3) | 514 (48.1) | 402 (37.9) | <0.0001 |
| 89 (8.4) | 112 (10.5) | 128 (12.1) | 0.020 |
| 314 (29.6) | 374 (35.0) | 476 (44.9) | <0.0001 |
| Physical exercise in the past month (n, %) | ||||
| 249 (23.5) | 258 (24.1) | 315 (29.7) | 0.003 |
| 612 (57.7) | 618 (57.8) | 581 (54.8) | 0.313 |
| 199 (18.8) | 193 (18.1) | 164 (15.5) | 0.132 |
| Poor sleep (n, %) | ||||
| 802 (75.7) | 817 (76.4) | 785 (74.1) | 0.474 |
| 182 (17.2) | 171 (16.0) | 191 (18.0) | 0.480 |
| 76 (7.2) | 81 (7.6) | 84 (7.9) | 0.835 |
| Central obesity (n, %) | 123 (11.6) | 248 (23.2) | 381 (35.9) | <0.0001 |
| Hypertension (n, %) | 255 (24.1) | 398 (37.2) | 519 (49.0) | <0.0001 |
| Diabetes mellitus (n, %) | 218 (20.6) | 366 (34.2) | 587 (55.4) | <0.0001 |
| Metabolic syndrome (n, %) | 27 (2.6) | 124 (11.6) | 587 (55.4) | <0.0001 |
| Chronic kidney disease (n, %) | 16 (1.5) | 41 (3.8) | 52 (4.9) | <0.0001 |
| Shift work (n, %) | 473 (44.6) | 455 (42.6) | 475 (44.8) | 0.573 |
| Framingham 10-year risk score (median, interquartile range) | 1.3 (0.5–2.7) | 2.5 (1.2–4.8) | 4.2 (2.1–7.4) | <0.0001 |
| Variable | First Tertile <4.547 | Second Tertile 4.547–4.810 | Third Tertile >4.810 | p-Value |
|---|---|---|---|---|
| No. | 1060 | 1069 | 1060 | |
| Systolic blood pressure (mmHg) | 119 ± 14 | 124 ± 15 | 128 ± 16 | <0.0001 |
| Diastolic blood pressure (mmHg) | 75 ± 9 | 79 ± 11 | 82 ± 11 | <0.0001 |
| Body mass index (kg/m2) | 23.6 ± 3.2 | 25.1 ± 3.3 | 26.3 ± 3.6 | <0.0001 |
| Waist circumference (cm) | 80.2 ± 8.3 | 84.4 ± 8.1 | 87.3 ± 8.5 | <0.0001 |
| Sodium (mEq/L) | 140.3 ± 1.6 | 140.3 ± 1.6 | 140.2 ± 1.7 | 0.051 |
| Potassium (mEq/L) | 4.02 ± 0.28 | 4.02 ± 0.27 | 4.02 ± 0.29 | 0.865 |
| Calcium (mg/dL) | 9.5 ± 0.3 | 9.6 ± 0.3 | 9.6 ± 0.4 | <0.0001 |
| Fasting glucose (mg/dL) | 94.4 ± 9.6 | 99.0 ± 13.2 | 109.8 ± 30.6 | <0.0001 |
| HbA1c (%) | 5.5 ± 0.4 | 5.6 ± 0.5 | 5.9 ± 1.0 | <0.0001 |
| Total cholesterol (mg/dL) | 181.3 ± 31.1 | 193.7 ± 31.3 | 205.7 ± 35.6 | <0.0001 |
| Triglyceride (mg/dL) | 69.5 (57.0–81.0) | 116.0 (102.0–133.0) | 208.0 (170.0–275.8) | <0.0001 |
| HDL cholesterol (mg/dL) | 51.6 ± 10.8 | 46.1 ± 9.1 | 41.8 ± 7.8 | <0.0001 |
| LDL cholesterol (mg/dL) | 104.2 ± 27.6 | 117.0 ± 27.8 | 116.0 ± 31.7 | <0.0001 |
| Aspartate aminotransferase (U/L) | 26.8 ± 9.0 | 30.9 ± 17.1 | 34.5 ± 18.2 | <0.0001 |
| Alanine aminotransferase (U/L) | 27.0 (20.0–37.0) | 33.0 (25.0–48.0) | 40.0 (30.0–59.0) | <0.0001 |
| Uric acid (mg/dL) | 6.2 ± 1.2 | 6.6 ± 1.3 | 6.9 ± 1.4 | <0.0001 |
| Creatinine (mg/dL) | 1.15 ± 0.17 | 1.17 ± 0.27 | 1.18 ± 0.47 | 0.149 |
| Albumin (g/dL) | 4.4 ± 0.2 | 4.5 ± 0.2 | 4.5 ± 0.2 | <0.0001 |
| Estimated GFR (ml/min/1.73 m2) | 79.6 ± 10.5 | 77.5 ± 10.9 | 77.0 ± 11.1 | <0.0001 |
| White blood cell count (×109/L) | 5.874 ± 1.528 | 6.296 ± 1.557 | 6.747 ± 1.646 | <0.0001 |
| Neutrophil count (×109/L) | 3492 ± 1239 | 3698 ± 1189 | 3930 ± 1238 | <0.0001 |
| Monocyte count (×109/L) | 333 ± 118 | 352 ± 116 | 371 ± 119 | <0.0001 |
| Lymphocyte count (×109/L) | 1863 ± 525 | 2042 ± 564 | 2226 ± 618 | <0.0001 |
| Ejection fraction (%) | 68.3 ± 5.4 | 68.3 ± 5.8 | 68.7 ± 5.5 | 0.366 |
| Left ventricular mass index (g/m2) | 91.5 ± 17.8 | 92.9 ± 17.6 | 95.0 ± 19.4 | 0.016 |
| ECG parameters | ||||
| Heart rate (bpm) | 64.4 ± 9.5 | 66.1 ± 9.2 | 68.8 ± 9.8 | <0.0001 |
| PR interval (ms) | 158.7 ± 39.5 | 158.1 ± 19.5 | 159.0 ± 20.5 | 0.764 |
| QRS duration (ms) | 93.9 ± 10.6 | 93.8 ± 13.1 | 95.2 ± 11.0 | 0.012 |
| QT interval (ms) | 395.4 ± 25.8 | 393.0 ± 24.5 | 390.7 ± 23.9 | 0.0002 |
| QTc interval (ms) | 406.3 ± 21.1 | 408.8 ± 22.8 | 415.2 ± 20.7 | <0.0001 |
| Variable | exp(B) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age | 1.05 | 1.01–1.09 | 0.006 |
| Smoking | 0.95 | 0.58–1.52 | 0.821 |
| Alcohol use | 0.68 | 0.40–1.13 | 0.141 |
| Lack of physical exercise | 1.46 | 0.89–2.34 | 0.128 |
| Shift work | 1.19 | 0.76–1.86 | 0.440 |
| Hypertension | 1.82 | 0.99–3.22 | 0.055 |
| Diabetes mellitus | 1.16 | 0.37–3.00 | 0.787 |
| Central obesity | 1.80 | 1.11–2.88 | 0.018 |
| Calcium | 0.99 | 0.55–1.86 | 0.975 |
| Estimated GFR | 0.10 | 0.98–1.02 | 0.957 |
| TyG index | 2.73 | 1.39–5.24 | 0.004 |
| Variable | Age | Smoking | Drinking | Betel Use | Shift Work | BMI | WC | SBP | DBP | AST | ALT | HbA1c | T-CHOL | HDL-C | LDL-C | TyG | Cr | eGFR | Albumin | WBC | Monocyte | Neutrophil | Lymphocyte | QTc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | −0.04 * | 0.03 | −0.01 | −0.07 ** | 0.00 | 0.04 * | 0.11 ** | 0.17 ** | 0.04 * | −0.04 * | 0.24 ** | 0.11 ** | −0.05 ** | 0.07 ** | 0.17 ** | 0.03 | −0.37 ** | −0.25 ** | −0.04 * | −0.06 ** | −0.02 | −0.07 ** | 0.17 ** |
| Smoking | 1 | 0.28 ** | 0.15 ** | 0.05 ** | 0.00 | 0.02 | −0.07 ** | −0.06 ** | 0.01 | 0.03 | 0.05 ** | −0.00 | −0.10 ** | −0.00 | 0.15 ** | −0.03 | 0.05 ** | −0.06 ** | 0.26 ** | 0.27 ** | 0.20 ** | 0.20 ** | 0.04 * | |
| Drinking | 1 | 0.13 ** | −0.00 | 0.04 * | 0.06 ** | −0.00 | 0.03 | 0.02 | −0.01 | 0.03 | 0.00 | 0.02 | −0.04 * | 0.11 ** | −0.02 | −0.00 | −0.04 * | 0.05 ** | 0.06 ** | 0.04 * | 0.04 * | 0.03 | ||
| Betel use | 1 | −0.02 | 0.00 | 0.02 | 0.00 | 0.00 | 0.03 | 0.02 | 0.02 | −0.01 | −0.02 | −0.06 ** | 0.11 ** | 0.00 | −0.01 | −0.02 | 0.04 * | 0.04 * | 0.02 | 0.06 ** | 0.01 | |||
| Shift work | 1 | 0.01 | 0.01 | 0.02 | 0.01 | −0.02 | 0.01 | −0.02 | −0.00 | −0.01 | 0.01 | 0.00 | −0.01 | 0.04 * | −0.01 | 0.06 ** | 0.05 ** | 0.04 * | 0.04 * | −0.00 | ||||
| BMI | 1 | 0.89 ** | 0.39 ** | 0.35 ** | 0.23 ** | 0.31 ** | 0.18 ** | 0.12 ** | −0.34 ** | 0.16 ** | 0.34 ** | 0.03 | −0.03 | −0.02 | 0.19 ** | 0.14 ** | 0.13 ** | 0.21 ** | 0.24 ** | |||||
| WC | 1 | 0.37 ** | 0.35 ** | 0.24 ** | 0.31 ** | 0.20 ** | 0.14 ** | −0.33 ** | 0.16 ** | 0.37 ** | 0.01 | 0.00 | −0.03 | 0.19 ** | 0.15 ** | 0.12 ** | 0.21 ** | 0.29 ** | ||||||
| SBP | 1 | 0.86 ** | 0.17 ** | 0.16 ** | 0.15 ** | 0.08 ** | −0.10 ** | 0.05 * | 0.25 ** | 0.09 ** | −0.09 ** | 0.10 ** | 0.13 ** | 0.05 ** | 0.13 ** | 0.08 ** | 0.29 ** | |||||||
| DBP | 1 | 0.18 ** | 0.17 ** | 0.16 ** | 0.14 ** | −0.09 ** | 0.08 ** | 0.29 ** | 0.05 ** | −0.12 ** | 0.08 ** | 0.12 ** | 0.05 ** | 0.12 ** | 0.07 ** | 0.29 ** | ||||||||
| AST | 1 | 0.83 ** | 0.10 ** | 0.09 ** | −0.08 ** | 0.05 ** | 0.22 ** | 0.01 | −0.02 | 0.04 * | 0.07 ** | 0.09 ** | 0.02 | 0.12 ** | 0.18 ** | |||||||||
| ALT | 1 | 0.11 ** | 0.12 ** | −0.17 ** | 0.12 ** | 0.25 ** | −0.02 | 0.04 * | 0.06 ** | 0.10 ** | 0.12 ** | 0.04 * | 0.15 ** | 0.17 ** | ||||||||||
| HbA1c | 1 | 0.15 ** | −0.13 ** | 0.11 ** | 0.35 ** | −0.00 | −0.03 | −0.05 ** | 0.12 ** | 0.06 ** | 0.08 ** | 0.15 ** | 0.14 ** | |||||||||||
| T-CHOL | 1 | 0.14 ** | 0.82 ** | 0.35 ** | −0.00 | −0.12 ** | 0.11 ** | 0.07 ** | 0.00 | 0.01 | 0.15 ** | 0.08 ** | ||||||||||||
| HDL-C | 1 | −0.05 ** | −0.44 ** | −0.04 * | 0.01 | 0.04 * | −0.14 ** | −0.11 ** | −0.09 ** | −0.15 ** | −0.11 ** | |||||||||||||
| LDL-C | 1 | 0.12 ** | −0.02 | −0.07 ** | 0.09 ** | 0.09 ** | 0.04 * | 0.04 * | 0.14 ** | 0.06 ** | ||||||||||||||
| TyG index | 1 | 0.04 * | −0.10 ** | 0.07 ** | 0.22 ** | 0.13 ** | 0.14 ** | 0.26 ** | 0.18 ** | |||||||||||||||
| Cr | 1 | −0.55 ** | −0.01 | −0.02 | 0.00 | −0.00 | −0.05 ** | 0.02 | ||||||||||||||||
| eGFR | 1 | 0.05 * | 0.05 ** | 0.04 * | 0.04 * | 0.05 ** | −0.03 | |||||||||||||||||
| Albumin | 1 | 0.07 ** | −0.01 | 0.07 ** | 0.07 ** | 0.04 * | ||||||||||||||||||
| WBC count | 1 | 0.67 ** | 0.91 ** | 0.63 ** | 0.14 ** | |||||||||||||||||||
| Monocyte count | 1 | 0.55 ** | 0.43 ** | 0.08 ** | ||||||||||||||||||||
| Neutrophil count | 1 | 0.27 ** | 0.12 ** | |||||||||||||||||||||
| Lymphocyte count | 1 | 0.11 ** | ||||||||||||||||||||||
| QTc interval | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-L.; Hsuan, C.-F.; Wu, C.-C.; Hung, W.-C.; Tsai, I.-T.; Wei, C.-T.; Yu, T.-H.; Lu, I.-C.; Chung, F.-M.; Lee, Y.-J.; et al. Association between Triglyceride Glucose Index and Corrected QT Prolongation in Chinese Male Steelworkers. Int. J. Environ. Res. Public Health 2021, 18, 4020. https://doi.org/10.3390/ijerph18084020
Lee T-L, Hsuan C-F, Wu C-C, Hung W-C, Tsai I-T, Wei C-T, Yu T-H, Lu I-C, Chung F-M, Lee Y-J, et al. Association between Triglyceride Glucose Index and Corrected QT Prolongation in Chinese Male Steelworkers. International Journal of Environmental Research and Public Health. 2021; 18(8):4020. https://doi.org/10.3390/ijerph18084020
Chicago/Turabian StyleLee, Thung-Lip, Chin-Feng Hsuan, Cheng-Ching Wu, Wei-Chin Hung, I-Ting Tsai, Ching-Ting Wei, Teng-Hung Yu, I-Cheng Lu, Fu-Mei Chung, Yau-Jiunn Lee, and et al. 2021. "Association between Triglyceride Glucose Index and Corrected QT Prolongation in Chinese Male Steelworkers" International Journal of Environmental Research and Public Health 18, no. 8: 4020. https://doi.org/10.3390/ijerph18084020
APA StyleLee, T.-L., Hsuan, C.-F., Wu, C.-C., Hung, W.-C., Tsai, I.-T., Wei, C.-T., Yu, T.-H., Lu, I.-C., Chung, F.-M., Lee, Y.-J., & Lu, Y.-C. (2021). Association between Triglyceride Glucose Index and Corrected QT Prolongation in Chinese Male Steelworkers. International Journal of Environmental Research and Public Health, 18(8), 4020. https://doi.org/10.3390/ijerph18084020






